An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma
Abstract
:1. Introduction
2. Immunotherapy Strategies for HCC
2.1. Multi-Kinase Inhibitors for HCC
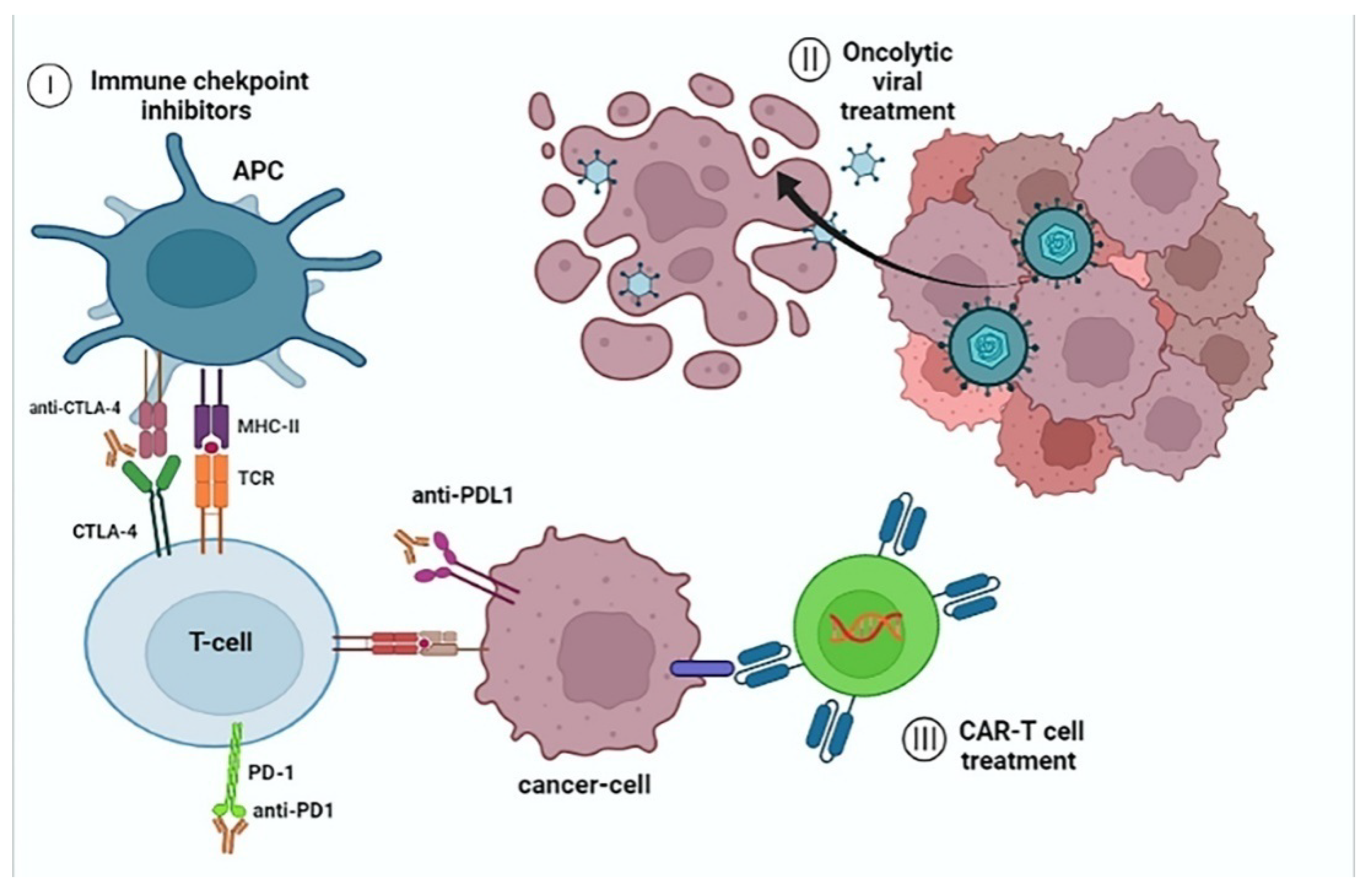
2.2. Immune Checkpoint Inhibition for HCC
2.3. Cell-Based Immunotherapy for HCC
2.4. Oncolytic Viral Therapy for HCC
2.5. Cancer Vaccines for HCC
2.6. Gut Microbiome Modulation for Optimal Anti-Tumor Response in HCC
3. Immunotherapy Strategies for CCA
3.1. Small-Molecule Kinase Inhibitors (SMKIs) for CCA
3.2. Immune Checkpoint Blockade in CCA
3.3. Cancer Vaccines in CCA
3.4. CAR-T Cell Therapy for CCA
3.5. The Implication of Gut-Microbiome in CCA
3.6. Mechanisms for Overcoming Immunoresistance
4. Adverse Effects of Immunotherapeutic and Targeted Agents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| ARID1A | AT-rich interactive domain-containing protein 1A gene |
| BAs | Bile acids |
| CAF | Cancer-Associated Fibroblast |
| CAR | Chimeric antigen receptor |
| CAR | Chimeric antigen receptor |
| CIKs | Cytokine-induced killer cells |
| CTL4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DC | Dendritic cells |
| EGFR | Epidermal growth factor receptor |
| FMT | Fecal microbiota transplant |
| GPC3 | Glypican-3 |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular Carcinoma |
| HCV | Hepatitis C virus |
| HSV | Herpes simplex virus 1 |
| iCCA | Intrahepatic cholangiocarcinoma |
| MDSC | Myeloid-derived suppressor cell |
| MHCI/II | Major histocompatibility complex I/II) |
| NASH | Non-alcoholic steatohepatitis |
| PD-1 | Programmed cell Death protein-1 |
| PD-L1 | Programmed cell Death protein Ligand-1 |
| PDGF-R | Platelet-derived growth factor |
| PSC | Primary sclerosing cholangitis |
| SMKIs | Small-molecule kinase inhibitors |
| TACE | Transarterial chemoembolization |
| TAM | Tumor-associated macrophage |
| TCR | T-cell receptor |
| TKI | Tyrosine kinase inhibitor |
| TLL | Tumour-infiltrating lymphocytes |
| TME | Tumour microenvironment |
| TNF | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
References
- Giannitrapani, L.; Zerbo, M.; Amodeo, S.; Pipitone, E.; Galia, M.; Li Cavoli, T.V.; Minissale, M.G.; Licata, A.; Schiavone, C.; Brancatelli, G.; et al. The Changing Epidemiology of Hepatocellular Carcinoma: Experience of a Single Center. Biomed Res. Int. 2020, 2020, 5309307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global Trends in Mortality from Intrahepatic and Extrahepatic Cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.B.-B.; Li, J.W.; Chang, P.-E.; Chow, K.-Y.; Tan, C.-K. Deciphering the Epidemiology of Hepatocellular Carcinoma through the Passage of Time: A Study of 1401 Patients across 3 Decades. Hepatol. Commun. 2017, 1, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Maemura, K.; Natsugoe, S.; Takao, S. Molecular Mechanism of Cholangiocarcinoma Carcinogenesis. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 754–760. [Google Scholar] [CrossRef]
