Paediatric Partial-Thickness Burn Therapy: A Meta-Analysis and Systematic Review of Randomised Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Methods for Identification of Records
2.2. Study Selection, Data Extraction and Management
2.3. Assessment of Methodological Quality of Included Records
2.4. Data Synthesis
3. Results
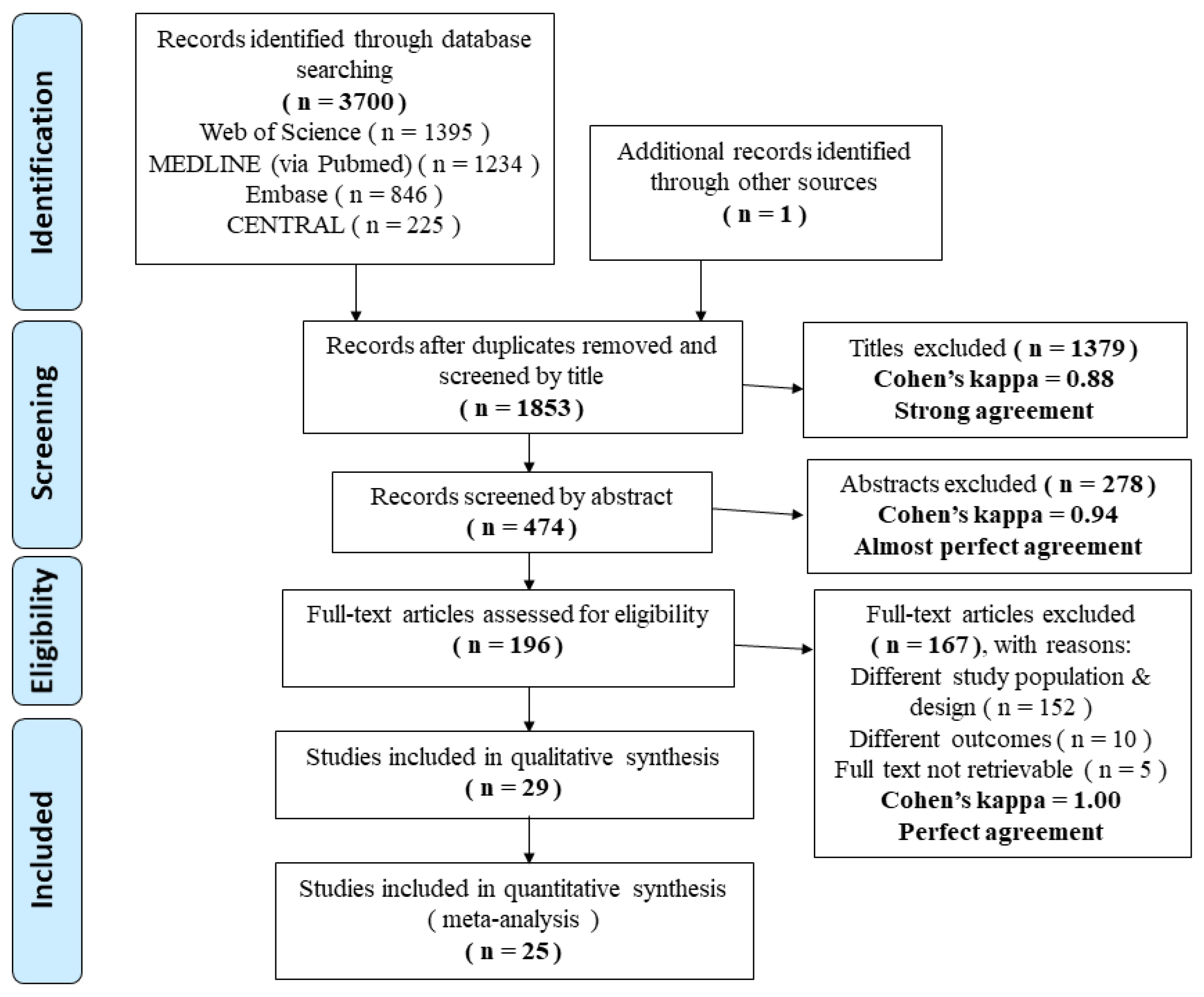
3.1. Search Results
3.2. Description of Included Studies
3.3. Methodological Quality of the Included Studies
3.4. Effects of Interventions
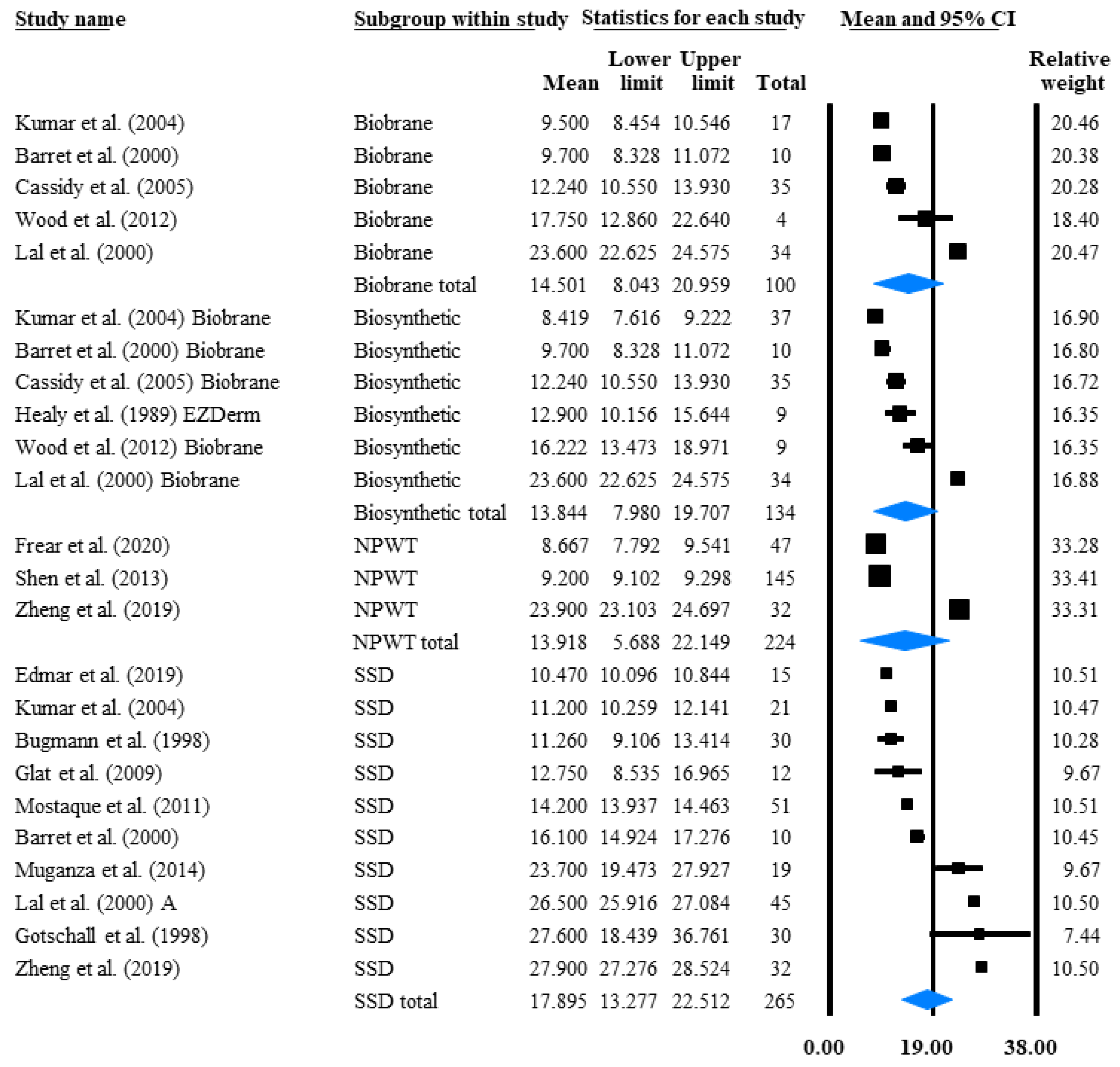
3.4.1. Time to Reepithelialisation (TTRE)
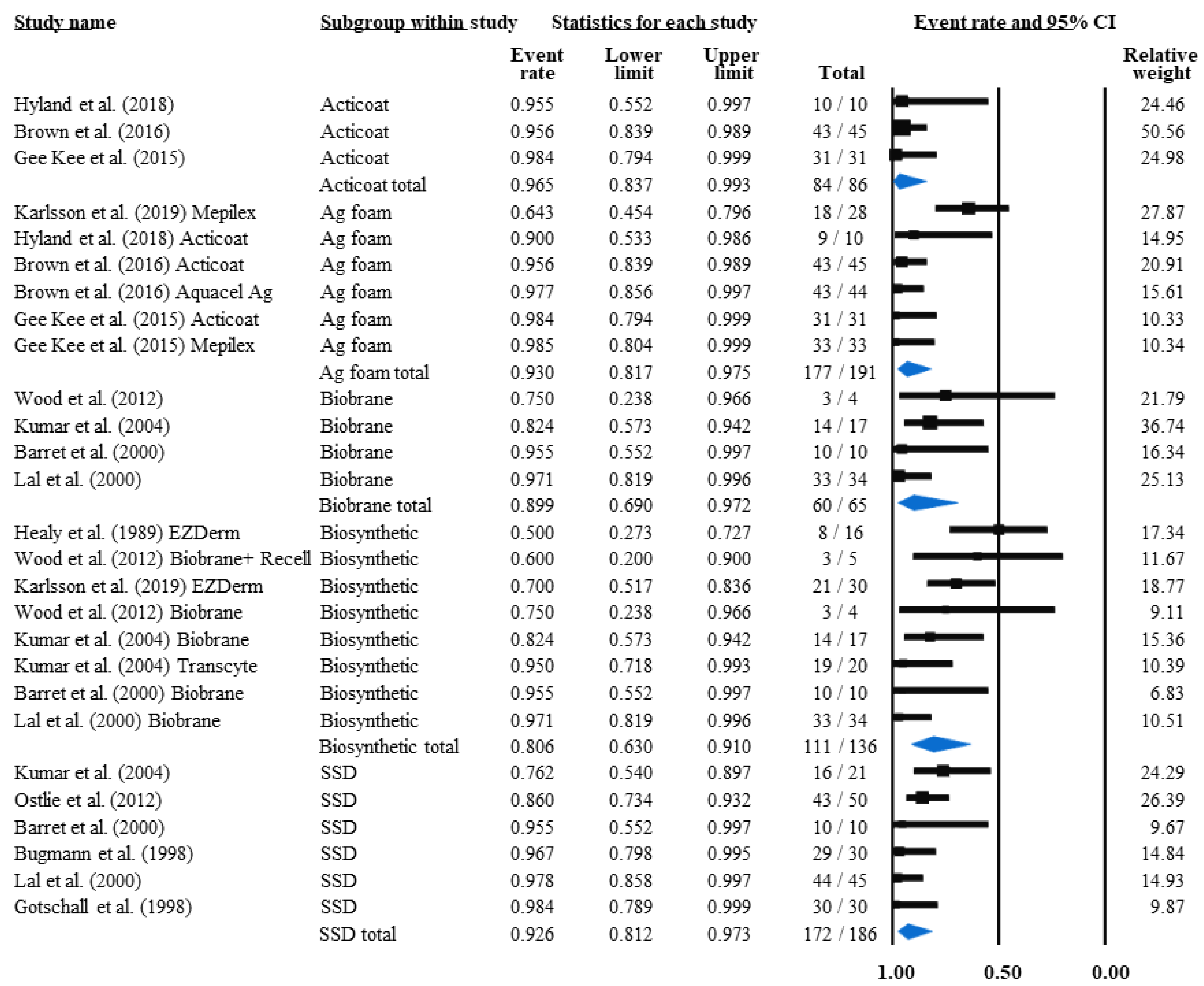
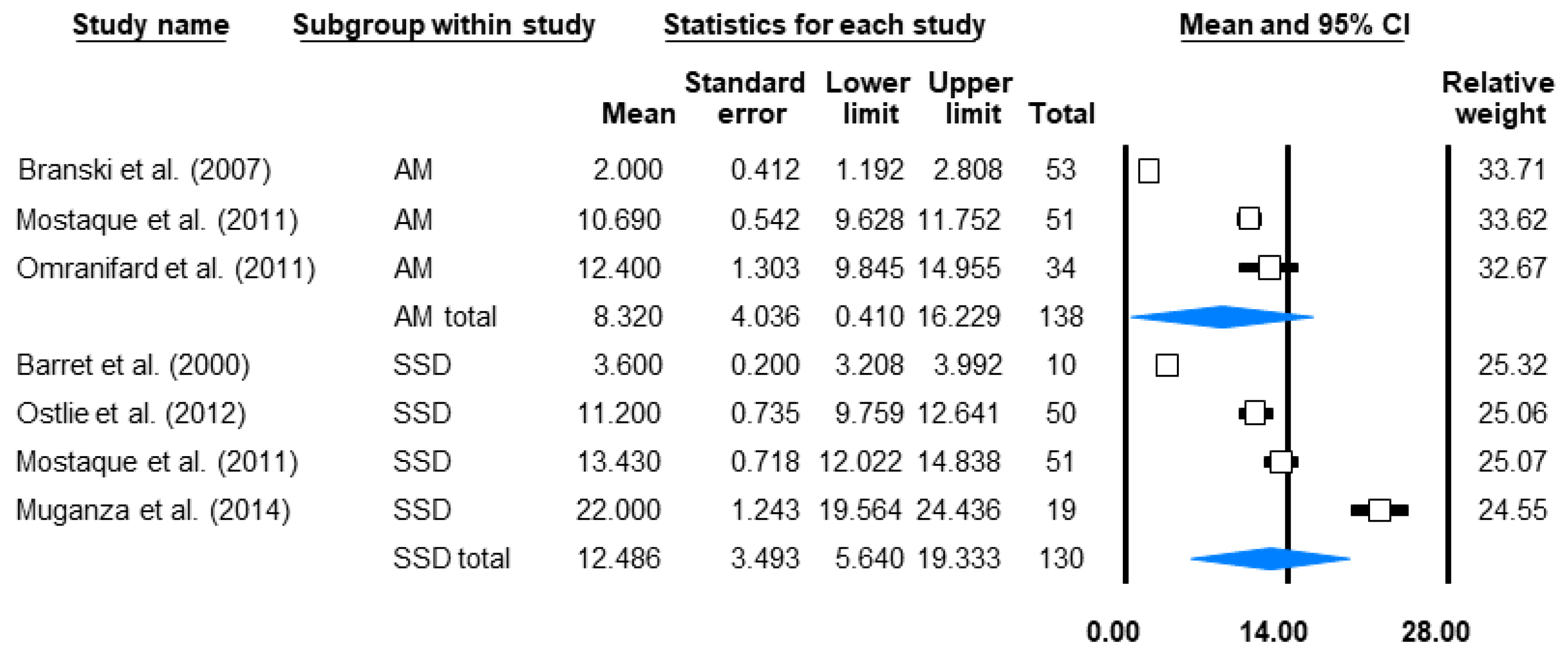
3.4.2. Grafting Rate and Non-Grafted Rate
3.4.3. Dressing Changes
3.4.4. Infection Rate and Non-Infected Rate
3.4.5. Length of Stay (LOS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forjuoh, S.; Gielen, A.; Arreola-Rissa, C.; El-Oteify, M.; Macpherson, A.; van Niekerk, A.; Peck, M.; Villaveces, A. World Report on Child Injury Prevention; Peden, M., Oyegbite, K., Ozanne-Smith, J., Hyder, A.A., Branche, C., Rahman, F.A.K.M., Rivara, F., Bartolomeos, K., Eds.; World Health Organisation: Geneva, Switzerland, 2008; pp. 79–101. Available online: https://apps.who.int/iris/bitstream/handle/10665/43851/9789241563574_eng.pdf;sequence=1 (accessed on 10 May 2021).
