Analysis of Antidepressant, Benzodiazepine Anxiolytic, and Hypnotic Use When Treating Depression, Anxiety, and Aggression in Pain Clinic Patients Treated for Neuropathic Pain
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, S.; Tubbs, R.S. Neuroanatomy and Neuropsychology of Pain. Cureus 2017, 9, e1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boadas-Vaello, P.; Homs, J.; Reina, F.; Carrera, A.; Verdú, E. Neuroplasticity of Supraspinal Structures Associated with Pathological Pain. Anat. Rec. 2017, 300, 1481–1501. [Google Scholar] [CrossRef] [Green Version]
- Dinakar, P.; Stillman, A.M. Pathogenesis of Pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef]
- Von Knorring, L.; Perris, C.; Eisemann, M.; Eriksson, U.; Perris, H. Pain as a symptom in depressive disorders. II. Relationship to personality traits as assessed by means of KSP. Pain 1983, 17, 377–384. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S. Emotion Regulation and Psychopathology: The Role of Gender. Annu. Rev. Clin. Psychol. 2012, 8, 161–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radat, F.; Margot-Duclot, A.; Attal, N. Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: A multicentre cohort study. Eur. J. Pain 2013, 17, 1547–1557. [Google Scholar] [CrossRef]
- Torta, R.; Ieraci, V.; Zizzi, F. A Review of the Emotional Aspects of Neuropathic Pain: From Comorbidity to Co-Pathogenesis. Pain Ther. 2017, 6, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Okifuji, A.; Turk, D.C.; Curran, S.L. Anger in chronic pain: Investigations of anger targets and intensity. J. Psychosom. Res. 1999, 47, 1–12. [Google Scholar] [CrossRef]
- LaMotte, A.D.; Taft, C.T.; Weatherill, R.P.; Casement, M.D.; Creech, S.K.; Milberg, W.P.; Fortier, C.B.; McGlinchey, R.E. Sleep problems and physical pain as moderators of the relationship between PTSD symptoms and aggression in returning veterans. Psychol. Trauma Theory Res. Pract. Policy 2017, 9, 113–116. [Google Scholar] [CrossRef]
- Agüera-Ortiz, L.; Failde, I.; Mico, J.A.; Cervilla, J.; López-Ibor, J.J. Pain as a symptom of depression: Prevalence and clinical correlates in patients attending psychiatric clinics. J. Affect. Disord. 2011, 130, 106–112. [Google Scholar] [CrossRef]
- Bair, M.; Robinson, R.; Katon, W.; Kroenke, K.; Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.S.; Jones, W.J.; Shen, J.; Robinson, R.L.; Weinberger, M.; Kroenke, K. Prevalence and impact of depression and pain in neurology outpatients. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1587–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherif, F.; Zouari, H.G.; Cherif, W.; Hadded, M.; Cheour, M.; Damak, R. Depression Prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Res. Manag. 2020, 2020, 7408508. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Suevelliance Report 2017-Neuropathic Pain in Adults: Pharmacological Management in Non-Specialist Settings (2013). NICE Guidel. CG173. Available online: https://www.ncbi.nlm.nhi.gov/books/NBK552078 (accessed on 5 February 2022).
