When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of RHF and Variables Evaluated
2.3. Study Design
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Incidence and Treatment of RHF
3.3. Risk Factors and Biomarkers for RHF and Impact on Survival
3.4. Pump Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | Hazard Ratio | Confidence Interval (95%) | p-Value |
|---|---|---|---|
| Baseline demographics | |||
| Baseline characteristics | |||
| Sex (female) | 0.86 | 0.30–2.45 | 0.78 |
| Age at implant | 0.99 | 0.97–1.03 | 0.92 |
| BMI (kg/m2) | 1.03 | 0.97–1.11 | 0.33 |
| INTERMACS level | 0.83 | ||
| 1 | ref | - | - |
| 2 | 1.24 | 0.45–3.43 | 0.67 |
| 3 | 0.78 | 0.23–2.58 | 0.68 |
| 4–7 | 1.27 | 0.51–3.16 | 0.61 |
| Cardiomyopathy (ischemic vs. dilative) | 0.98 | 0.45–2.12 | 0.96 |
| Strategy | 0.56 | ||
| Destination therapy | 1.82 | 0.79–4.14 | 0.16 |
| Bridge to transplantation | 1.26 | 0.46–3.47 | 0.65 |
| Bridge to candidacy | ref | - | - |
| Bridge to recovery | 0.00 | - | 0.98 |
| Intraoperative variables | |||
| Device (HeartMate 3 vs. HVAD) | 2.51 | 1.16–5.46 | 0.02 |
| Implantation technique, less invasive | 1.50 | 0.73–3.07 | 0.27 |
| Implantation with CPB | |||
| Concomitant temporary RVAD implantation | 1.69 | 0.83–3.45 | 0.15 |
| Concomitant valve surgery | 0.95 | ||
| AVR | 1.31 | 0.31–5.53 | 0.72 |
| TVR | 1.17 | 0.41–3.37 | 0.77 |
| AVR + TVR | 0.00 | - | 0.98 |
| Other | 0.49 | 0.07–3.61 | 0.48 |
| None | ref | - | - |
| Comorbidities/medication | |||
| Prae ECLS | 0.98 | 0.42–2.30 | 0.96 |
| Mechanical ventilation | 0.29 | 0.04–2.15 | 0.23 |
| Diabetes mellitus | 1.13 | 0.53–2.41 | 0.75 |
| Hypertension | 0.67 | 0.31–1.46 | 0.31 |
| Myocardial infarction | 1.08 | 0.51–2.32 | 0.84 |
| Beta blocker use | 1.83 | 0.79–4.20 | 0.16 |
| Laboratory parameters | |||
| Hemoglobin (g/dL) | 1.20 | 0.92–1.57 | 0.18 |
| Hematocrit (%) | 1.01 | 0.96–1.06 | 0.65 |
| Platelets (g/L) | 0.99 | 0.99–1.00 | 0.23 |
| Leucocytes (g/L) | 1.02 | 0.93–1.10 | 0.72 |
| Serum creatinine (mg/dL) | 1.25 | 0.79–1.96 | 0.35 |
| BUN (mg/dL) | 1.00 | 0.98–1.02 | 0.97 |
| Total bilirubin (mg/dL) | 1.04 | 0.93–1.15 | 0.50 |
| Gamma GT (U/L) | 0.99 | 0.99–1.00 | 0.34 |
| CRP (mg/dL) | 0.98 | 0.91–1.05 | 0.53 |
| LDH (U/L) | 1.00 | 1.00–1.00 | 0.24 |
| proBNP (pg/mL) | 1.00 | 1.00–1.00 | 0.46 |
| Albumin (g/L) | 0.97 | 0.92–1.02 | 0.21 |
| CK (U/L) | 1.00 | 1.00–1.00 | 0.13 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 1.03 | 0.99–1.07 | 0.10 |
| PCWP (mmHg) | 1.01 | 0.91–1.11 | 0.86 |
| CVP/PCWP ratio | 2.50 | 0.98–31.59 | 0.48 |
| Systolic pulmonary artery pressure (mmHg) | 1.00 | 0.98–1.02 | 0.99 |
| Mean arterial blood pressure (mmHg) | 1.01 | 0.98–1.03 | 0.53 |
| Cardiac output (L/min) | 0.89 | 0.58–1.39 | 0.63 |
| Heart rate (bpm) | 0.99 | 0.98–1.01 | 0.96 |
| Early post-op | |||
| Laboratory parameters | |||
| Hemoglobin (g/dL) | 1.02 | 0.88–1.18 | 0.82 |
| Hematocrit (%) | 1.06 | 0.98–1.15 | 0.15 |
| Platelets (g/L) | 0.99 | 0.99–1.00 | 0.44 |
| Leucocytes (g/L) | 1.02 | 0.96–1.09 | 0.50 |
| Serum creatinine (mg/dL) | 1.13 | 0.72–1.77 | 0.61 |
| BUN (mg/dL) | 1.00 | 0.98–1.02 | 0.90 |
| Total bilirubin (mg/dL) | 1.03 | 0.90–1.18 | 0.70 |
| Gamma GT (U/L) | 0.99 | 0.99–1.00 | 0.48 |
| CRP (mg/dL) | 1.00 | 0.91–1.10 | 0.98 |
| LDH (U/L) | 1.00 | 1.00–1.00 | 0.81 |
| proBNP (pg/mL) | 1.00 | 1.00–1.00 | 0.82 |
| Albumin (g/L) | 1.01 | 0.94–1.09 | 0.81 |
| CK (U/L) | 1.00 | 1.00–1.00 | 0.20 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 1.11 | 1.03–1.19 | 0.004 |
| PCWP (mmHg) | 1.06 | 0.87–1.30 | 0.56 |
| CVP/PCWP ratio | - | - | - |
| Systolic pulmonary artery pressure (mmHg) | 1.01 | 0.98–1.04 | 0.37 |
| Mean arterial blood pressure (mmHg) | 1.00 | 0.97–1.03 | 0.94 |