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 2014, 40, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Ohgi, S.; Gu, S. Pediatric burn rehabilitation: Philosophy and strategies. Burn. Trauma 2013, 1, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Rutter, N. The immature skin. Eur. J. Pediatr. 1996, 155, S18–S20. [Google Scholar] [CrossRef] [PubMed]
- Visscher, M.O.; Carr, A.N.; Narendran, V. Premature infant skin barrier maturation: Status at full-term corrected age. J. Perinatol. 2021, 41, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.J. Pediatrics. Skin 2004, 113, 1114–1119. [Google Scholar]
- Dewar, D.J.; Magson, C.L.; Fraser, J.F.; Crighton, L.; Kimble, R.M. Hot Beverage Scalds in Australian Children. J. Burn Care Rehabil. 2004, 25, 224–227. [Google Scholar] [CrossRef]
- Shupp, J.W.; Nasabzadeh, T.J.; Rosenthal, D.S.; Jordan, M.H.; Fidler, P.; Jeng, J.C. A review of the local pathophysiologic bases of burn wound progression. J. Burn Care Res. 2010, 31, 849–873. [Google Scholar] [CrossRef]
- Evers, L.H.; Bhavsar, D.; Mailänder, P. The biology of burn injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef]
- Passaretti, D.; Billmire, D.A. Management of Pediatric Burns. J. Craniofacial Surg. 2003, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Helvig, E. Pediatric burn injuries. AACN Clin. Issues Crit. Care Nurs. 1993, 4, 433–442. [Google Scholar] [PubMed]
- Schiestl, C.; Meuli, M.; Trop, M.; Neuhaus, K. Management of Burn Wounds. Eur. J. Pediatr. Surg. 2013, 23, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.P.; Kamel, R.A.; Caterson, E.; Eriksson, E. Clinical Impact upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Lima Júnior, E.M.; Filho, M.O.d.M.; Forte, A.J.; Costa, B.A.; Fechine, F.V.; Alves, A.P.N.N.; de Moraes, M.E.A.; Rocha, M.B.S.; Júnior, F.R.S.; Soares, M.F.A.D.N.; et al. Pediatric Burn Treatment Using Tilapia Skin as a Xenograft for Superficial-Partial Thickness Wounds: A Pilot Study. J. Burn Care Res. 2020, 41, 241–247. [Google Scholar] [CrossRef]
- Caruso, D.M.; Foster, K.N.; Blome-Eberwein, S.A.; Twomey, J.A.; Herndon, D.N.; Luterman, A.; Silverstein, P.; Antimarino, J.R.; Bauer, G.J. Randomized Clinical Study of Hydrofiber Dressing with Silver or Silver Sulfadiazine in the Management of Partial-Thickness Burns. J. Burn Care Res. 2006, 27, 298–309. [Google Scholar] [CrossRef]
- Barret, J.P.; Dziewulski, P.; Ramzy, P.I.; Wolf, S.; Desai, M.H.; Herndon, D.N. Biobrane versus 1% Silver Sulfadiazine in Second-Degree Pediatric Burns. Plast. Reconstr. Surg. 2000, 105, 62–65. [Google Scholar] [CrossRef]
- Ostlie, D.J.; Juang, D.; Aguayo, P.; Pettiford-Cunningham, J.P.; Erkmann, E.A.; Rash, D.E.; Sharp, S.W.; Sharp, R.J.; Peter, S.D.S. Topical silver sulfadiazine vs. collagenase ointment for the treatment of partial thickness burns in children: A prospective randomized trial. J. Pediatr. Surg. 2012, 47, 1204–1207. [Google Scholar] [CrossRef]
- Mostaque, A.K.; Rahman, K.B.M.A. Comparisons of the Effects of Biological Membrane (Amnion) and Silver Sulfadiazine in the Management of Burn Wounds in Children. J. Burn Care Res. 2011, 32, 200–209. [Google Scholar] [CrossRef]
- Kumar, R.J.; Kimble, R.M.; Boots, R.; Pegg, S.P. Treatment of partial-thickness burns: A prospective, randomized trial using TranscyteTM. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef]
- Lal, S.; Barrow, R.E.; Wolf, S.; Chinkes, D.L.; Hart, D.W.; Heggers, J.P.; Herndon, D.N. Biobrane® improves wound healing in burned children without increased risk of infection. Shock 2000, 14, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Gotschall, C.S.; Morrison, M.I.S.; Eichelberger, M.R. Prospective, Randomized Study of the Efficacy of Mepitel on Children With Partial-Thickness Scalds. J. Burn Care Res. 1998, 19, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Bugmann, P.; Taylor, S.; Gyger, D.; Lironi, A.; Genin, B.; Vunda, A.; La Scala, G.; Birraux, J.; Le Coultre, C. A silicone-coated nylon dressing reduces healing time in burned paediatric patients in comparison with standard sulfadiazine treatment: A prospective randomized trial. Burns 1998, 24, 609–612. [Google Scholar] [CrossRef]
- Glat, P.M.; Kubat, W.D.; Hsu, J.F.; Copty, T.; Burkey, B.A.; Davis, W.; Goodwin, I. Randomized Clinical Study of SilvaSorb (R) Gel in Comparison to Silvadene (R) Silver Sulfadiazine Cream in the Management of Partial-Thickness Burns. J. Burn Care Res. 2009, 30, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Muganza, A.; Cullingworth, L. A Prospective Single-Centre Randomised Controlled Study to Compare the Time to Healing of Partial Thickness Burn Wounds Treated with Versajet, Biobrane and Acticoat to Conventional Therapy. Glob. J. Med. Res. 2015, 14. Available online: https://medicalresearchjournal.org/index.php/GJMR/article/view/865/775 (accessed on 8 November 2020).
- Zheng, X.P.; Chen, J.; Chen, T.S.; Jiang, Y.N.; Shen, T.; Xiao, S.C.; Hu, X.Y. Preliminary effect observation on the application of micro-negative pressure in children with small-area deep partial-thickness burn. Zhonghua Shaoshang Zazhi = Chin. J. Burn. 2019, 35, 720–725. [Google Scholar]
- BMJ. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. 2021, p. n71. Available online: https://www.bmj.com/content/bmj/372/bmj.n71.full.pdf (accessed on 12 January 2021).
