Osteogenic Effect of Pregabalin in Human Primary Mesenchymal Stem Cells, Osteoblasts, and Osteosarcoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. HMSC Isolation
2.2. HOB Isolation
2.3. MG63 Cells
2.4. Cell Culture Conditions
2.5. Cell Proliferation
2.6. Cell Metabolism
2.7. Measurement of Alkaline Phosphatase (ALP) Activity
2.8. Histochemical Stains
2.9. Software and Statistical Analysis
3. Results
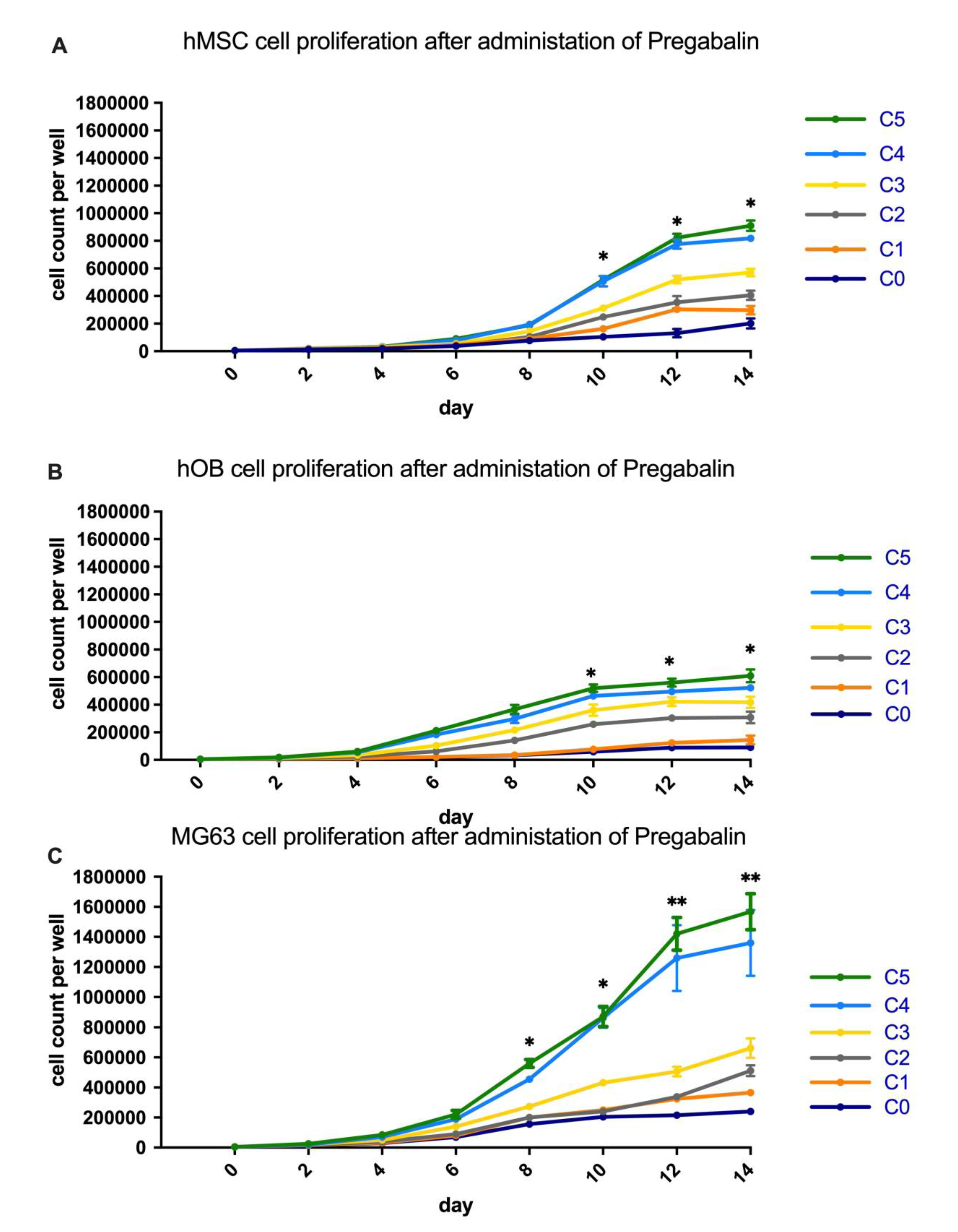
3.1. Analysis of Cell Proliferation in Pregabalin Treated hMSC, hOB, and MG63
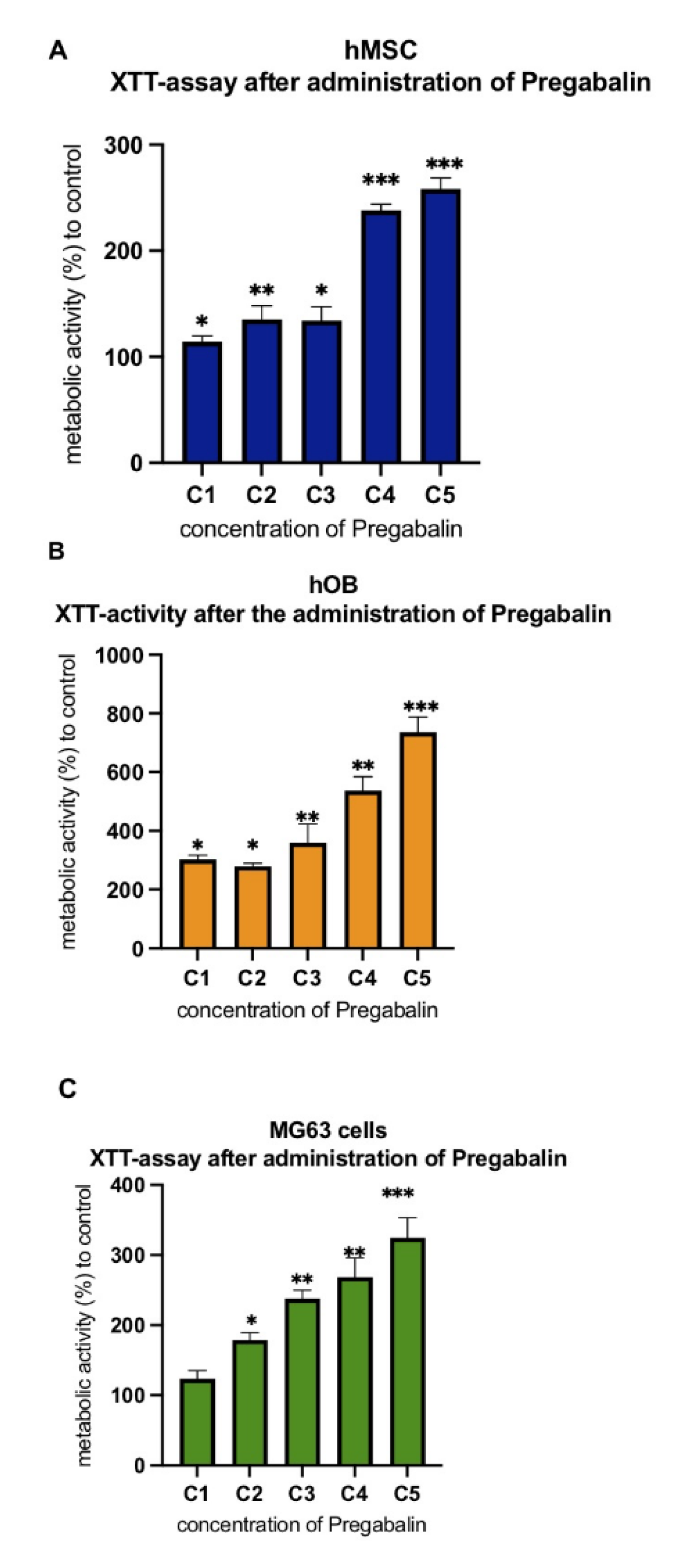
3.2. Evaluation of Cell Metabolism after Treatment with Pregabalin
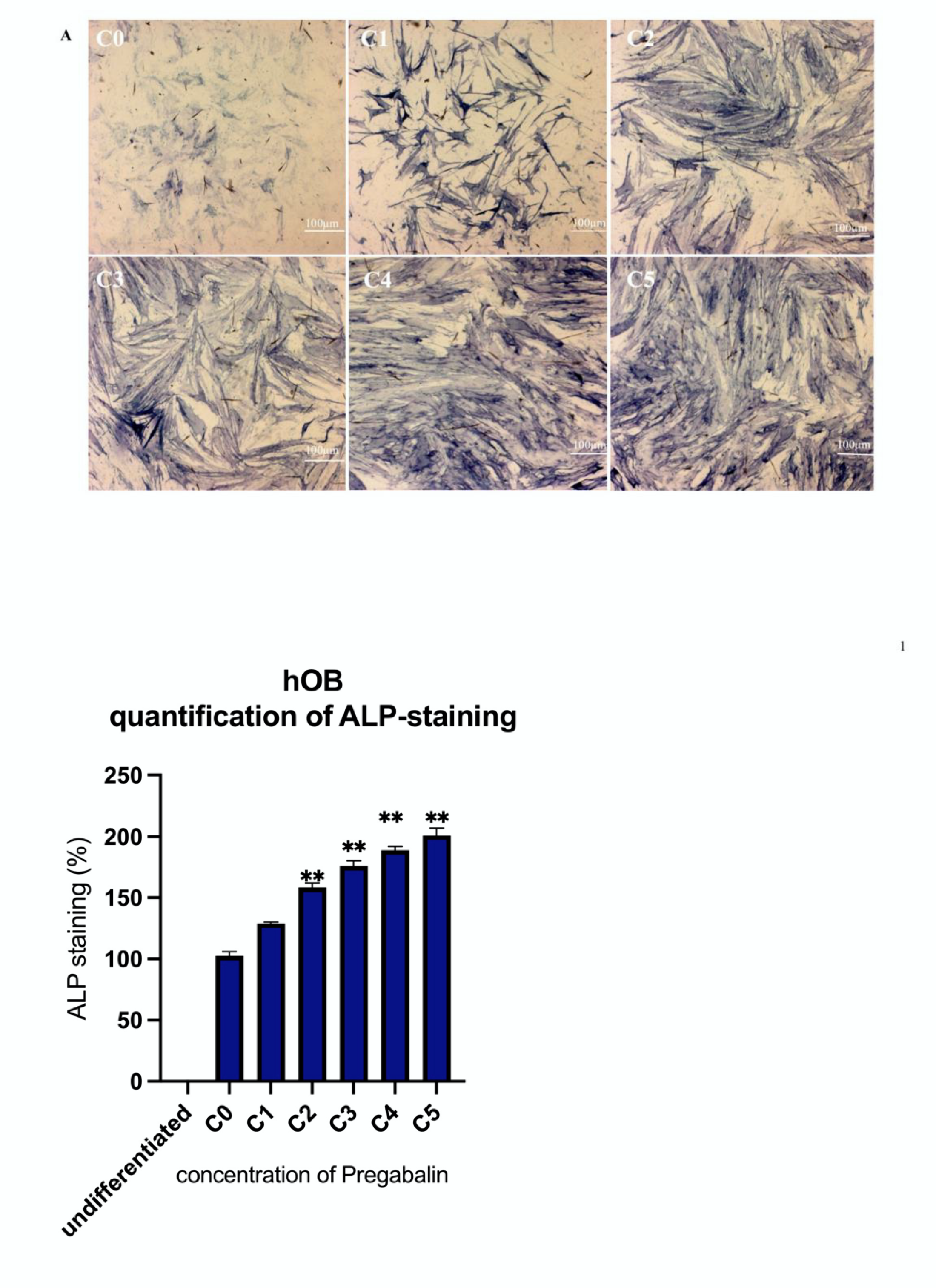
3.3. Evaluation of Alkaline Phosphatase Activity in Pregabalin Treated hMSC, hOB, and MG63 Cells
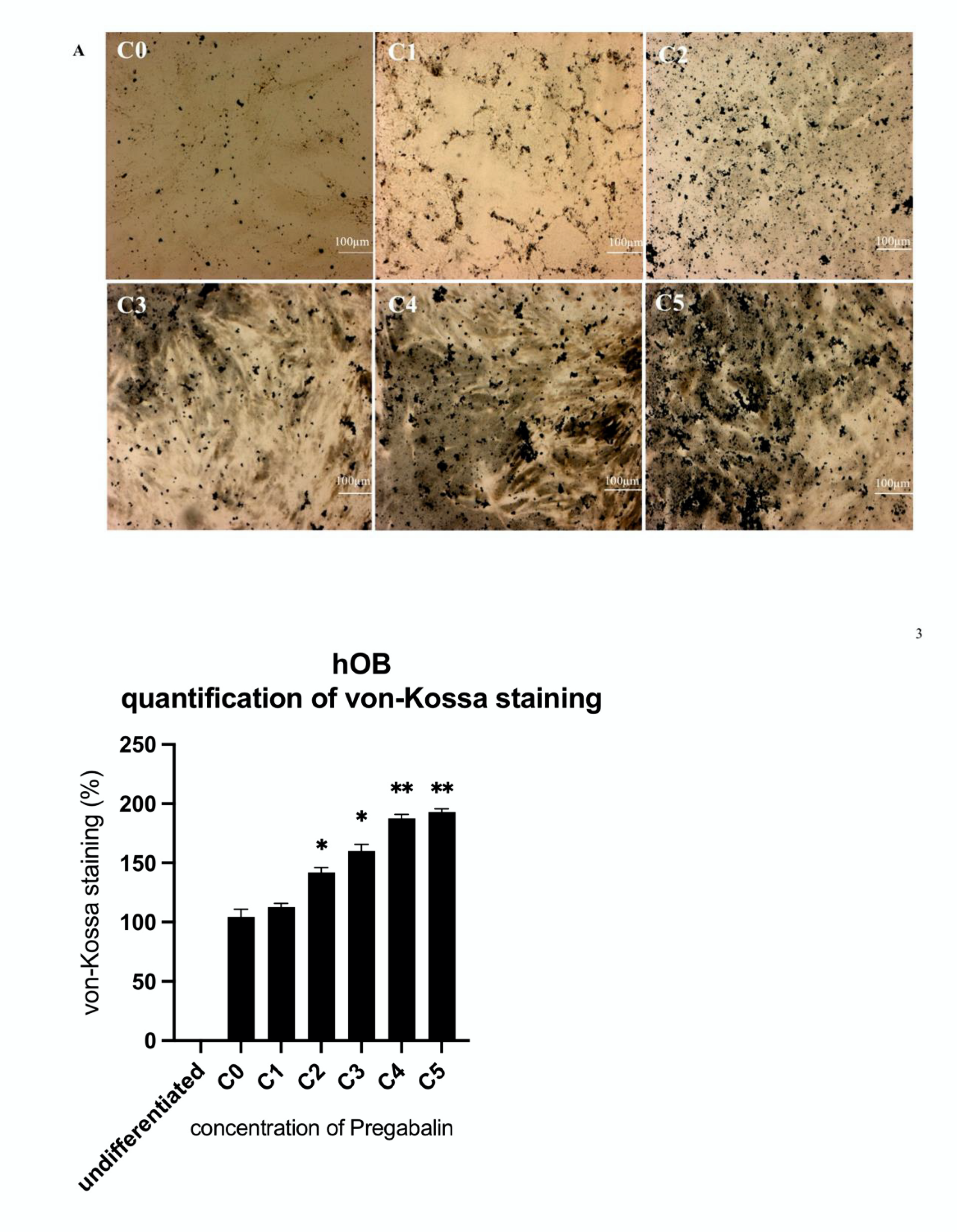
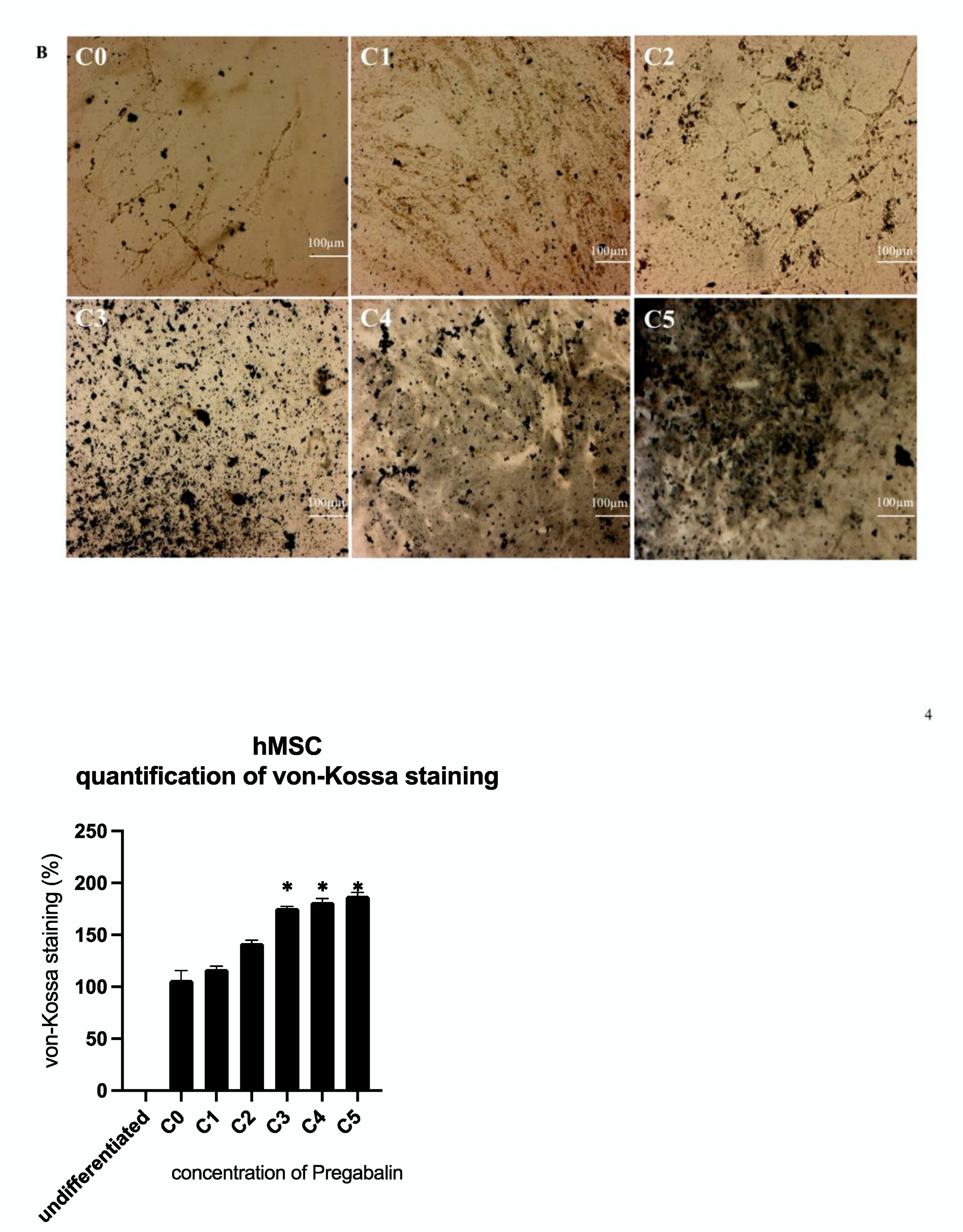
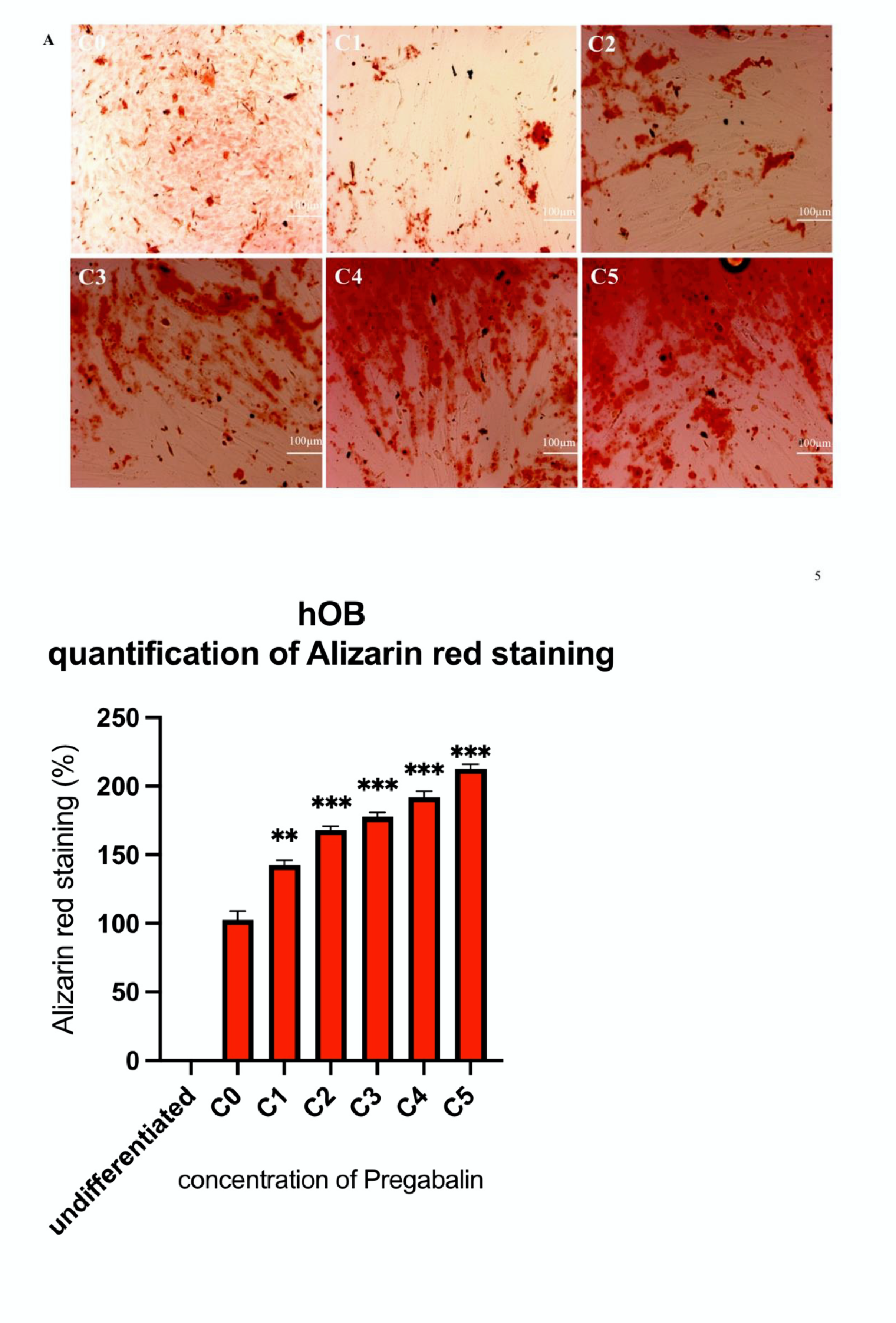