| Cardiac output (L/min) | 0.85 | 0.59–1.21 | 0.37 |
| Heart rate (bpm) | 0.97 | 0.96–0.99 | 0.001 |
| 30-days post-op | |||
| Laboratory parameters | |||
| Hemoglobin (g/dL) | 0.67 | 0.51–0.90 | 0.01 |
| Hematocrit (%) | 0.94 | 0.87–1.02 | 0.16 |
| Platelets (g/L) | 0.99 | 0.99–1.00 | 0.02 |
| Leucocytes (g/L) | 1.05 | 1.01–1.10 | 0.02 |
| Serum creatinine (mg/dL) | 1.11 | 0.82–1.49 | 0.51 |
| BUN (mg/dL) | 1.00 | 0.98–1.02 | 0.80 |
| Total bilirubin (mg/dL) | 1.13 | 1.03–1.24 | 0.01 |
| Gamma GT (U/L) | 1.00 | 0.99–1.00 | 0.95 |
| CRP (mg/dL) | 1.10 | 1.04–1.17 | 0.001 |
| LDH (U/L) | 1.00 | 0.99–1.00 | 0.97 |
| proBNP (pg/mL) | 1.00 | 0.99–1.00 | 0.70 |
| Albumin (g/L) | 0.94 | 0.89–1.00 | 0.07 |
| CK (U/L) | 0.99 | 0.98–1.01 | 0.29 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 0.99 | 0.94–1.06 | 0.89 |
| PCWP (mmHg) | - | - | - |
| CVP/PCWP ratio | - | - | - |
| Systolic pulmonary artery pressure (mmHg) | - | - | - |
| Mean arterial blood pressure (mmHg) | 0.98 | 0.95–1.01 | 0.23 |
| Cardiac output (L/min) | - | - | - |
| Heart rate (bpm) | - | - | - |
| Variable n (%), Median (IQR) or Mean ± SD | HeartMate 3 (n = 70) | HVAD (n = 75) | p-Value |
|---|---|---|---|
| Baseline characteristics | |||
| Sex (female) | 7 (10.0) | 13 (17.3) | 0.23 |
| Age at implant (years) | 64.5 (13.0) | 59.0 (15.0) | 0.048 |
| BMI (kg/m2) | 27.1 (7.7) | 25.4 (6.1) | 0.14 |
| INTERMACS level | |||
| 1 | 14 (20.0) | 27 (37.0) | |
| 2 | 13 (18.6) | 17 (23.3) | 0.017 |
| 3 | 12 (17.1) | 14 (19.2) | |
| 4–7 | 31 (44.3) | 15 (20.5) | |
| Cardiomyopathy, ischemic | 39 (55.7) | 45 (61.6) | 0.28 |
| Strategy | |||
| Destination therapy | 26 (37.1) | 22 (30.1) | |
| Bridge to transplantation | 12 (17.1) | 18 (24.7) | 0.49 |
| Bridge to candidacy | 32 (45.7) | 32 (43.8) | |
| Bridge to recovery | 0 (0.0) | 1 (1.4) | |
| Intraoperative variables | |||
| Implantation technique, less invasive | 19 (27.1) | 35 (47.3) | 0.016 |
| Implantation with CPB | 65 (94.2) | 46 (61.3) | <0.001 |
| Concomitant temporary RVAD implantation | 22 (31.4) | 23 (30.7) | 0.99 |
| Concomitant valve surgery | |||
| AVR | 4 (5.7) | 3 (4.0) | |
| TVR | 11 (15.7) | 6 (8.0) | |
| AVR + TVR | 0 (0.0) | 1 (1.3) | 0.52 |
| Other | 4 (5.7) | 5 (6.7) | |
| None | 51 (72.9) | 60 (80.0) | |
| Comorbidities | |||
| Prae ECLS | 11 (16.4) | 24 (32.9) | 0.032 |
| Mechanical ventilation | 5 (9.3) | 10 (19.6) | 0.17 |
| Diabetes mellitus | 24 (34.3) | 20 (27.0) | 0.37 |
| Hypertension | 30 (42.9) | 25 (33.8) | 0.31 |
| Myocardial infarction | 50 (71.4) | 47 (63.5) | 0.38 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 14.0 (7.5) | 15.0 (7.0) | 0.15 |
| PCWP (mmHg) | 20.3 ± 7.1 | 22.4 ± 7.8 | 0.37 |
| CVP/PCWP ratio | 0.78 ± 0.3 | 0.75 ± 0.3 | 0.73 |
| Systolic pulmonary artery pressure (mmHg) | 44.5 ± 20.9 | 39.4 ± 16.9 | 0.12 |
| Mean arterial blood pressure (mmHg) | 69.5 (17.3) | 73.0 (14.0) | 0.40 |
| Cardiac output (L/min) | 3.4 (1.5) | 3.6 (1.8) | 0.67 |
| Heart rate (bpm) | 87.5 (39.0) | 83.0 (28.0) | 0.92 |
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Lima, B.; Mack, M.; Gonzalez-Stawinski, G.V. Ventricular assist devices: The future is now. Trends Cardiovasc. Med. 2015, 25, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, N.K.; Smith, D.E.; Moazami, N. The Achilles’ heel of left ventricular assist device therapy: Right ventricle. Curr. Opin. Organ Transplan. 2018, 23, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Lampert, B.C.; Teuteberg, J.J. Right ventricular failure after left ventricular assist devices. J. Heart Lung Transp. 2015, 34, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandel, M.; Krabatsch, T.; Falk, V. Left ventricular vs. biventricular mechanical support: Decision making and strategies for avoidance of right heart failure after left ventricular assist device implantation. Int. J. Cardiol. 2015, 198, 241–250. [Google Scholar] [CrossRef]