- Cuschieri, S. The CONSORT statement. Saudi J. Anaesth. 2019, 13, 27. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. Available online: www.training.cochrane.org/handbook (accessed on 15 February 2021).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Rashaan, Z.; Krijnen, P.; Klamer, R.R.M.; Schipper, I.B.; Dekkers, O.; Breederveld, R.S. Nonsilver treatment vs. silver sulfadiazine in treatment of partial-thickness burn wounds in children: A systematic review and meta-analysis. Wound Repair Regen. 2014, 22, 473–482. [Google Scholar] [CrossRef]
- Jiaao, Y.; Kai, S.; Hua, J.Z.; Chun, Z.J.; Hai, N.Z.; Jing, L.H.; Wei, W.W.; Qiang, L.J. Treatment of deep second-degree facial burns in pediatric population with recombinant human GM-CSF hydrogel. Wound Repair Regen. 2011, 19, A29. [Google Scholar]
- Shen, C.-A.; Chai, J.-K.; Tuo, X.-Y.; Cai, J.-H.; Li, D.-J.; Zhang, L.; Zhu, H.; Cai, J.-D. Efficacy observation on application of negative pressure therapy in the treatment of superficial partial-thickness scald wound in children. Zhonghua Shaoshang Zazhi = Chin. J. Burn. 2013, 29, 14–17. [Google Scholar]
- Hartel, M.; Illing, P.; Mercer, J.B.; Lademann, J.; Daeschlein, G.; Hoffmann, G. Therapy of acute wounds with water-filtered infrared-A (wIRA). GMS Krankenhhyg. Interdiszip. 2007, 2, Doc53. [Google Scholar] [PubMed]
- Hayashida, K.; Akita, S. Quality of pediatric second-degree burn wound scars following the application of basic fibroblast growth factor: Results of a randomized, controlled pilot study? Ostomy Wound Manag. 2012, 58, 32–36. [Google Scholar]
- Venkatachalapathy, T.S. A Comparative Study of Paediatric Thermal Burns Treated with Topical Heparin and without Heparin. Indian J. Surg. 2014, 76, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.Q.; Li, H.M.; Meng, C.Y. Repair of second degree facial burns in children using recombinant human epidermal growth factor. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 1974–1975. [Google Scholar]
- Branski, L.K.; Herndon, D.N.; Celis, M.M.; Norbury, W.B.; Masters, O.E.; Jeschke, M.G. Amnion in the treatment of pediatric partial-thickness facial burns. Burns 2008, 34, 393–399. [Google Scholar] [CrossRef]
- Omranifard, M.; Ansari, M.; Jazebi, N.; Rouzbahani, R.; Akbari, M. The comparison between long-term results of treatment with amnion graft and skin graft in children second degree burn wounds. J. Isfahan Med. Sch. 2011, 29, 126. [Google Scholar]
- Cassidy, C.; Peter, S.D.S.; Lacey, S.; Beery, M.; Ward-Smith, P.; Sharp, R.J.; Ostlie, D.J. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: A prospective, randomized trial. Burns 2005, 31, 890–893. [Google Scholar] [CrossRef]
- Wood, F.; Martin, L.; Lewis, D.; Rawlins, J.; McWilliams, T.; Burrows, S.; Rea, S. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane® synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns 2012, 38, 830–839. [Google Scholar] [CrossRef]
- Healy, C.; Boorman, J. Comparison of E-Z Derm and Jelonet dressings for partial skin thickness burns. Burns 1989, 15, 52–54. [Google Scholar] [CrossRef]
- Frear, C.C.; Cuttle, L.; McPhail, S.M.; Chatfield, M.D.; Kimble, R.M.; Griffin, B.R. Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children. Br. J. Surg. 2020, 107, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Gee Kee, E.L.; Kimble, R.M.; Cuttle, L.; Khan, A.; Stockton, K.A. Randomized controlled trial of three burns dressings for partial thickness burns in children. Burns 2015, 41, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Dalziel, S.R.; Herd, E.; Johnson, K.; She, R.W.; Shepherd, M. A Randomized Controlled Study of Silver-Based Burns Dressing in a Pediatric Emergency Department. J. Burn Care Res. 2016, 37, e340–e347. [Google Scholar] [CrossRef]
- Karlsson, M.; Elmasry, M.; Steinvall, I.; Sjöberg, F.; Olofsson, P.; Thorfinn, J. Superiority of silver-foam over porcine xenograft dressings for treatment of scalds in children: A prospective randomised controlled trial. Burns 2019, 45, 1401–1409. [Google Scholar] [CrossRef]
- Hyland, E.J.; D’Cruz, R.; Menon, S.; Harvey, J.G.; La Hei, E.; Lawrence, T.; Waddell, K.; Nash, M.; Holland, A.J. Biobrane™ versus acticoat™ for the treatment of mid-dermal pediatric burns: A prospective randomized controlled pilot study. Int. J. Burn. Trauma 2018, 8, 63–67. [Google Scholar]
- Gee Kee, E.L.; Kimble, R.M.; Cuttle, L.; Stockton, K.A. Scar outcome of children with partial thickness burns: A 3 and 6 month follow up. Burns 2016, 42, 97–103. [Google Scholar] [CrossRef]
- Gee Kee, E.L.; Stockton, K.A.; Kimble, R.M.; Cuttle, L.; McPhail, S.M. Cost-effectiveness of silver dressings for paediatric partial thickness burns: An economic evaluation from a randomized controlled trial. Burns 2017, 43, 724–732. [Google Scholar] [CrossRef]
- Nuñez-Gutiérrez, H.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Combined Use of Allograft and Autograft Epidermal Cultures in Therapy of Burns. Plast. Reconstr. Surg. 1996, 98, 929–939. [Google Scholar] [CrossRef]
- Barbosa, E.; Faintuch, J.; Moreira, E.A.M.; Da Silva, V.R.G.; Pereima, M.J.L.; Fagundes, R.L.M.; Filho, D.W. Supplementation of Vitamin E, Vitamin C, and Zinc Attenuates Oxidative Stress in Burned Children: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Burn Care Res. 2009, 30, 859–866. [Google Scholar] [CrossRef]
- Lockhart, S.P.; Rushworth, A.; Azmy, A.A.F.; Raine, P.A.M. Topical silver sulphadiazine: Side effects and urinary excretion. Burns 1983, 10, 9–12. [Google Scholar] [CrossRef]