3.4. Evaluation of Osteogenic Differentiation Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Zhang, Q.; Lin, Z.; Gao, F. Prediction of Epilepsy Based on Tensor Decomposition and Functional Brain Network. Brain Sci. 2021, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Druschky, K.; Bleich, S.; Grohmann, R.; Engel, R.R.; Kleimann, A.; Stübner, S.; Greil, W.; Toto, S. Use and safety of antiepileptic drugs in psychiatric inpatients-data from the AMSP study. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Carney, P.R.; Bussing, R.; Segal, R.; Cottler, L.B.; Winterstein, A.G. Trends in Antiepileptic Drug Use in Children and Adolescents with Epilepsy. Pediatr. Neurol. 2017, 74, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.D.; Johnson, K.C.; Robbins, J.; Larson, J.C.; Curb, J.D.; Watson, K.; Gass, M.; Lacroix, A.Z. Antiepileptic drug use, falls, fractures, and BMD in postmenopausal women: Findings from the women’s health initiative (WHI). J. Bone Miner Res. 2010, 25, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, A.A.; Dussault, P.M.; Thakore-James, M.; Gagnon, D.; Baker, E.; Davis, S.A.; Houranieh, A.M. Prevention of bone loss and vertebral fractures in patients with chronic epilepsy—Antiepileptic drug and osteoporosis prevention trial. Epilepsia 2013, 54, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, F.; Zhang, Y.; Guo, Y.; Ding, M. Association between use of antiepileptic drugs and fracture risk: A systematic review and meta-analysis. Bone 2014, 64, 246–253. [Google Scholar] [CrossRef]
- Collins, N.; Maher, J.; Cole, M.; Baker, M.; Callaghan, N. A prospective study to evaluate the dose of vitamin D required to correct low 25-hydroxyvitamin D levels, calcium, and alkaline phosphatase in patients at risk of developing antiepileptic drug-induced osteomalacia. Q. J. Med. 1991, 178, 113–122. [Google Scholar]
- Pascussi, J.M.; Robert, A.; Nguyen, M.; Walrant-Debray, O.; Garabedian, M.; Martin, P.; Pineau, T.; Saric, J.; Navarro, F.; Maurel, P.; et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J. Clin. Investig. 