- Frankfurter, C.; Molinero, M.; Vishram-Nielsen, J.K.; Foroutan, F.; Mak, S.; Rao, V.; Billia, F.; Orchanian-Cheff, A.; Alba, A.C. Predicting the Risk of Right Ventricular Failure in Patients Undergoing Left Ventricular Assist Device Implantation. Circ. Heart Fail. 2020, 13, e006994. [Google Scholar] [CrossRef]
- Sabashnikov, A.; Mohite, P.N.; Zych, B.; García, D.; Popov, A.-F.; Weymann, A.; Patil, N.P.; Hards, R.; Capoccia, M.; Wahlers, T.; et al. Outcomes and Predictors of Early Mortality After Continuous-Flow Left Ventricular Assist Device Implantation as a Bridge to Transplantation. ASAIO J. 2014, 60, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Atluri, P.; Goldstone, A.B.; Fairman, A.S.; MacArthur, J.W.; Shudo, Y.; Cohen, J.E.; Acker, A.L.; Hiesinger, W.; Howard, J.L.; Acker, M.A.; et al. Predicting Right Ventricular Failure in the Modern, Continuous Flow Left Ventricular Assist Device Era. Ann. Thorac. Surg. 2013, 96, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Lo Coco, V.; De Piero, M.E.; Massimi, G.; Chiarini, G.; Raffa, G.M.; Kowalewski, M.; Maessen, J.; Lorusso, R. Right Ventricular Failure after Left Ven-tricular Assist Device Implantation: A Review of the Literature. J. Thorac. Dis. 2021, 13, 1256–1269. [Google Scholar] [CrossRef]
- Pasrija, C.; Sawan, M.A.; Sorensen, E.; Voorhees, H.; Shah, A.; Strauss, E.; Ton, V.-K.; DiChiacchio, L.; Kaczorowski, D.J.; Griffith, B.P.; et al. Less invasive left ventricular assist device implantation may reduce right ventricular failure. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 592–598. [Google Scholar] [CrossRef]
- Chiang, Y.P.; Cox, D.; Schroder, J.N.; Daneshmand, M.A.; Blue, L.J.; Patel, C.B.; Devore, A.D.; Bishawi, M.; Milano, C.A. Stroke risk following implantation of current generation centrifugal flow left ventricular assist devices. J. Card. Surg. 2019, 35, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-M.; Mehaffey, J.H.; Meyers, S.L.; Cantor, R.S.; Starling, R.C.; Kirklin, J.K.; Jacobs, J.P.; Kern, J.; Uchino, K.; Yarboro, L.T. Cerebrovascular Events in Patients With Centrifugal-Flow Left Ventricular Assist Devices: Propensity Score–Matched Analysis From the Intermacs Registry. Circulation 2021, 144, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.V.; Nersesian, G.; Lewin, D.; Özbaran, M.; de By, T.M.M.H.; Stein, J.; Pya, Y.; Gummert, J.; Ramjankhan, F.; Zembala, M.O.; et al. Propensity score-based analysis of long-term follow-up in patients supported with durable centrifugal left ventricular assist devices: The EUROMACS analysis. Eur. J. Cardio-Thoracic Surg. 2021, 60, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Numan, L.; Ramjankhan, F.Z.; Oberski, D.L.; Oerlemans, M.I.; Aarts, E.; Gianoli, M.; Van Der Heijden, J.J.; De Jonge, N.; Van Der Kaaij, N.P.; Meuwese, C.L.; et al. Propensity score-based analysis of long-term outcome of patients on HeartWare and HeartMate 3 left ventricular assist device support. ESC Heart Fail. 2021, 8, 1596–1603. [Google Scholar] [CrossRef]
- Li, S.; Beckman, J.A.; Cheng, R.; Ibeh, C.; Creutzfeldt, C.J.; Bjelkengren, J.; Herrington, J.; Stempien-Otero, A.; Lin, S.; Levy, W.C.; et al. Comparison of Neurologic Event Rates Among HeartMate II, HeartMate 3, and HVAD. ASAIO J. 2019, 66, 620–624. [Google Scholar] [CrossRef]
- Interagency Registry for Mechanically Assisted Circulatory Support—Appendix A—Adverse Event Definitions 5.0. Available online: https://www.uab.edu/medicine/intermacs/intermacs-documents (accessed on 19 February 2022).