- Fuller, F.W. The side effects of silver sulfadiazine. J. Burn Care Res. 2009, 30, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Vloemans, A.F.P.M.; Hermans, M.H.E.; van der Wal, M.B.A.; Liebregts, J.; Middelkoop, E. Optimal treatment of partial thickness burns in children: A systematic review. Burns 2014, 40, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Yi, H.S. Diagnostic accuracy of laser Doppler imaging in burn depth assessment: Systematic review and meta-analysis. Burns 2016, 42, 1369–1376. [Google Scholar] [CrossRef]
- Oaks, R.J.; Cindass, R. Silver Sulfadiazine. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Singh, A.; Halder, S.; Chumber, S.; Misra, M.C.; Sharma, L.K.; Srivastava, A.; Menon, G.R. Meta-analysis of Randomized Controlled Trials on Hydrocolloid Occlusive Dressing Versus Conventional Gauze Dressing in the Healing of Chronic Wounds. Asian J. Surg. 2004, 27, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Williams, C. Mepitel. Br. J. Nurs. 1995, 4, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Payne, W.G.; Smith, D.J.; Robson, M.C. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int. Wound J. 2007, 4, 114–122. [Google Scholar] [CrossRef]
- Chaganti, P.; Gordon, I.; Chao, J.H.; Zehtabchi, S. A systematic review of foam dressings for partial thickness burns. Am. J. Emerg. Med. 2019, 37, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.; Edwards-Jones, V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Richetta, A.G.; Cantisani, C.; Li, V.W.; Mattozzi, C.; Melis, L.; De Gado, F.; Giancristoforo, S.; Silvestri, E.; Calvieri, S. Hydrofiber dressing and wound repair: Review of the literature and new patents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 150–154. [Google Scholar] [CrossRef]
- Barnea, Y.; Weiss, J.; Gur, E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag®) in wound care. Ther. Clin. Risk Manag. 2010, 6, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demircan, M.; Yetis, M.I.; Cicek, T.; Bayrakci, E.; Tasci, A. Comparison of five different times for change of a silver-containing soft silicone foam dressing in pediatric partial thickness burns. J. Burn Care Res. 2015, 36, S193. [Google Scholar]
- Karlsson, M.; Steinvall, I.; Sjöberg, F.; Olofsson, P.; Elmasry, M. Burn scar outcome at six and 12 months after injury in children with partial thickness scalds: Effects of dressing treatment. Burns 2020, 46, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Mccarty, S.; Hamberg, K. Silver-containing foam dressings with Safetac: A review of the scientific and clinical data. J. Wound Care 2017, 26, S1–S32. [Google Scholar] [CrossRef] [Green Version]
- Joshi, C.J.; Hassan, A.; Carabano, M.; Galiano, R.D. Up-to-date role of the dehydrated human amnion/chorion membrane (AMNIOFIX) for wound healing. Expert Opin. Biol. Ther. 2020, 20, 1125–1131. [Google Scholar] [CrossRef]
- Palanker, N.D.; Lee, C.-T.; Weltman, R.L.; Tribble, G.D.; van der Hoeven, R.; Hong, J.; Wang, B. Antimicrobial Efficacy Assessment of Human Derived Composite Amnion-Chorion Membrane. Sci. Rep. 2019, 9, 15600. [Google Scholar] [CrossRef] [Green Version]
- Osama, M. Use of Nile Tilapia (Oreochromisniloticus) skin in the management of skin burns. J. Pak. Med. Assoc. 2017, 67, 1955. [Google Scholar]
- Wasiak, J.; Cleland, H. Burns: Dressings. BMJ Clin. Evid. 2015, 2015, 1903. [Google Scholar]
- Whitaker, I.S.; Prowse, S.; Potokar, T.S. A Critical Evaluation of the Use of Biobrane as a Biologic Skin Substitute: A Versatile Tool for the Plastic and Reconstructive Surgeon. Ann. Plast. Surg. 2008, 60, 333–337. [Google Scholar] [CrossRef]
- Troy, J.; Karlnoski, R.; Downes, K.; Brown, K.S.; Cruse, C.W.; Smith, D.J.; Payne, W.G. The Use of EZ Derm® in Partial-Thickness Burns: An Institutional Review of 157 Patients. Eplasty 2013, 13, e14. [Google Scholar]
- Lukish, J.R.; Eichelberger, M.R.; Newman, K.D.; Pao, M.; Nobuhara, K.; Keating, M.; Golonka, N.; Pratsch, G.; Misra, V.; Valladares, E.; et al. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J. Pediatr. Surg. 2001, 36, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.C.; Carolan-Rees, G. ReCell® Spray-On Skin System for Treating Skin Loss, Scarring and Depigmentation after Burn Injury: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2019, 17, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Almeland, S.K.; Dheansa, B.; Fuchs, P.; Governa, M.; Hoeksema, H.; Korzeniowski, T.; Lumenta, D.B.; Marinescu, S.; Martinez-Mendez, J.R.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns 2020, 46, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.N.; Herman, I.M. Collagenase Promotes the Cellular Responses to Injury and Wound Healing In Vivo. J. Burn. Wounds 2005, 4, e8. [Google Scholar]
- Kaya, O.; Orhan, E.; Sapmaz-Metin, M.; Topçu-Tarladaçalışır, Y.; Gündüz, Ö.; Aydın, B. The effects of epidermal growth factor on early burn-wound progression in rats. Dermatol. Ther. 2020, 33, e13196. [Google Scholar] [CrossRef]
- Gibran, N.; Isik, F.F.; Heimbach, D.M.; Gordon, D. Basic Fibroblast Growth Factor in the Early Human Burn Wound. J. Surg. Res. 1994, 56, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Sun, H.; Han, C.; Wang, X.; Yu, W. Topically applied rhGM-CSF for the wound healing: A systematic review. Burns 2011, 37, 729–741. [Google Scholar] [CrossRef]
- Oremus, M.; Hanson, M.; Whitlock, R.; Young, E.; Gupta, A.; Cin, A.D.; Archer, C.; Raina, P. The uses of heparin to treat burn injury. Évid. Rep. Technol. Assess. 2006, 148, 1–58. [Google Scholar]
- Rizzo, J.A.; Rowan, M.P.; Driscoll, I.R.; Chung, K.K.; Friedman, B.C. Vitamin C in Burn Resuscitation. Crit. Care Clin. 2016, 32, 539–546. [Google Scholar] [CrossRef]