- Haanpää, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; Iannetti, G.D.; et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011, 152, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N.; et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Carsten Schultheis, B.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, 2015, CD008242. [Google Scholar] [CrossRef]
- Szczudlik, A.; Dobrogowski, J.; Wordliczek, J.; Stępień, A.; Krajnik, M.; Leppert, W.; Woroń, J.; Przeklasa-Muszyńska, A.; Kocot-Kępska, M.; Zajączkowska, R.; et al. Diagnosis and management of neuropathic pain: Review of literature and recommendations of the Polish Association for the Study of Pain and the Polish Neurological Society—Part one. Neurol. Neurochir. Pol. 2014, 48, 262–271. [Google Scholar] [CrossRef]
- Szok, D.; Tajti, J.; Nyári, A.; Vécsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019. [Google Scholar] [CrossRef]
- Koen, N.; Stein, D.J. Pharmacotherapy of anxiety disorders: A critical review. Dialogues Clin. Neurosci. 2011, 13, 423–437. [Google Scholar] [PubMed]
- Chokroverty, S. Diagnosis and treatment of sleep disorders caused by co-morbid disease. Neurology 2000, 54, S8–S15. [Google Scholar] [PubMed]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Sindrup, S.H.; Jensen, T.S. Antidepressants in Neuropathic Pain. In Encyclopedia of Pain; Springer: Berlin/Heidelberg, Germany, 2013; pp. 175–179. [Google Scholar]
- Shin, S.W.; Lee, J.S.; Abdi, S.; Lee, S.J.; Kim, K.H. Antipsychotics for patients with pain. Korean J. Pain 2019, 32, 3–11. [Google Scholar] [CrossRef]
- Cubała, W.J. Czynniki wpływające na ryzyko wystąpienia działań niepożądanych w trakcie leczenia zolpidemem. Psychiatria 2007, 4, 124–127. [Google Scholar]
- Bomalaski, M.N.; Claflin, E.S.; Townsend, W.; Peterson, M.D. Zolpidem for the treatment of neurologic disorders: A systematic review. JAMA Neurol. 2017, 74, 1130–1139. [Google Scholar] [CrossRef]
- Repka, I.; Wordliczek, J. Emotional state versus quality of life of patients with chronic neuropathic pain. Postępy Nauk Med. 2013, 26, 730–736. [Google Scholar]
- Nicholson, B.; Verma, S. Comorbidities in chronic neuropathic pain. Pain Med. 2004, 5, S9–S27. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, J.P.; Kerklaan, J.; Huygen, F.J.P.M.; Bouma, P.A.D.; Sturkenboom, M.C.J.M. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008, 137, 681–688. [Google Scholar] [CrossRef]
- FDA News Release. FDA Requires Strong Warnings for Opioid Analgesics, Prescription Opioid Cough Products, and Benzodiazepine Labeling Related to Serious Risks and Death from Combined Use. 2016. Available online: https://www.fda.gov/news-events/press-announcements/fda-requires-strong-warnings-opioid-analgesics-prescription-opioid-cough-products-and-benzodiazepine (accessed on 5 February 2022).