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Renlund, D.G.; et al. Risk Factors Predictive of Right Ventricular Failure after Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2010, 105, 1030–1035. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Baek, S.H. Optimal Use of Beta-Blockers for Congestive Heart Failure. Circ. J. 2016, 80, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Imamura, T.; Mehta, P.; Nguyen, A.; Chung, B.; Narang, N.; Rodgers, D.; Raikhelkar, J.; Smith, B.; Song, T.; Ota, T.; et al. Neurohormonal Blockade During Left Ventricular Assist Device Support. ASAIO J. 2019, 66, 881–885. [Google Scholar] [CrossRef]
- Provencher, S.; Herve, P.; Jais, X.; Lebrec, D.; Humbert, M.; Simonneau, G.; Sitbon, O. Deleterious Effects of β-Blockers on Exercise Ca-pacity and Hemodynamics in Patients with Portopulmonary Hypertension. Gastroenterology 2006, 130, 120–126. [Google Scholar] [CrossRef]
- Rame, J.E.; Pagani, F.D.; Kiernan, M.S.; Oliveira, G.H.; Birati, E.Y.; Atluri, P.; Gaffey, A.; Grandin, E.W.; Myers, S.L.; Collum, C.; et al. Evolution of Late Right Heart Failure with Left Ventricular Assist Devices and Association With Outcomes. J. Am. Coll. Cardiol. 2021, 78, 2294–2308. [Google Scholar] [CrossRef]
- Schmitto, J.D.; Molitoris, U.; Haverich, A.; Strueber, M. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: Upper hemisternotomy combined with anterolateral thoracotomy. J. Thorac. Cardiovasc. Surg. 2012, 143, 511–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riebandt, J.; Schlöglhofer, T.; Moayedifar, R.; Wiedemann, D.; Wittmann, F.; Angleitner, P.; Dimitrov, K.; Tschernko, E.; Laufer, G.; Zimpfer, D. Less Invasive Left Ventricular Assist Device Implantation Is Safe and Reduces Intraoperative Blood Product Use: A Propensity Score Analysis VAD Implantation Techniques and Blood Product Use. ASAIO J. 2020, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Argiriou, M.; Kolokotron, S.-M.; Sakellaridis, T.; Argiriou, O.; Charitos, C.; Zarogoulidis, P.; Katsikogiannis, N.; Kougioumtzi, I.; Machairiotis, N.; Tsiouda, T.; et al. Right heart failure post left ventricular assist device implantation. J. Thorac. Dis. 2014, 6, S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, Y.; McCarthy, P.M.; Smedira, N.G.; Banbury, M.K.; Navia, J.L.; Feng, J.; Hsu, A.P.; Yeager, M.L.; Buda, T.; Hoercher, K.J.; et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation 2002, 106, I-198–I-202. [Google Scholar] [CrossRef]