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M.; Diagnosis Group; Systemic Therapy Group. Management Consensus Guideline for Hepatocellular Carcinoma: 2020 Update on Surveillance, Diagnosis, and Systemic Treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2021, 120, 1051–1060. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. Role of Autophagy in Cholangiocarcinoma: An Autophagy-Based Treatment Strategy. World J. Gastrointest. Oncol. 2021, 13, 1229–1243. [Google Scholar] [CrossRef]
- Suk, W.A.; Bhudhisawasdi, V.; Ruchirawat, M. The Curious Case of Cholangiocarcinoma: Opportunities for Environmental Health Scientists to Learn about a Complex Disease. J. Environ. Public Health 2018, 2018, 2606973. [Google Scholar] [CrossRef]
- Lendvai, G.; Szekerczés, T.; Illyés, I.; Dóra, R.; Kontsek, E.; Gógl, A.; Kiss, A.; Werling, K.; Kovalszky, I.; Schaff, Z.; et al. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol. Oncol. Res. 2020, 26, 3–15. [Google Scholar] [CrossRef]
- Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part I: Classification, Diagnosis and Staging. Dig. Liver Dis. 2020, 52, 1282–1293. [Google Scholar] [CrossRef]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, Histomorphological and Molecular Classification of Cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen Vaccine: An Emerging Tumor Immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.-Y.; Gong, W. Immunotherapy in Cholangiocarcinoma: From Concept to Clinical Trials. Surg. Pract. Sci. 2021, 5, 100028. [Google Scholar] [CrossRef]
- Fritz, J.M.; Lenardo, M.J. Development of Immune Checkpoint Therapy for Cancer. J. Exp. Med. 2019, 216, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Ghisoni, E.; Genta, S.; Scotto, G.; Tuninetti, V.; Turinetto, M.; Valabrega, G. Immuno-Metabolism and Microenvironment in Cancer: Key Players for Immunotherapy. Int. J. Mol. Sci. 2020, 21, 4414. [Google Scholar] [CrossRef]