- Donatelli, N.S.; Somes, J. Opioids and Benzodiazepines—A Drug Problem for the Older Adult. J. Emerg. Nurs. 2019, 45, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Micó, J.A.; Ardid, D.; Berrocoso, E.; Eschalier, A. Antidepressants and pain. Trends Pharmacol. Sci. 2006, 27, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.R. The epidemiology and treatment of depression when it coexists with somatoform disorders, somatization, or pain. Gen. Hosp. Psychiatry 1992, 14, 265–272. [Google Scholar] [CrossRef]
- Langley, P.C.; Van Litsenburg, C.; Cappelleri, J.C.; Carroll, D. The burden associated with neuropathic pain in Western Europe. J. Med. Econ. 2013, 16, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Melek, L.; Smith, J.; Karamat, A.; Renton, T. Comparison of the Neuropathic Pain Symptoms and Psychosocial Impacts of Trigeminal Neuralgia and Painful Posttraumatic Trigeminal Neuropathy. J. Oral Facial Pain Headache 2019, 33, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Fava, M.; Mallinckrodt, C.H.; Detke, M.J.; Watkin, J.G.; Wohlreich, M.M. The effect of duloxetine on painful physical symptoms in depressed patients: Do improvements in these symptoms result in higher remission rates? J. Clin. Psychiatry 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Wohlreich, M.M.; Sullivan, M.D.; Mallinckrodt, C.H.; Chappell, A.S.; Oakes, T.M.; Watkin, J.G.; Raskin, J. Duloxetine for the treatment of recurrent major depressive disorder in elderly patients: Treatment outcomes in patients with comorbid arthritis. Psychosomatics 2009, 50, 402–412. [Google Scholar] [CrossRef]
- Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [Green Version]
- Slee, A.; Nazareth, I.; Bondaronek, P.; Liu, Y.; Cheng, Z.; Freemantle, N. Pharmacological treatments for generalised anxiety disorder: A systematic review and network meta-analysis. Lancet 2019, 393, 768–777. [Google Scholar] [CrossRef]
- Gauntlett-Gilbert, J.; Gavriloff, D.; Brook, P. Benzodiazepines may be worse than opioids negative medication effects in severe chronic pain. Clin. J. Pain 2016, 32, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.; Benveniste, H.; McLellan, A.T. Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med. 2018, 69, 451–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, H.; Khalilzadeh, E.; Vafaei Saiah, G. Pain-induced aggression and changes in social behavior in mice. Aggress. Behav. 2020, 47, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Goedhard, L.E.; Stolker, J.J.; Heerdink, E.R.; Nijman, H.L.I.; Olivier, B.; Egberts, T.C.G. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: A systematic review. J. Clin. Psychiatry 2006, 67, 1013–1024. [Google Scholar] [CrossRef]