2005, 115, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Menon, B.; Harinarayan, C.V. The effect of anti epileptic drug therapy on serum 25-hydroxyvitamin D and parameters of calcium and bone metabolism—A longitudinal study. Seizure 2010, 19, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, L.A. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004, 5, 3–15. [Google Scholar] [CrossRef]
- Petty, S.J.; Milligan, C.J.; Todaro, M.; Richards, K.L.; Kularathna, P.K.; Pagel, C.N.; French, C.R.; Hill-Yardin, E.L.; O’Brien, T.J.; Wark, J.D.; et al. The antiepileptic medications carbamazepine and phenytoin inhibit native sodium currents in murine osteoblasts. Epilepsia 2016, 57, 1398–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialer, M. Why are antiepileptic drugs used for nonepileptic conditions? Epilepsia 2012, 53, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Nakken, K.O.; Taubøll, E. Bone loss associated with use of antiepileptic drugs. Expert Opin. Drug Saf. 2010, 9, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, A.; Murphy, S.; Cione, E.; Piro, L.; Sarro, G.; Gallelli, L. Antiepileptic Drugs and Bone Health: Current Concepts. Psychopharmacol. Bull. 2020, 50, 36–44. [Google Scholar] [PubMed]
- Hant, F.N.; Bolster, M.B. Drugs that may harm bone: Mitigating the risk. Cleve Clin. J. Med. 2016, 83, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.P.; Angelotti, T.; Fauman, E. Pharmacology and mechanism of action of pregabalin: The calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007, 73, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K. Old versus New: Why Do We Need New Antiepileptic Drugs? J. Epilepsy Res. 2014, 4, 39–44. [Google Scholar] [CrossRef]
- Rocha, S.; Ferraz, R.; Prudêncio, C.; Fernandes, M.H.; Costa-Rodrigues, J. Differential effects of antiepileptic drugs on human bone cells. J. Cell Physiol. 2019, 234, 19691–19701. [Google Scholar] [CrossRef]
- İmre, E.; Çiftdemir, M.; Taştekin, E. Effects of pregabalin on spinal fusion. Eur. Spine J. 2020, 29, 332–339. [Google Scholar] [CrossRef]