- Butt, H.; Mehmood, A.; Ali, M.; Tasneem, S.; Tarar, M.N.; Riazuddin, S. Vitamin E preconditioning alleviates in vitro thermal stress in cultured human epidermal keratinocytes. Life Sci. 2019, 239, 116972. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Matthew, S. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonca, D.A.; Papini, R.; Price, P.E. Negative-pressure wound therapy: A snapshot of the evidence. Int. Wound J. 2006, 3, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Hartel, M.; Mercer, J.B. Heat for wounds—Water-filtered infrared-A (wIRA) for wound healing—A review. Ger. Med. Sci. 2016, 14, Doc08. [Google Scholar] [CrossRef] [PubMed]

| Publication Data | Intervention | Demography | Aetiology | Burn Depth | Age (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year of Publication | Country | No. of Patients | Female (%) | Scald (%) | Contact (%) | Flame (%) | Mean | SD | Min (Months) | Max | ||
| Barbosa et al. [53] | 2009 | Brazil | Vitamin C&E + Zn | 17 | 35.3 | NR | NR | NR | II | 4.51 | 4.32 | NR | NR |
| placebo | 15 | 33.3 | NR | NR | NR | 4.53 | 3.74 | NR | NR | ||||
| Barret et al. [18] | 2000 | USA, Texas | Biobrane | 10 | 30 | 80 | 0 | 20 | II | 3.1 | 0.5′ | NR | 17 |
| SSD (Silvadene) | 10 | 20 | 70 | 0 | 30 | 3.7 | 0.6′ | NR | 17 | ||||
| Branski et al. [40] | 2007 | USA, Texas | Nystatin + PMB | 49 | 28.57 | 45 | 0 | 55 | II | 7 | 4 | NR | NR |
| AM + Nystatin + PMB | 53 | 30.19 | 43 | 0 | 57 | 7 | 4 | NR | NR | ||||
| Brown et al. [47] | 2016 | New Zealand | Acticoat | 41 | 46.67 | 91 | 9 | 0 | II | 4.3 | 4 | NR | 15 |
| Aquacel Ag foam | 40 | 43.18 | 95 | 5 | 0 | 3 | 3.5 | NR | 15 | ||||
| Bugmann et al. [24] | 1998 | Switzerland | Mepitel | 36 | 46.34 | 68.3 | 26.8 | 4.87 | II | 3.29 | 3.09 | 3 | 15 |
| SSD (Flamazin) | 30 | 42.86 | 60 | 25.7 | 11.43 | 3.43 | 3.7 | 3 | 15 | ||||
| Caruso et al. [17] | 2006 | USA, Arizona | Aquacel Ag foam | 13 | NR | NR | NR | NR | II/A + MD | NR | NR | 2 | 16 |
| SSD | 19 | NR | NR | NR | NR | NR | NR | 2 | 16 | ||||
| Cassidy et al. [42] | 2005 | USA, Kansas | Duoderm | 37 | NR | NR | NR | NR | II/A + MD | NR | NR | 36 | 18 |
| Biobrane | 35 | NR | NR | NR | NR | NR | NR | 36 | 18 | ||||
| Frear et al. [45] | 2020 | Australia | Acticoat + Mepitel | 54 | 42.59 | 65 | 33 | 2 | II | 4 * | NR | 12 ** | 9^ |
| NPWT+ Acticoat + Mepitel | 47 | 59.57 | 60 | 36 | 4 | 4 * | NR | 12 ** | 8^ | ||||
| Gee Kee et al. [46] | 2015 | Australia | Acticoat | 31 | 41.94 | 58.1 | 35.5 | 3.2 | II | 1 | NR | 1 | 5 |
| Acticoat + Mepitel | 32 | 34.38 | 62.5 | 34.4 | 3.1 | 1 | NR | 1 | 4 | ||||
| Mepilex | 33 | 51.52 | 54.5 | 42.4 | 0 | 1 | NR | 1 | 4 | ||||
| Glat et al. [25] | 2009 | USA, Pennsylvania | Silvasorb | 12 | NR | NR | NR | NR | II/A + MD | 3.58 | 2.43 | 13 | 5 |
| SSD (Silvadene) | 12 | NR | NR | NR | NR | 1.9 | 1.13 | 9 | 9 | ||||
| Gotschall et al. [23] | 1998 | USA, Wa-shington | Mepitel | 33 | NR | 100 | 0 | 0 | II | NR | NR | NR | 12 |
| SSD | 30 | NR | 100 | 0 | 0 | NR | NR | NR | 12 | ||||
| Hartel et al. [36] | 2007 | Germany | wIRA(75%) + VIS | 10 | NR | NR | NR | NR | II/A | NR | NR | NR | NR |
| VIS (placebo) | 10 | NR | NR | NR | NR | NR | NR | NR | NR | ||||
| Hayashida et al. [37] | 2012 | Japan | bFGF | 15 | NR | 66.7 | 13.3 | 20 | II | NR | NR | 8 | 2.67 |
| placebo (Ekzalb) | 15 | NR | 73.3 | 6.7 | 20 | NR | NR | 8 | 2.67 | ||||
| Healy et al. [44] | 1989 | UK | EZDerm | 9 | NR | NR | NR | NR | 2.6 | 0.6′ | NR | NR | |
| Hyland et al. [49] | 2018 | Australia | Biobrane + Acticoat | 10 | 30 | NR | NR | NR | MD | NR | NR | 0 | 16 |
| Acticoat | 10 | 20 | NR | NR | NR | NR | NR | 0 | 16 | ||||
| Jiaao et al. [34] | 2010 | China | rhGM-CSF | 15 | NR | NR | NR | NR | II/B | 5.3 | NR | NR | NR |
| placebo (hydrogel matrix) | 15 | NR | NR | NR | NR | 5.3 | NR | NR | NR | ||||
| Karlsson et al. [48] | 2019 | Sweden | Ezderm | 30 | 36.67 | 100 | 0 | 0 | II | 1.75 * | NR | 11 ** | 4.92 ^ |
| Mepilex | 28 | 42.86 | 100 | 0 | 0 | 1.42 * | NR | 8 ** | 2.92 ^ | ||||
| Kumar et al. [21] | 2004 | Australia | Biobrane | 17 | NR | NR | NR | NR | II | 3.6 | NR | NR | NR |
| Transcyte | 20 | NR | NR | NR | NR | 3.6 | NR | NR | NR | ||||
| SSD (Silvazin) | 21 | NR | NR | NR | NR | 3.6 | NR | NR | NR | ||||
| Lal et al. [22] | 2000 | USA, Texas | Biobrane | 34 | 44.12 | 100 | 0 | 0 | II/A | 2.8 | 0.5′ | 0 | 17 |
| SSD | 45 | 33.33 | 100 | 0 | 0 | 3.4 | 0.6′ | 0 | 17 | ||||
| Liang et al. [39] | 2007 | China | rhEGF | 30 | NR | NR | NR | NR | II/A | NR | NR | NR | 14 |
| 30 | NR | NR | NR | NR | II/B | NR | NR | NR | 14 | ||||
| placebo (saline gauze) | 30 | NR | NR | NR | NR | II/A | NR | NR | NR | 14 | |||
| 30 | NR | NR | NR | NR | II/B | NR | NR | NR | 14 | ||||
| Lima Júnior et al. [16] | 2019 | Brazil | Tilapia | 15 | 33.3 | 93.3 | 0 | 6.67 | II/A | 5.67 | 3.66 | 24 | 12 |
| SSD | 15 | 46.67 | 80 | 0 | 20 | 5.2 | 2.7 | 24 | 12 | ||||
| Mostaque et al. [20] | 2011 | Bangladesh | AM | 51 | 52.9 | 82.4 | 0 | 17.6 | II/A | 3.61 | 2.31 | 0.03 | 12 |
| 22 | NR | NR | NR | NR | II/B | NR | NR | 0.03 | 12 | ||||
| SSD | 51 | 51 | 49 | 0 | 51 | II/A | 4.03 | 2.4 | 0.03 | 12 | |||
| 36 | NR | NR | NR | NR | II/B | NR | NR | 0.03 | 12 | ||||
| Muganza et al. [26] | 2014 | South Africa | Biobrane + Acticoat | 26 | 46.15 | NR | NR | NR | II | 2.3 * | NR | 20.4 ** | 4.1 ^ |
| SSD | 19 | 57.89 | NR | NR | NR | 2.7 * | NR | 19.2 ** | 4.1 ^ | ||||
| Omranifard et al. [41] | 2011 | Iran | AM | 34 | 29.41 | NR | NR | NR | II/B | 5.4 | 7.5 | NR | 18 |
| autograft | 32 | 34.38 | NR | NR | NR | 4.4 | 6.9 | NR | 18 | ||||
| Ostlie et al. [19] | 2012 | USA, Kansas | Collagenase + PMB | 50 | 42 | NR | NR | NR | II | 4.8 | 4.5 | 2 | 18 |
| SSD | 50 | 30 | NR | NR | NR | 5.1 | 4.5 | 2 | 18 | ||||