- Terzic, D.; Putnik, S.; Nestorovic, E.; Jovicic, V.; Lazovic, D.; Rancic, N.; Milicevic, V.; Ivanisevic, D.; Karan, R.; Mikic, A. Impact of Right Heart Failure on Clinical Outcome of Left Ventricular Assist Devices (LVAD) Implantation: Single Center Experience. Healthcare 2022, 10, 114. [Google Scholar] [CrossRef]
- Dandel, M.; Potapov, E.; Krabatsch, T.; Stepanenko, A.; Löw, A.; Vierecke, J.; Knosalla, C.; Hetzer, R. Load Dependency of Right Ventricular Performance Is a Major Factor to be Considered in Decision Making Before Ventricular Assist Device Implantation. Circulation 2013, 128, S14–S23. [Google Scholar] [CrossRef] [Green Version]
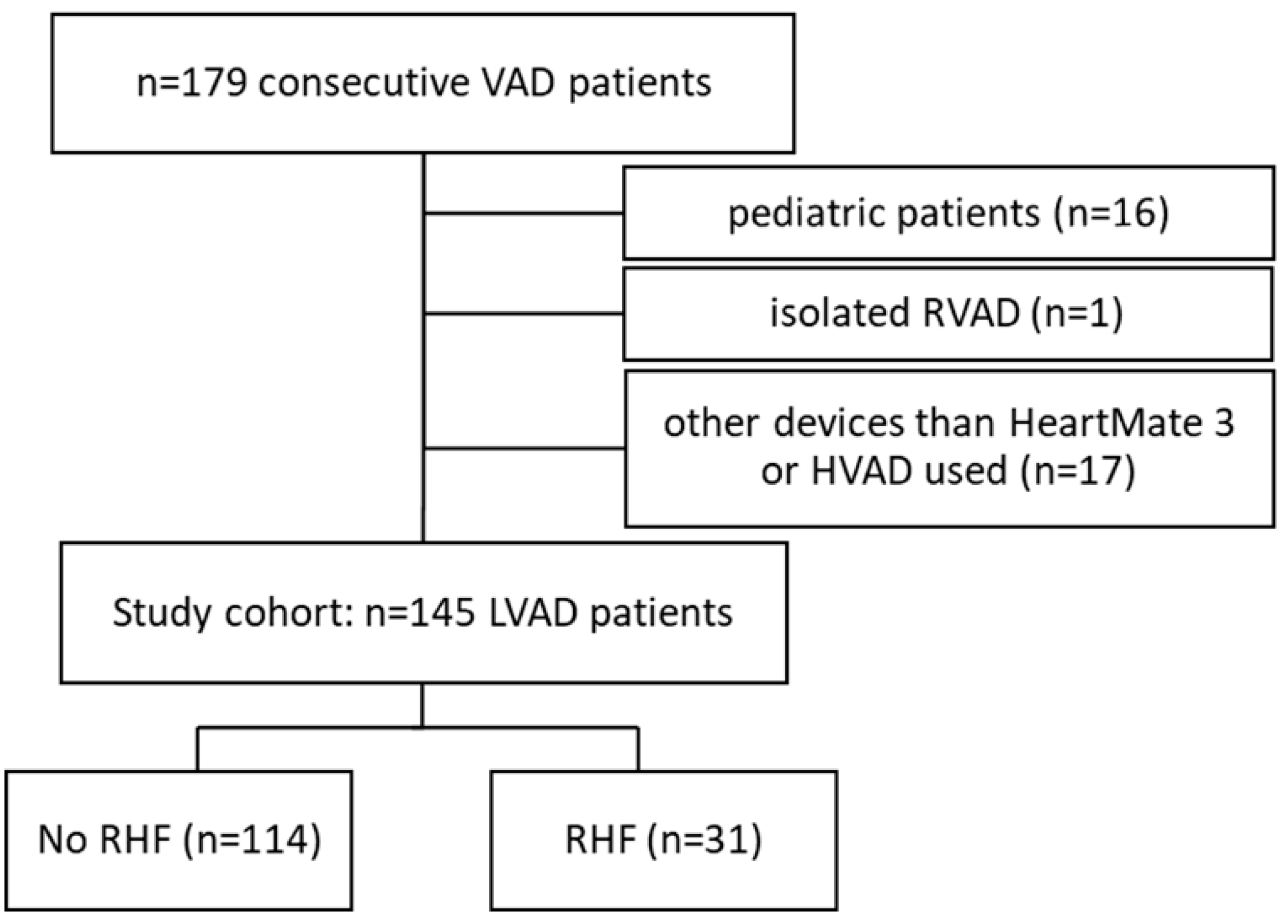
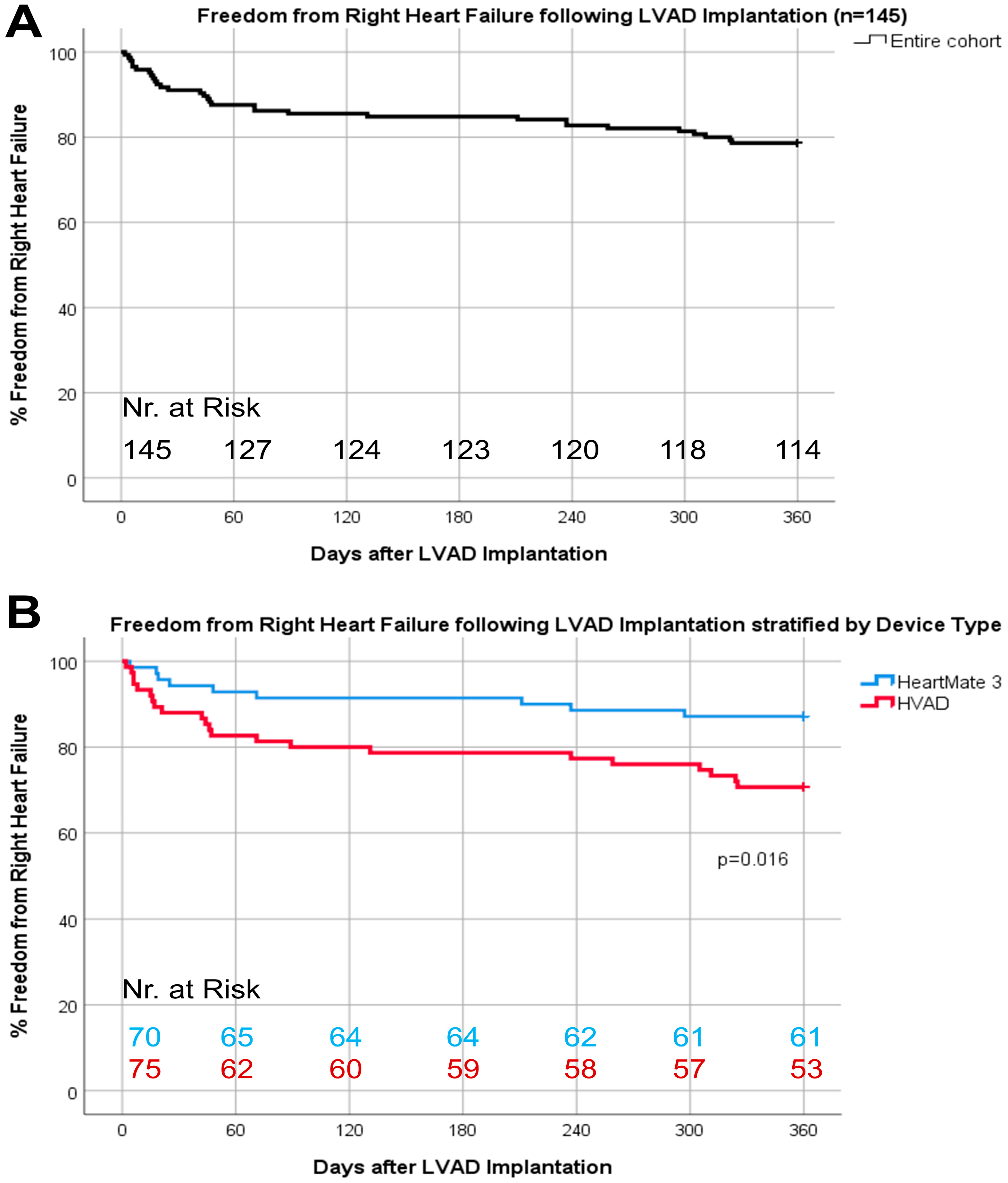
| Variable n (%), Median (IQR) or Mean ± SD | No RHF (n = 114) | RHF (n = 31) | p-Value |
|---|---|---|---|
| Baseline characteristics | |||
| Sex (female) | 16 (14.0) | 4 (12.9) | 1.00 |
| Age at implant (years) | 60.5 (13.0) | 66.0 (20.0) | 0.36 |
| BMI (kg/m2) | 26.1 (7.0) | 27.6 (8.7) | 0.36 |
| INTERMACS level | |||
| 1 | 33 (29.0) | 8 (25.8) | |
| 2 | 23 (20.2) | 7 (22.6) | 0.83 |
| 3 | 22 (19.3) | 5 (16.2) | |
| 4–7 | 36 (31.5) | 11 (35.4) | |
| Cardiomyopathy, ischemic | 66 (58.9) | 18 (58.1) | 0.90 |
| Strategy | |||
| Destination therapy | 35 (30.7) | 14 (45.2) | |
| Bridge to transplantation | 24 (21.1) | 6 (19.4) | 0.46 |
| Bridge to candidacy | 54 (47.4) | 11 (35.5) | |
| Bridge to recovery | 1 (0.9) | 0 (0.0) | |
| Intraoperative variables | |||
| Device | |||
| HeartMate 3 | 61 (53.5) | 9 (29.0) | 0.03 |
| HVAD | 53 (46.5) | 22 (71.0) | |
| Implantation technique, less invasive | 40 (35.1) | 14 (46.7) | 0.29 |
| Implantation with CPB | 89 (78.8) | 22 (71.0) | 0.07 |
| Concomitant temporary RVAD implantation | 32 (28.1) | 13 (41.9) | 0.19 |
| Concomitant valve surgery | |||
| AVR | 5 (4.4) | 2 (6.5) | |
| TVR | 13 (11.4) | 4 (12.9) | |
| AVR + TVR | 1 (0.9) | 0 (0.0) | 0.89 |
| Other | 8 (7.0) | 1 (3.2) | |
| None | 87 (76.3) | 24 (77.4) | |
| Comorbidities | |||
| Prae ECLS | 28 (25.0) | 7 (25.0) | 1.00 |
| Mechanical ventilation | 14 (16.5) | 1 (5.0) | 0.29 |