- Eatrides, J.; Wang, E.; Kothari, N.; Kim, R. Role of Systemic Therapy and Future Directions for Hepatocellular Carcinoma. Cancer Control 2017, 24, 1073274817729243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent Progress in Treatment of Hepatocellular Carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-X.; Schwabe, R.F. The Gut Microbiome and Liver Cancer: Mechanisms and Clinical Translation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 527–539. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sakabe, T.; Abe, H.; Tanii, M.; Takahashi, H.; Chiba, A.; Yanagida, E.; Shibamoto, Y.; Ogasawara, M.; Tsujitani, S.-I.; et al. Dendritic Cell-Based Immunotherapy Targeting Synthesized Peptides for Advanced Biliary Tract Cancer. J. Gastrointest. Surg. 2013, 17, 1609–1617. [Google Scholar] [CrossRef]
- Sen, S.; Shroff, R.T. Emerging Targeted and Immunotherapies in Cholangiocarcinoma. Oncol. Hematol. Rev. 2019, 15, 71. [Google Scholar] [CrossRef]
- Lau, D.K.; Mouradov, D.; Wasenang, W.; Luk, I.Y.; Scott, C.M.; Williams, D.S.; Yeung, Y.H.; Limpaiboon, T.; Iatropoulos, G.F.; Jenkins, L.J.; et al. Genomic Profiling of Biliary Tract Cancer Cell Lines Reveals Molecular Subtypes and Actionable Drug Targets. iScience 2019, 21, 624–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3. Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.L.; Yeo, W.; Mo, F.; Li, L.; Lee, K.; Hui, P.; Ma, B.; Mok, T.S.K.; Chan, A.T.C.; Lai, P.B.S.; et al. A Phase Ii Study on Combination of Axitinib and Transarterial Chemoembolization (Tace) for Treatment of Inoperable Hepatocellular Carcinoma (Hcc). Ann. Oncol. 2014, 25 (Suppl. S4), iv247. [Google Scholar] [CrossRef]
- Mei, Q.; Chen, M.; Lu, X.; Li, X.; Duan, F.; Wang, M.; Luo, G.; Han, W. An Open-Label, Single-Arm, Phase I/II Study of Lower-Dose Decitabine Based Therapy in Patients with Advanced Hepatocellular Carcinoma. Oncotarget 2015, 6, 16698–16711. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-J.; Kim, T.-Y.; Feng, Y.-H.; Chao, Y.; Lin, D.-Y.; Ryoo, B.-Y.; Huang, D.C.-L.; Schnell, D.; Hocke, J.; Loembé, A.-B.; et al. A Phase I/Randomized Phase II Study to Evaluate the Safety, Pharmacokinetics, and Efficacy of Nintedanib versus Sorafenib in Asian Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2018, 7, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus Sorafenib in Advanced Hepatocellular Carcinoma (CheckMate 459): A Randomised, Multicentre, Open-Label, Phase 3. Trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Wu, L.; Tang, Q.; Yin, X.; Yan, D.; Tang, M.; Xin, J.; Pan, Q.; Ma, C.; Yan, S. The Therapeutic Potential of Adipose Tissue-Derived Mesenchymal Stem Cells to Enhance Radiotherapy Effects on Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2019, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.S.; Kichloo, A.; Singh, J.; Albosta, M.; Lekkala, M. Current Immunotherapy in Gastrointestinal Malignancies A Review. J. Investig. Med. 2021, 69, 689–696. [Google Scholar] [CrossRef]