| Venkatachalapathy et al. [38] | 2012 | India | Heparin | 50 | NR | NR | NR | NR | II | NR | NR | NR | NR |
| Sulphur-based cream | 50 | NR | NR | NR | NR | NR | NR | NR | NR | ||||
| Shen et al. [35] | 2013 | China | NPWT | 145 | NR | 100 | 0 | 0 | II/A | NR | NR | NR | NR |
| Wood et al. [43] | 2012 | Australia | Biobrane | 4 | 50 | 100 | 0 | 0 | II | 4.95 | 3.91 | 8 | 9 |
| Biobrane + ReCell | 5 | 40 | 100 | 0 | 0 | 1.32 | 0.55 | 8 | 9 | ||||
| Zheng et al. [27] | 2019 | China | NPWT | 32 | 43.75 | NR | NR | NR | II/B | 3.9 | 1.6 | NR | NR |
| SSD | 32 | 37.5 | NR | NR | NR | 3.8 | 1.7 | NR | NR | ||||
| Depth: II | Publication Data | No. of Patients (n) | TBSA(%) | TTRE (Days) | TBSA%/TTRE | TTRE Red% | ||
|---|---|---|---|---|---|---|---|---|
| Intervention | Author (Year of Publication) | Mean | SD | Mean | SD | |||
| Acticoat + Mepitel SUM | 86 | 1.42 | 10.61 | 0.14 | ||||
| Acticoat + Mepitel | Frear et al. (2020) [45] | 54 | * 1.35 | 0.76 | * 10.7 | 4.57 | 0.13 | |
| Acticoat + Mepitel | Gee Kee et al. (2015) [46] | 32 | * 1.53 | 1.94 | * 10.35 | 3.91 | 0.15 | |
| NPWT + Acticoat + Mepitel | Frear et al. (2020) [45] | 47 | * 1.5 | 0.76 | * 8.71 | 3.06 | 0.17 | |
| EZDerm SUM | 39 | 4.26 | 18.75 | 0.23 | ||||
| EZDerm | Healy et al. (1989) [44] | 9 | 1.8 | 3.75 | 12.9 | 4.2 | 0.14 | |
| EZDerm | Karlsson et al. (2019) [48] | 30 | ** 5 | NR | 20.5 | NR | 0.24 | |
| Acticoat SUM | 41 | 3.23 | 14.18 | 0.23 | ||||
| Acticoat | Hyland et al. (2018) [49] | 10 | ** 8.5 | NR | 26.5 | NR | 0.32 | |
| Acticoat | Gee Kee et al. (2015) [46] | 31 | 1.53 | 1.94 | 10.21 | 5.47 | 0.15 | |
| Mepilex SUM | 61 | 2.85 | 10.29 | 0.28 | ||||
| Mepilex | Karlsson et al. (2019) [48] | 28 | ** 5 | NR | ** 15 | NR | 0.33 | |
| Mepilex | Gee Kee et al. (2015) [46] | 33 | * 1.03 | 1.16 | 6.29 | 3.1 | 0.16 | |
| Biobrane + ReCell | Wood et al. (2012) [43] | 5 | 5.2 | 3.19 | 15 | 3.54 | 0.35 | |
| SSD SUM | 110 | 7.21 | 18.29 | 0.39 | ||||
| SSD | Gotschall et al. (1998) [23] | 30 | 5.1 | 2.2 | 27.6 | NR | 0.19 | |
| SSD | Muganza et al. (2014) [26] | 19 | 21 | 7.1 | 23.7 | 9.4 | 0.89 | |
| SSD | Barret et al. (2000) [18] | 10 | 7.8 | 2.85 | 16.1 | 2.21 | 0.48 | |
| SSD | Bugmann et al. (1998) [24] | 30 | 1.92 | 2.05 | 11.26 | 6.02 | 0.17 | |
| SSD | Kumar et al. (2004) [21] | 21 | 5 | NR | 11.2 | NR | 0.45 | |
| placebo (Ekzalb) | Hayashida et al. (2012) [37] | 15 | 8.3 | 2.9 | 17.5 | 3.1 | 0.47 | |
| bFGF | Hayashida et al. (2012) [37] | 15 | 7 | 2.6 | 13.8 | 2.4 | 0.51 | 21.14 |
| Mepitel SUM | 69 | 4.97 | 8.98 | 0.55 | ||||
| Mepitel | Gotschall et al. (1998) [23] | 33 | 6.8 | 3.4 | 10.5 | NR | 0.65 | |
| Mepitel | Bugmann et al. (1998) [24] | 36 | 3.29 | 3.09 | 7.58 | 3.12 | 0.43 | |
| Biobrane SUM | 31 | 6.65 | 10.63 | 0.63 | ||||
| Biobrane | Wood et al. (2012) [43] | 4 | 8 | 5.23 | 17.75 | 4.99 | 0.45 | |
| Biobrane | Barret et al. (2000) [18] | 10 | 8.9 | 15.5 | 9.7 | 2.21 | 0.92 | |
| Biobrane | Kumar et al. (2004) [21] | 17 | 5 | NR | 9.5 | NR | 0.53 | |
| Transcyte | Kumar et al. (2004) [21] | 20 | 5 | NR | 7.5 | NR | 0.66 | |
| Biobrane + Acticoat SUM | 36 | 18.11 | 20.95 | 0.87 | ||||
| Biobrane + Acticoat | Muganza et al. (2014) [26] | 26 | 22 | 7.5 | 21.7 | 9 | 1.01 | |
| Biobrane + Acticoat | Hyland et al. (2018) [49] | 10 | ** 8 | NR | 19 | NR | 0.42 | |
| Nystatin + PMB | Branski et al. (2007) [40] | 49 | 11 | 6 | 8 | 2 | 1.38 | |
| AM + Nystatin +PMB | Branski et al. (2007) [40] | 53 | 12 | 7 | 6 | 2 | 2 | |
| Vitamin C&E + Zinc + TT | Barbosa et al. (2009) [53] | 15 | 16.2 | 5.3 | 7.5 | NR | 2.16 | 23.67 |
| Intervention and Burn Depth | Publication Data | No. of Patients (n) | Grafted | |
|---|---|---|---|---|
| Author (Year of Publication) | No. (n) | % | ||
| placebo (VIS) II/A | Hartel et al. (2007) [36] | 24 | 14 | 58.33 |
| wIRA(75%) + VIS II/A | Hartel et al. (2007) [36] | 21 | 11 | 52.38 |
| placebo (Ekzalb) II | Hayashida et al. (2012) [37] | 15 | 5 | 33.33 |
| bFGF II | Hayashida et al. (2012) [37] | 15 | 5 | 33.33 |
| Collagenase + PMB II | Ostlie et al. (2012) [19] | 50 | 16 | 32 |
| EZDerm II | 46 | 11 | 23.91 | |
| EZDerm | Healy et al. (1989) [44] | 16 | 7 | 43.75 |
| EZDerm | Karlsson et al. (2019) [48] | 30 | 4 | 13.33 |
| SSD II | 180 | 29 | 21.48 | |
| Acticoat II | 43 | 9 | 20.93 | |
| Acticoat | Hyland et al. (2018) [49] | 10 | 7 | 70 |
| Acticoat | Gee Kee et al. (2015) [46] | 33 | 2 | 6.06 |
| Nystatin + PMB II | Branski et al. (2007) [40] | 59 | 10 | 16.95 |
| SSD II + II/A | 135 | 29 | 16.1 | |
| SSD | Ostlie et al. (2012) [19] | 50 | 18 | 36 |
| SSD | Kumar et al. (2004) [21] | 21 | 5 | 23.81 |
| SSD | Barret et al. (2000) [18] | 10 | 0 | 0 |
| SSD | Bugmann et al. (1998) [24] | 35 | 5 | 14.28 |
| SSD | Muganza et al. (2014) [26] | 19 | 1 | 5.26 |
| SSD II/A | Lal et al. (2000) [22] | 45 | 0 | 0 |
| Biobrane + Acticoat II | 36 | 5 | 13.89 | |
| Biobrane + Acticoat | Hyland et al. (2018) [49] | 10 | 4 | 40 |
| Biobrane + Acticoat | Muganza et al. (2014) [26] | 26 | 1 | 3.85 |
| AM + Nystatin + PMB II | Branski et al. (2007) [40] | 61 | 8 | 13.11 |
| Biobrane II | 31 | 4 | 12.9 | |
| Mepitel II | Bugmann et al. (1998) [24] | 41 | 5 | 12.19 |
| Acticoat + Mepitel II | 88 | 6 | 6.82 | |
| Acticoat + Mepitel | Frear et al. (2020) [45] | 54 | 4 | 7.41 |
| Acticoat + Mepitel | Gee Kee et al. (2015) [46] | 34 | 2 | 5.89 |
| Biobrane II + II/A | 65 | 4 | 6.15 | |
| Biobrane | Wood et al. (2012) [43] | 4 | 1 | 25 |
| Biobrane | Kumar et al. (2004) [21] | 17 | 3 | 17.65 |
| Biobrane | Barret et al. (2000) [18] | 10 | 0 | 0 |
| Biobrane II/A | Lal et al. (2000) [22] | 34 | 0 | 0 |
| Transcyte II | Kumar et al. (2004) [21] | 20 | 1 | 5 |
| Mepilex II | 61 | 2 | 3.28 | |
| Mepilex | Karlsson et al. (2019) [48] | 28 | 2 | 7.14 |