| Diabetes mellitus | 34 (29.8) | 10 (33.3) | 0.82 |
| Hypertension | 46 (40.4) | 9 (30.0) | 0.40 |
| Myocardial infarction | 77 (67.5) | 20 (66.7) | 1.00 |
| Laboratory parameters | |||
| Hemoglobin (g/dL) | 11.3 (4.2) | 11.9 (3.6) | 0.65 |
| Hematocrit (%) | 34.0 (11.0) | 36.6 (9.9) | 0.57 |
| Platelets (g/L) | 212.0 (124.0) | 190.5 (135.3) | 0.24 |
| Leucocytes (g/L) | 8.2 (3.7) | 8.8 (5.2) | 0.92 |
| Serum creatinine (mg/dL) | 1.3 (0.9) | 1.5 (0.8) | 0.31 |
| BUN (mg/dL) | 27.5 (23.2) | 27.2 (20.3) | 0.95 |
| Total bilirubin (mg/dL) | 1.0 (1.3) | 1.5 (1.5) | 0.03 |
| Gamma GT (U/L) | 98.0 (114.0) | 72.0 (81.0) | 0.06 |
| CRP (mg/dL) | 2.4 (4.4) | 1.8 (3.1) | 0.63 |
| LDH (U/L) | 244.0 (171.0) | 251.0 (130.8) | 0.65 |
| proBNP (pg/mL) | 9408.5 ± 7570.0 | 7687.1 ± 5817.4 | 0.45 |
| Albumin (g/L) | 33.9 (11.2) | 34.5 (10.3) | 0.49 |
| CK (U/L) | 66.5 (96.3) | 68.0 (104.0) | 0.78 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 14.0 (7.0) | 16.5 (7.0) | 0.01 |
| PCWP (mmHg) | 21.4 ± 7.4 | 22.0 ± 8.1 | 0.84 |
| CVP/PCWP ratio | 0.75 ± 0.3 | 0.83 ± 0.3 | 0.50 |
| Systolic pulmonary artery pressure (mmHg) | 42.0 ± 20.0 | 41.7 ± 15.8 | 0.94 |
| Mean arterial blood pressure (mmHg) | 71.5 (15.0) | 73.0 (18.0) | 0.62 |
| Cardiac output (L/min) | 3.5 (1.7) | 3.5 (2.3) | 0.86 |
| Heart rate (bpm) | 82.0 (35.0) | 81.0 (35.0) | 0.90 |
| Variable, n (%), Median (IQR) or Mean ± SD | No RHF (n = 114) | RHF (n = 31) | p-Value |
|---|---|---|---|
| Early post-op | |||
| Laboratory parameters | |||
| Hemoglobin (g/dL) | 10.8 (1.6) | 11.2 (1.5) | 0.18 |
| Hematocrit (%) | 31.8 (5.4) | 33.4 (3.9) | 0.13 |
| Platelets (g/L) | 151.5 (101.5) | 135.0 (93.5) | 0.50 |
| Leucocytes (g/L) | 12.2 (6.7) | 13.4 (7.4) | 0.49 |
| Serum creatinine (mg/dL) | 1.3 (0.8) | 1.4 (0.5) | 0.19 |
| BUN (mg/dL) | 25.1 (18.8) | 25.1 (13.1) | 0.78 |
| Total bilirubin (mg/dL) | 1.8 (2.1) | 2.8 (2.6) | 0.21 |
| Gamma GT (U/L) | 52.0 (61.0) | 39.0 (38.8) | 0.06 |
| CRP (mg/dL) | 1.6 (3.2) | 1.7 (3.3) | 0.32 |
| LDH (U/L) | 331.0 (146.0) | 386.0 (249.0) | 0.25 |
| proBNP (pg/mL) | 4462.0 (4454.0) | 5376.0 (3423.0) | 0.92 |
| Albumin (g/L) | 27.6 ± 4.9 | 27.8 ± 5.0 | 0.81 |
| CK (U/L) | 414.0 (446.0) | 369.0 (468.0) | 0.64 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 13.0 (4.0) | 14.0 (4.0) | 0.047 |