- Nakatake, R.; Kaibori, M.; Nakamura, Y.; Tanaka, Y.; Matushima, H.; Okumura, T.; Murakami, T.; Ino, Y.; Todo, T.; Kon, M. Third-Generation Oncolytic Herpes Simplex Virus Inhibits the Growth of Liver Tumors in Mice. Cancer Sci. 2018, 109, 600–610. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, C.; Ren, W.; Ju, F.; Xu, Z.; Liu, H.; Yu, Z.; Chen, J.; Zhang, J.; Liu, P.; et al. Intravenous Injections of a Rationally Selected Oncolytic Herpes Virus as a Potent Virotherapy for Hepatocellular Carcinoma. Mol. Ther. Oncolytics 2019, 15, 153–165. [Google Scholar] [CrossRef] [Green Version]
- de Graaf, J.F.; de Vor, L.; Fouchier, R.A.M.; van den Hoogen, B.G. Armed Oncolytic Viruses: A Kick-Start for Anti-Tumor Immunity. Cytokine Growth Factor Rev. 2018, 41, 28–39. [Google Scholar] [CrossRef]
- Buonaguro, L. Human Hepatocellular Carcinoma (HCC). Cancers 2020, 12, 3739. [Google Scholar] [CrossRef]
- Cavalluzzo, B.; Mauriello, A.; Ragone, C.; Manolio, C.; Tornesello, M.L.; Buonaguro, F.M.; Tvingsholm, S.A.; Hadrup, S.R.; Tagliamonte, M.; Buonaguro, L. Novel Molecular Targets for Hepatocellular Carcinoma. Cancers 2021, 14, 140. [Google Scholar] [CrossRef]
- Ikeda, M.; Okusaka, T.; Ohno, I.; Mitsunaga, S.; Kondo, S.; Ueno, H.; Morizane, C.; Gemmoto, K.; Suna, H.; Ushida, Y.; et al. Phase I Studies of Peptide Vaccine Cocktails Derived from GPC3, WDRPUH and NEIL3 for Advanced Hepatocellular Carcinoma. Immunotherapy 2021, 13, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Sakai, M.; Yoshikawa, T.; Ofuji, K.; Nakatsura, T. A Glypican-3-Derived Peptide Vaccine against Hepatocellular Carcinoma. Oncoimmunology 2012, 1, 1448–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-C.; Wang, H.-C.; Hung, C.-F.; Huang, P.-F.; Lia, C.-R.; Chen, M.-F. Vaccination of Advanced Hepatocellular Carcinoma Patients with Tumor Lysate-Pulsed Dendritic Cells: A Clinical Trial. J. Immunother. 2005, 28, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Kong, F.-H.; Liu, X.; Wang, X.-B. Immunotherapy with Dendritic Cells and Cytokine-Induced Killer Cells for Hepatocellular Carcinoma: A Meta-Analysis. World J. Gastroenterol. 2019, 25, 3649–3663. [Google Scholar] [CrossRef]
- Calmeiro, J.; Carrascal, M.A.; Tavares, A.R.; Ferreira, D.A.; Gomes, C.; Cruz, M.T.; Falcão, A.; Neves, B.M. Pharmacological Combination of Nivolumab with Dendritic Cell Vaccines in Cancer Immunotherapy: An Overview. Pharmacol. Res. 2021, 164, 105309. [Google Scholar] [CrossRef]