| Mepilex | Gee Kee et al. (2015) [46] | 33 | 0 | 0 |
| NPWT + Acticoat + Mepitel II | Frear et al. (2020) [45] | 47 | 1 | 2.13 |
| Biobrane + ReCell II | Wood et al. (2012) [43] | 5 | 0 | 0 |
| Intervention and Burn Depth | Publication Data | No. of Patients | Dressing Changes | |
|---|---|---|---|---|
| Author (Year of Publication) | Mean | SD | ||
| SSD II + II/A | 198 | 25.16 | ||
| Silvasorb II/A+ MD | Glat et al. (2009) [25] | 12 | 13.5 | 4.7 |
| Collagenase + PMB II | Ostlie et al. (2012) [19] | 50 | 11 | 4.1 |
| SSD II ex. Mostaque | 132 | 9.56 | ||
| SSD | Mostaque et al. (2011) [20] | 51 | 65.53 | 18.23 |
| SSD | Glat et al. (2009) [25] | 12 | 13.42 | 8.26 |
| SSD | Ostlie et al. (2012) [19] | 50 | 11 | 3.8 |
| SSD | Muganza et al. (2014) [26] | 19 | 10.7 | 3.8 |
| SSD | Kumar et al. (2004) [21] | 21 | 9.2 | NR |
| SSD | Bugmann et al. (1998) [24] | 30 | 5.13 | 2.9 |
| SSD II/A | Lima Júnior et al. (2019) [16] | 15 | 9.27 | 1.39 |
| Biobrane + Acticoat II | 36 | 6.87 | ||
| Biobrane + Acticoat | Muganza et al. (2014) [26] | 26 | 7.6 | 4.8 |
| Biobrane + Acticoat | Hyland et al. (2018) [49] | 10 | * 5 | NR |
| Nystatin + PMB II | Branski et al. (2007) [40] | 49 | 6 | 3 |
| EZDerm II | Karlsson et al. (2019) [48] | 30 | * 5 | NR |
| Biobrane + ReCell II | Wood et al. (2012) [43] | 5 | 4.8 | 1.3 |
| Mepilex II | Karlsson et al. (2019) [48] | 28 | * 4 | NR |
| Mepitel II | Bugmann et al. (1998) [24] | 36 | 3.64 | 1.5 |
| Biobrane II | 21 | 3.37 | ||
| Biobrane | Wood et al. (2012) [43] | 4 | 7.5 | 2.64 |
| Biobrane | Kumar et al. (2004) [21] | 17 | 2.4 | NR |
| Acticoat + Mepitel II | Frear et al. (2020) [45] | 54 | 3 | 1.48 |
| Tilapia II/A | Lima Júnior et al. (2019) [16] | 15 | 3 | 0.76 |
| Acticoat II | 51 | 2.69 | ||
| Acticoat | Hyland et al. (2018) [49] | 10 | * 5.5 | NR |
| Acticoat | Brown et al. (2016) [47] | 41 | 2 | 0.2 |
| NPWT + Acticoat + Mepitel II | Frear et al. (2020) [45] | 47 | 2.43 | 0.86 |
| NPWT II/A | Shen et al. (2013) [35] | 145 | 2.05 | 0.22 |
| Transcyte II | Kumar et al. (2004) [21] | 20 | 1.5 | NR |
| AM II | Mostaque et al. (2011) [20] | 51 | 1.33 | 0.55 |
| Aquacel Ag II | Brown et al. (2016) [47] | 40 | 1 | 0.1 |
| AM + Nystatin + PMB II | Branski et al. (2007) [40] | 53 | 0.5 | 2 |
| Intervention and Burn Depth | Publication Data | No. of Patients (n) | Infected | |
|---|---|---|---|---|
| Author (Year of Publication) | No. (n) | % | ||
| Biobrane + Acticoat II | Hyland et al. (2018) [49] | 10 | 6 | 60 |
| Biobrane + ReCell II | Wood et al. (2012) [43] | 5 | 2 | 40 |
| EZDerm II | 46 | 17 | 36.96 | |
| EZDerm | Healy et al. (1989) [44] | 16 | 8 | 50 |
| EZDerm | Karlsson et al. (2019) [48] | 30 | 9 | 30 |
| NPWT II/A | Shen et al. (2013) [35] | 145 | 39 | 26.9 |
| Mepilex II | 61 | 10 | 16.39 | |
| Mepilex | Karlsson et al. (2019) [48] | 28 | 10 | 35.72 |
| Mepilex | Gee Kee et al. (2015) [46] | 33 | 0 | 0 |
| Biobrane II | 31 | 4 | 12.9 | |
| SSD II | 141 | 13 | 9.22 | |
| Biobrane II + II/A | 65 | 5 | 7.69 | |
| Biobrane | Wood et al. (2012) [43] | 4 | 1 | 25 |
| Biobrane | Kumar et al. (2004) [21] | 17 | 3 | 17 |
| Biobrane | Barret et al. (2000) [18] | 10 | 0 | 0 |
| Biobrane II/A | Lal et al. (2000) [22] | 34 | 1 | 2.9 |
| SSD II + II/A | 186 | 14 | 7.53 | |
| SSD | Kumar et al. (2004) [21] | 21 | 5 | 24 |
| SSD | Ostlie et al. (2012) [19] | 50 | 7 | 14 |
| SSD | Bugmann et al. (1998) [24] | 30 | 1 | 3.33 |
| SSD | Gotschall et al. (1998) [23] | 30 | 0 | 0 |
| SSD | Barret et al. (2000) [18] | 10 | 0 | 0 |
| SSD II/A | Lal et al. (2000) [22] | 45 | 1 | 2.2 |
| Transcyte II | Kumar et al. (2004) [21] | 20 | 1 | 5 |
| Mepitel II | 72 | 3 | 4.17 | |
| Mepitel | Gotschall et al. (1998) [23] | 36 | 3 | 8.3 |
| Mepitel | Bugmann et al. (1998) [24] | 36 | 0 | 0 |
| Nystatin + PMB II | Branski et al. (2007) [40] | 49 | 2 | 4.08 |
| Acticoat II | 86 | 3 | 3.49 | |
| Acticoat | Brown et al. (2016) [47] | 45 | 2 | 4.44 |
| Acticoat | Hyland et al. (2018) [49] | 10 | 1 | 10 |
| Acticoat | Gee Kee et al. (2015) [46] | 31 | 0 | 0 |
| Aquacel Ag II | Brown et al. (2016) [47] | 44 | 1 | 2.27 |
| Collagenase + PMB II | Ostlie et al. (2012) [19] | 50 | 1 | 2 |
| AM + Nystatin + PMB II | Branski et al. (2007) [40] | 53 | 1 | 1.89 |
| Acticoat + Mepitel II | Gee Kee et al. (2015) [46] | 32 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőrincz, A.; Váradi, A.; Hegyi, P.; Rumbus, Z.; Tuba, M.; Lamberti, A.G.; Varjú-Solymár, M.; Párniczky, A.; Erőss, B.; Garami, A.; et al. Paediatric Partial-Thickness Burn Therapy: A Meta-Analysis and Systematic Review of Randomised Controlled Trials. Life 2022, 12, 619. https://doi.org/10.3390/life12050619
Lőrincz A, Váradi A, Hegyi P, Rumbus Z, Tuba M, Lamberti AG, Varjú-Solymár M, Párniczky A, Erőss B, Garami A, et al. Paediatric Partial-Thickness Burn Therapy: A Meta-Analysis and Systematic Review of Randomised Controlled Trials. Life. 2022; 12(5):619. https://doi.org/10.3390/life12050619
Chicago/Turabian StyleLőrincz, Aba, Alex Váradi, Péter Hegyi, Zoltán Rumbus, Máté Tuba, Anna Gabriella Lamberti, Margit Varjú-Solymár, Andrea Párniczky, Bálint Erőss, András Garami, and et al. 2022. "Paediatric Partial-Thickness Burn Therapy: A Meta-Analysis and Systematic Review of Randomised Controlled Trials" Life 12, no. 5: 619. https://doi.org/10.3390/life12050619
APA StyleLőrincz, A., Váradi, A., Hegyi, P., Rumbus, Z., Tuba, M., Lamberti, A. G., Varjú-Solymár, M., Párniczky, A., Erőss, B., Garami, A., & Józsa, G. (2022). Paediatric Partial-Thickness Burn Therapy: A Meta-Analysis and Systematic Review of Randomised Controlled Trials. Life, 12(5), 619. https://doi.org/10.3390/life12050619