| PCWP (mmHg) | 18.2 ± 7.6 | 19.9 ± 10.1 | 0.33 |
| CVP/PCWP ratio | 0.7 (0.5) | 0.8 (0.5) | 0.23 |
| Systolic pulmonary artery pressure (mmHg) | 43.0 (18.0) | 42.0 (24.0) | 0.86 |
| Mean arterial blood pressure (mmHg) | 73.0 (14.0) | 72.0 (12.0) | 0.83 |
| Cardiac output (L/min) | 5.3 (1.7) | 4.8 (2.3) | 0.17 |
| Heart rate (bpm) | 94.5 (21.0) | 85.0 (32.0) | 0.003 |
| 30-days post-op | |||
| Laboratory parameters | |||
| Hemoglobin (g/dL) | 10.1 (2.1) | 9.3 (1.6) | 0.01 |
| Hematocrit (%) | 30.5 (6.2) | 28.4 (5.3) | 0.03 |
| Platelets (g/L) | 306.0 (168.0) | 229.0 (205.0) | 0.01 |
| Leucocytes (g/L) | 8.9 (4.3) | 10.9 (8.6) | 0.08 |
| Serum creatinine (mg/dL) | 0.9 (0.5) | 1.0 (0.9) | 0.27 |
| BUN (mg/dL) | 14.7 (14.6) | 17.0 (17.7) | 0.22 |
| Total bilirubin (mg/dL) | 0.6 (0.6) | 0.8 (1.2) | 0.04 |
| Gamma GT (U/L) | 117.0 (152.0) | 97.0 (126.0) | 0.79 |
| CRP (mg/dL) | 4.3 (5.1) | 7.1 (6.2) | 0.28 |
| LDH (U/L) | 282.0 (121.5) | 338.0 (173.0) | 0.18 |
| proBNP (pg/mL) | 2948.0 (6792.1) | - | - |
| Albumin (g/L) | 31.8 ± 9.9 | 28.9 ± 5.8 | 0.08 |
| CK (U/L) | 35.0 (24.0) | 30.0 (32.5) | 0.34 |
| Hemodynamic parameters | |||
| CVP (mmHg) | 10.0 (7.0) | 12.0 (9.0) | 0.39 |
| PCWP (mmHg) | - | - | - |
| CVP/PCWP ratio | - | - | - |
| Systolic pulmonary artery pressure (mmHg) | - | - | - |
| Mean arterial blood pressure (mmHg) | 87.0 (17.0) | 82.0 (12.0) | 0.83 |
| Cardiac output (L/min) | - | - | - |
| Heart rate (bpm) | 93.4 ± 14.4 | 85.8 ± 15.1 | 0.14 |
| Variables | Hazard Ratio | Confidence Interval (95%) | p-Value |
|---|---|---|---|
| Beta blocker use preoperative (no vs. yes) | 2.18 | 0.68–6.98 | 0.19 |
| Hemoglobin preoperative (per unit) | 0.29 | 0.06–1.59 | 0.16 |
| CK preoperative (per unit) | 1.00 | 0.99–1.01 | 0.56 |
| Concomitant temporary RVAD support (no vs. yes) | 1.76 | 0.39–8.02 | 0.47 |
| Device (HeartMate 3 vs. HVAD) | 4.61 | 1.12–18.98 | 0.03 |
| Heart rate early post-op (per bpm) | 0.96 | 0.93–0.99 | 0.02 |
| CVP early post-op (per mmHg) | 1.21 | 1.05–1.39 | 0.01 |
| Hematocrit early post-op (per unit) | 1.29 | 0.76–2.21 | 0.35 |
| Platelets 30 d post-op (per unit) | 0.99 | 0.99–1.00 | 0.25 |
| Leukocytes 30 d post-op (per unit) | 0.96 | 0.84–1.10 | 0.57 |
| CRP 30 d post-op (per unit) | 1.17 | 0.96–1.43 | 0.11 |
| Total bilirubin 30 d post-op (per unit) | 1.03 | 0.53–2.01 | 0.93 |