- Teng, C.-F.; Wang, T.; Wu, T.-H.; Lin, J.-H.; Shih, F.-Y.; Shyu, W.-C.; Jeng, L.-B. Combination Therapy with Dendritic Cell Vaccine and Programmed Death Ligand 1 Immune Checkpoint Inhibitor for Hepatocellular Carcinoma in an Orthotopic Mouse Model. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922034. [Google Scholar] [CrossRef]
- Wu, L.; Feng, J.; Li, J.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. The Gut Microbiome-Bile Acid Axis in Hepatocarcinogenesis. Biomed. Pharmacother. 2021, 133, 111036. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile Acid Nuclear Receptor FXR and Digestive System Diseases. Acta Pharm. Sin. B. 2015, 5, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.Y.; Suk, K.T. The Role of the Gut Microbiome in Liver Cirrhosis Treatment. Int. J. Mol. Sci. 2020, 22, 199. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Greten, T.F. Gut Microbiome in HCC—Mechanisms, Diagnosis and Therapy. J. Hepatol. 2020, 72, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut Microbiome Affects the Response to Anti-PD-1 Immunotherapy in Patients with Hepatocellular Carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temraz, S.; Nassar, F.; Kreidieh, F.; Mukherji, D.; Shamseddine, A.; Nasr, R. Hepatocellular Carcinoma Immunotherapy and the Potential Influence of Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 7800. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierrard, J.; Seront, E. Impact of the Gut Microbiome on Immune Checkpoint Inhibitor Efficacy—A Systematic Review. Curr. Oncol. 2019, 26, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, S.; Gores, G.J. Emerging Molecular Therapeutic Targets for Cholangiocarcinoma. J. Hepatol. 2017, 67, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in Previously Treated Patients with Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusions or Rearrangements: Mature Results from a Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in Advanced or Inoperable FGFR2 Gene Fusion-Positive Intrahepatic Cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Shroff, R.T.; Yarchoan, M.; O’Connor, A.; Gallagher, D.; Zahurak, M.L.; Rosner, G.; Ohaji, C.; Sartorius-Mergenthaler, S.; Parkinson, R.; Subbiah, V.; et al. Erratum: The Oral VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Cholangiocarcinoma. Br. J. Cancer 2018, 118, e2. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.H.; Uson Junior, P.L.S.; Masci, P.; Kosiorek, H.; Halfdanarson, T.R.; Mody, K.; Babiker, H.; DeLeon, T.; Sonbol, M.B.; Gores, G.; et al. A Pilot Study of Pan-FGFR Inhibitor Ponatinib in Patients with FGFR-Altered Advanced Cholangiocarcinoma. Investig. New Drugs 2022, 40, 134–141. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Futibatinib, an Investigational Agent for the Treatment of Intrahepatic Cholangiocarcinoma: Evidence to Date and Future Perspectives. Expert Opin. Investig. Drugs 2021, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Krook, M.A.; Lenyo, A.; Wilberding, M.; Barker, H.; Dantuono, M.; Bailey, K.M.; Chen, H.-Z.; Reeser, J.W.; Wing, M.R.; Miya, J.; et al. Efficacy of FGFR Inhibitors and Combination Therapies for Acquired Resistance in FGFR2-Fusion Cholangiocarcinoma. Mol. Cancer Ther. 2020, 19, 847–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-Institutional Phase II Study of Selumetinib in Patients with Metastatic Biliary Cancers. J. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised Phase II Trial (SWOG S1310) of Single Agent MEK Inhibitor Trametinib Versus 5-Fluorouracil or Capecitabine in Refractory Advanced Biliary Cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Pilla, L.; Elvevi, A.; Longarini, R.; Rossi, R.E.; Bidoli, P.; Invernizzi, P. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, S.; Saleh, M.; Bendell, J.; Infante, J.R.; Jones, S.; Kurkjian, C.D.; Moore, K.M.; Kazakin, J.; Abbadessa, G.; Wang, Y.; et al. A Phase I Dose Escalation Study of Oral C-MET Inhibitor Tivantinib (ARQ 197) in Combination with Gemcitabine in Patients with Solid Tumors. Ann. Oncol. 2014, 25, 1416–1421. [Google Scholar] [CrossRef]
- Salati, M.; Caputo, F.; Baldessari, C.; Galassi, B.; Grossi, F.; Dominici, M.; Ghidini, M. IDH Signalling Pathway in Cholangiocarcinoma: From Biological Rationale to Therapeutic Targeting. Cancers 2020, 12, 3310. [Google Scholar] [CrossRef]
- Shahda, S.; Noonan, A.M.; Bekaii-Saab, T.S.; O’Neil, B.H.; Sehdev, A.; Shaib, W.L.; Helft, P.R.; Loehrer, P.J.; Tong, Y.; Liu, Z.; et al. A Phase II Study of Pembrolizumab in Combination with MFOLFOX6 for Patients with Advanced Colorectal Cancer. J. Clin. Oncol. 2017, 35 (Suppl. S15), 3541. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.-Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and Safety of Pembrolizumab for the Treatment of Advanced Biliary Cancer: Results from the KEYNOTE-158 and KEYNOTE-028 Studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-Institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Boilève, A.; Hilmi, M.; Gougis, P.; Cohen, R.; Rousseau, B.; Blanc, J.-F.; Ben Abdelghani, M.; Castanié, H.; Dahan, L.; Tougeron, D.; et al. Triplet Combination of Durvalumab, Tremelimumab, and Paclitaxel in Biliary Tract Carcinomas: Safety Run-in Results of the Randomized IMMUNOBIL PRODIGE 57 Phase II Trial. Eur. J. Cancer 2021, 143, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.G.; Sanchon-Sanchez, P.; Cives-Losada, C.; Del Carmen, S.; González-Santiago, J.M.; Monte, M.J.; Macias, R.I.R. Novel Pharmacological Options in the Treatment of Cholangiocarcinoma: Mechanisms of Resistance. Cancers 2021, 13, 2358. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Oh, D.-Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.-T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase I Study of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Pretreated Biliary Tract Cancer. J. Immunother. Cancer 2020, 8, e000564. [Google Scholar] [CrossRef]
- Han, S.; Lee, S.Y.; Wang, W.-W.; Tan, Y.B.; Sim, R.H.Z.; Cheong, R.; Tan, C.; Hopkins, R.; Connolly, J.; Shuen, W.H.; et al. A Perspective on Cell Therapy and Cancer Vaccine in Biliary Tract Cancers (BTCs). Cancers 2020, 12, 3404. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Conboy, C.B.; Gores, G.J.; Rizvi, S. Immunobiology of Cholangiocarcinoma. JHEP Rep. 2019, 1, 297–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, B.; Ren, T.; Wang, X.; Wang, H.; Zou, Y.; Sun, Y.; Liu, S.; Ren, Z.; Yu, Z. Dysbiosis in the Human Microbiome of Cholangiocarcinoma. Front. Physiol. 2021, 12, 715536. [Google Scholar] [CrossRef]
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.K.; Min, S.K.; Lee, W.H. 16S RDNA Microbiome Composition Pattern Analysis as a Diagnostic Biomarker for Biliary Tract Cancer. World J. Surg. Oncol. 2020, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Koustas, E.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. The Resistance Mechanisms of Checkpoint Inhibitors in Solid Tumors. Biomolecules 2020, 10, 666. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28 (Suppl. S4), iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, Z.; Yan, H.; He, Q.; Yang, X.; Luo, P. A Comprehensive Review of Clinical Cardiotoxicity Incidence of FDA-Approved Small-Molecule Kinase Inhibitors. Front. Pharmacol. 2020, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Jin, Y.; Yan, H.; Xu, Z.; Yang, B.; He, Q.; Luo, P. Hepatotoxicity of FDA-Approved Small Molecule Kinase Inhibitors. Expert Opin. Drug Saf. 2021, 20, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yan, H.; Xu, Z.; Yang, B.; He, Q.; Wang, X.; Luo, P. Cutaneous Toxicity of FDA-Approved Small-Molecule Kinase Inhibitors. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
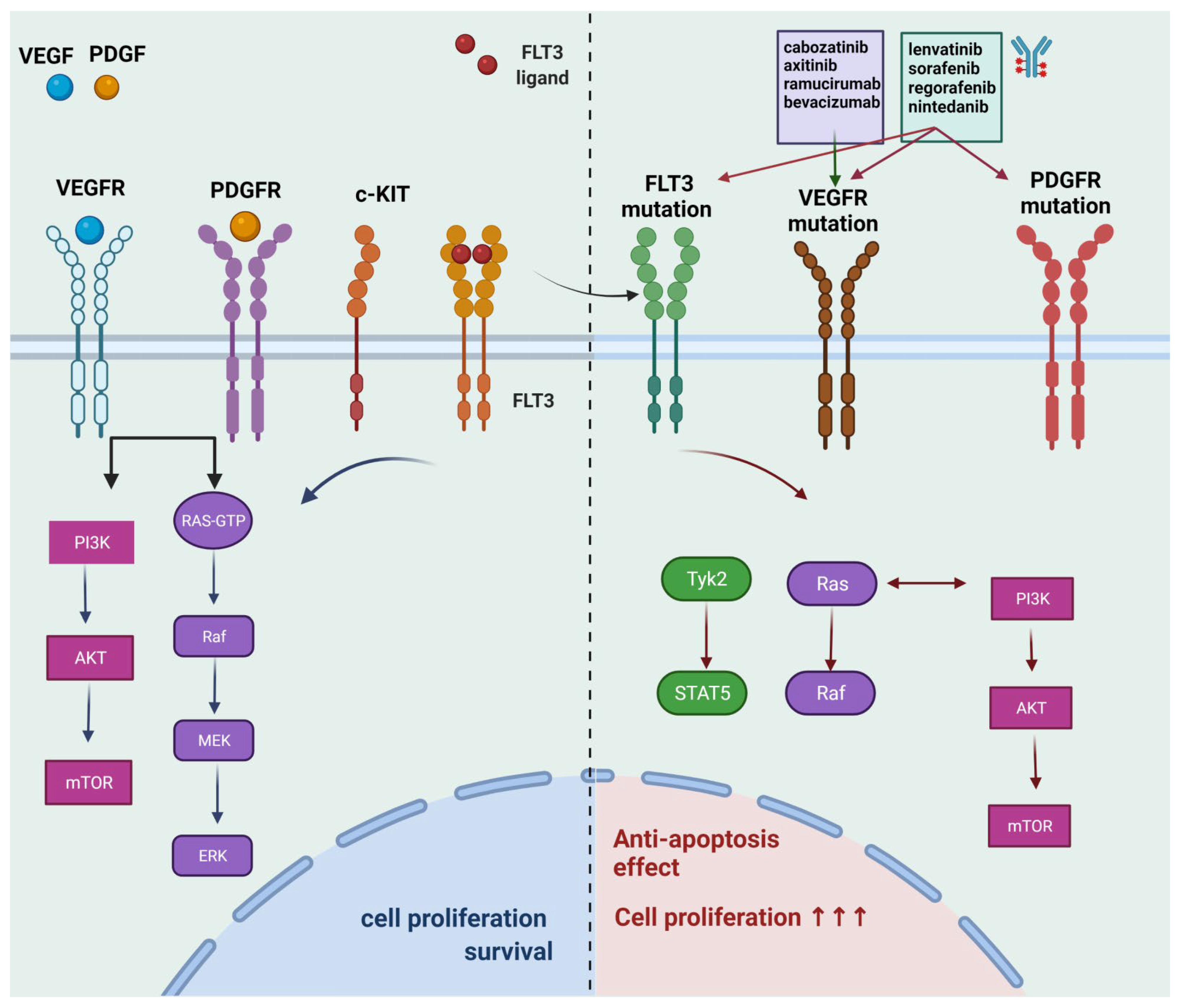
| Pharmaceutical Agent | Molecular Target | Phase of Clinical Trial |
|---|---|---|
| Immune checkpoint inhibitors | ||
| Pembrolizumab | PD-1 | phase III trial [33] |
| Nivolumab | PD-1 | phase III trial [32] |