- Akin, B.; Kelle, B.; Kozanoglu, E. The Effect of Pregabalin on Bone Metabolism. J. Clin. Densitom. 2021, 12, 1094–6950. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Mancini, R.; Pellegrini, M.; Concetta Rotolo, M.; Luna, A.; Falcon, M. Postmortem analysis of quetiapine and pregabalin in human bone. Leg. Med. 2020, 46, 101717. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Rossi, V.; Baugher, P.J. Aminolevulinic Acid-Mediated Photodynamic Therapy Causes Cell Death in MG-63 Human Osteosarcoma Cells. Photomed. Laser Surg. 2016, 34, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Bockbrader, H.N.; Radulovic, L.L.; Posvar, E.L.; Strand, J.C.; Alvey, C.W.; Busch, J.A.; Randinitis, E.J.; Corrigan, B.W.; Haig, G.M.; Boyd, R.A.; et al. Clinical pharmacokinetics of pregabalin in healthy volunteers. J. Clin. Pharmacol. 2010, 50, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Heath, J.K.; Yoon, K.; Noda, M.; Rodan, S.B. Diversity of the osteoblastic phenotype. Ciba Found Symp. 1988, 136, 78–91. [Google Scholar] [CrossRef]
- Ko, A.; Kong, J.; Samadov, F.; Mukhamedov, A.; Kim, Y.M.; Lee, Y.J.; Nam, S.O. Bone health in pediatric patients with neurological disorders. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 15–23. [Google Scholar] [CrossRef]
- Simko, J.; Karesova, I.; Kremlacek, J.; Eva, Z.; Horacek, J.; Fekete, S.; Malakova, J.; Zivna, H.; Palicka, V. The effect of gabapentin and pregabalin on bone turnover and bone strength: A prospective study in Wistar rats. Pharmacol. Rep. 2019, 71, 1213–1218. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; Mostafa, T.M.; Allah, H.M.S.; Akef, G.H. Levetiracetam and lamotrigine effects as mono- and polytherapy on bone mineral density in epileptic patients. Arq Neuropsiquiatr. 2018, 76, 452–458. [Google Scholar] [CrossRef]
- Lee, R.H.; Lyles, K.W.; Colón-Emeric, C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am. J. Geriatr. Pharmacother. 2010, 8, 34–46. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA) 2021. Lyrica. 