| Albumin 30 d post-op (per unit) | 1.05 | 0.93–1.19 | 0.40 |
| Variables | Hazard Ratio | Confidence Interval (95%) | p-Value |
|---|---|---|---|
| RHF (no vs. yes) | 1.00 | 0.99–1.01 | 0.49 |
| Beta blocker use preoperative (no vs. yes) | 1.46 | 0.54–3.92 | 0.46 |
| Age (per year) | 1.05 | 0.99–1.11 | 0.06 |
| Concomitant valve surgery (no vs. yes) | 2.84 | 0.98–8.26 | 0.06 |
| INTERMACS profile | |||
| 1 | Ref | Ref | 0.13 |
| 2 | 0.98 | 0.31–3.13 | 0.97 |
| 3 | 0.22 | 0.04–1.26 | 0.09 |
| 4–7 | 0.26 | 0.05–1.26 | 0.09 |
| CVP/PCWP ratio early post-op (per unit) | 0.91 | 0.48–1.71 | 0.76 |
| Hematocrit early post-op (per unit) | 1.03 | 0.91–1.16 | 0.67 |
| Creatinine early post-op (per unit) | 1.44 | 0.79–2.62 | 0.23 |
| BUN early post-op (per unit) | 1.03 | 0.99–1.06 | 0.17 |
| Total bilirubin early post-op (per unit) | 1.19 | 0.97–1.47 | 0.09 |
| Gamma GT early post-op (per unit) | 1.00 | 0.99–1.00 | 0.51 |
| Albumin early post-op (per unit) | 0.96 | 0.87–1.06 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlöglhofer, T.; Wittmann, F.; Paus, R.; Riebandt, J.; Schaefer, A.-K.; Angleitner, P.; Granegger, M.; Aigner, P.; Wiedemann, D.; Laufer, G.; et al. When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation. Life 2022, 12, 459. https://doi.org/10.3390/life12030459
Schlöglhofer T, Wittmann F, Paus R, Riebandt J, Schaefer A-K, Angleitner P, Granegger M, Aigner P, Wiedemann D, Laufer G, et al. When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation. Life. 2022; 12(3):459. https://doi.org/10.3390/life12030459
Chicago/Turabian StyleSchlöglhofer, Thomas, Franziska Wittmann, Robert Paus, Julia Riebandt, Anne-Kristin Schaefer, Philipp Angleitner, Marcus Granegger, Philipp Aigner, Dominik Wiedemann, Günther Laufer, and et al. 2022. "When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation" Life 12, no. 3: 459. https://doi.org/10.3390/life12030459
APA StyleSchlöglhofer, T., Wittmann, F., Paus, R., Riebandt, J., Schaefer, A.-K., Angleitner, P., Granegger, M., Aigner, P., Wiedemann, D., Laufer, G., Schima, H., & Zimpfer, D. (2022). When Nothing Goes Right: Risk Factors and Biomarkers of Right Heart Failure after Left Ventricular Assist Device Implantation. Life, 12(3), 459. https://doi.org/10.3390/life12030459