| Atezolizumab | PD-L1 | phase III trial [31] |
| Multikinase inhibitors | ||
| Lenvatinib | PDGF-R, PDGF-R, c-Kit, VEGFR, Raf, Flt3 | phase III trial [18] |
| Sorafenib | RET, PDGF-R, c-Kit, VEGFR, Raf, Flt3 | phase III trial [24,25] |
| Regorafenib | RET, PDGF-R, KIT, VEGFR, RAF, Flt3, TIE2 | phase III trial [27] |
| Cabozantinib | AXL, VEGFR, MET | phase III trial [26] |
| Nintedanib | RET, PDGF-R, c-Kit, VEGFR, Raf, Flt3 | phase II trial [30] |
| Decitabine | DNA methylationphase | phase trial I/II [29] |
| Tyrosine kinase inhibitors | ||
| Axitinib | VEGFR | phase II trial [28] |
| Various monoclonal antibodies | ||
| Ramucirumab | VEGFR | phase III trial [D136] |
| Bevacizumab | VEGFR | phase II trial [31] |
| Pharmaceutical Agent | Molecular Target | Phase of Clinical Trial |
|---|---|---|
| Immune checkpoint inhibitors | ||
| Pembrolizumab | PD-1 | phase II trial [69] |
| Nivolumab | PD-1 | phase II trial [70] |
| Durvalumab | PD-L1 | phase I/II trial [71] |
| Tremelimumab | CTLA-4 | phase II trial [71] |
| Bintrafusp alfa | PD-1, TGF-b | phase I trial [73] |
| Small-molecule kinase inhibitors | ||
| Infigratinib | FGFR | phase II trial [57] |
| Pazopanib | PDGF, VEGFR, c-kit | phase I trial [59] |
| Ponatinib | PDGF, VEGFR2, Scr, FGFR1 | phase II trial [60] |
| Futibatinib | FGFR | phase III trial [61] |
| Tivantinib | MET | phase I trial [65] |
| Trametinib | MEK1/2 | phase II trial [64] |
| Selumetinib | MEK1/22 | phase II trial [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifylli, E.-M.; Koustas, E.; Papadopoulos, N.; Sarantis, P.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Karamouzis, M.V. An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma. Life 2022, 12, 665. https://doi.org/10.3390/life12050665
Trifylli E-M, Koustas E, Papadopoulos N, Sarantis P, Aloizos G, Damaskos C, Garmpis N, Garmpi A, Karamouzis MV. An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma. Life. 2022; 12(5):665. https://doi.org/10.3390/life12050665
Chicago/Turabian StyleTrifylli, Eleni-Myrto, Evangelos Koustas, Nikolaos Papadopoulos, Panagiotis Sarantis, Georgios Aloizos, Christos Damaskos, Nikolaos Garmpis, Anna Garmpi, and Michalis V. Karamouzis. 2022. "An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma" Life 12, no. 5: 665. https://doi.org/10.3390/life12050665
APA StyleTrifylli, E.-M., Koustas, E., Papadopoulos, N., Sarantis, P., Aloizos, G., Damaskos, C., Garmpis, N., Garmpi, A., & Karamouzis, M. V. (2022). An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma. Life, 12(5), 665. https://doi.org/10.3390/life12050665