1995–2022 European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lyrica (accessed on 20 November 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagener, N.; Di Fazio, P.; Böker, K.O.; Matziolis, G. Osteogenic Effect of Pregabalin in Human Primary Mesenchymal Stem Cells, Osteoblasts, and Osteosarcoma Cells. Life 2022, 12, 496. https://doi.org/10.3390/life12040496
Wagener N, Di Fazio P, Böker KO, Matziolis G. Osteogenic Effect of Pregabalin in Human Primary Mesenchymal Stem Cells, Osteoblasts, and Osteosarcoma Cells. Life. 2022; 12(4):496. https://doi.org/10.3390/life12040496
Chicago/Turabian StyleWagener, Nele, Pietro Di Fazio, Kai Oliver Böker, and Georg Matziolis. 2022. "Osteogenic Effect of Pregabalin in Human Primary Mesenchymal Stem Cells, Osteoblasts, and Osteosarcoma Cells" Life 12, no. 4: 496. https://doi.org/10.3390/life12040496
APA StyleWagener, N., Di Fazio, P., Böker, K. O., & Matziolis, G. (2022). Osteogenic Effect of Pregabalin in Human Primary Mesenchymal Stem Cells, Osteoblasts, and Osteosarcoma Cells. Life, 12(4), 496. https://doi.org/10.3390/life12040496