- Felthous, A.R.; Stanford, M.S. A proposed algorithm for the pharmacotherapy of impulsive aggression. J. Am. Acad. Psychiatry Law 2015, 43, 456–467. [Google Scholar]
- Weissman, M.M.; Klerman, G.L. Sex Differences and the Epidemiology of Depression. Arch. Gen. Psychiatry 1977, 34, 98–111. [Google Scholar] [CrossRef]
- Sundbom, L.T.; Bingefors, K.; Hedborg, K.; Isacson, D. Are men under-treated and women over-treated with antidepressants? Findings from a cross-sectional survey in Sweden. BJPsych Bull. 2017, 41, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Brody, D.J.; Gu, Q. Antidepressant Use Among Adults: United States, 2015–2018. NCHS Data Brief 2020, 377, 1–8. [Google Scholar]
- Cayley, W.E. Antidepressants for the treatment of neuropathic pain. Am. Fam. Physician 2006, 7, 1933–1936. [Google Scholar]
- Baltenberger, E.P.; Buterbaugh, W.M.; Martin, B.S.; Thomas, C.J. Review of antidepressants in the treatment of neuropathic pain. Ment. Health Clin. 2015, 5, 123–133. [Google Scholar] [CrossRef]
- Keers, R.; Aitchison, K.J. Gender differences in antidepressant drug response. Int. Rev. Psychiatry 2010, 22, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Cochran, S.V.; Rabinowitz, F.E. Gender-sensitive recommendations for assessment and treatment of depression in men. Prof. Psychol. Res. Pract. 2003, 34, 132–140. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.U.; Kendler, K.S. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States: Results From the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Bendelow, G. Chronic pain patients and the biomedical model of pain. Virtual Mentor 2013, 15, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Humo, M.; Lu, H.; Yalcin, I. The molecular neurobiology of chronic pain–induced depression. Cell Tissue Res. 2019, 377, 21–43. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Liu, Y. Depression as a mediator of quality of life in patients with neuropathic pain: A cross-sectional study. J. Adv. Nurs. 2019, 75, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Tarride, J.E.; Moulin, D.E.; Lynch, M.; Clark, A.J.; Stitt, L.; Gordon, A.; Morley-Forster, P.K.; Nathan, H.; Smyth, C.; Toth, C.; et al. Impact on health-related quality of life and costs of managing chronic neuropathic pain in academic pain centres: Results from a one-year prospective observational Canadian study. Pain Res. Manag. 2015, 20, 327–333. [Google Scholar] [CrossRef]
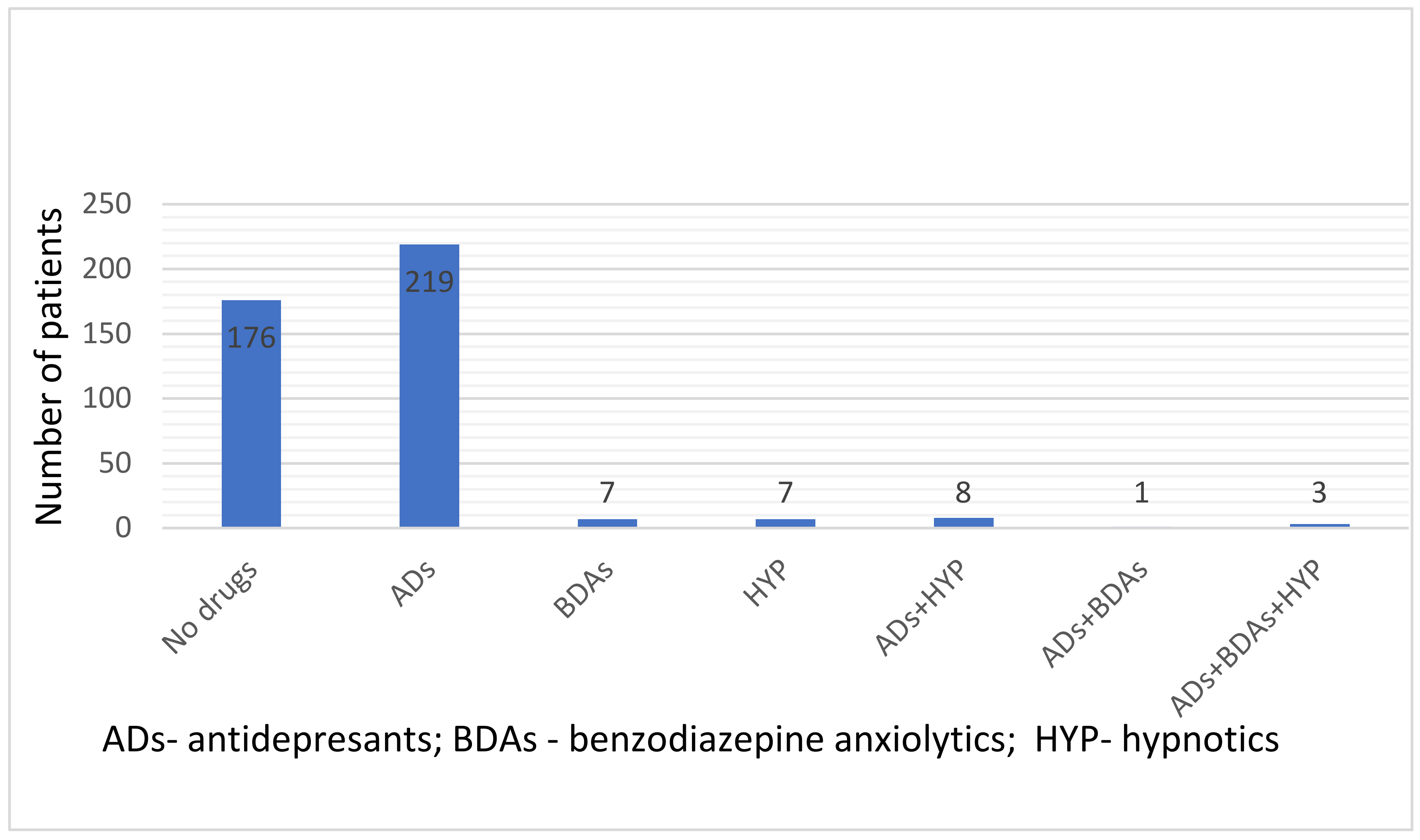

| ND | ADs | BDAs | HYP | Total (n) | |
|---|---|---|---|---|---|
| Male | 67 (43.7%) | 76 (49.67%) | 7 (4.58%) | 7 (4.58%) | 153 (100%) |
| Female | 109 (40.67%) | 155 (57.84%) | 4 (1.49%) | 11 (4.10%) | 268 (100%) |
| p | p = 0.79 | p = 0.11 | p = 0.06 | p = 0.82 | 421 |
| Median M (n = 153) | Median F (n = 268) | U | Z | p | |
|---|---|---|---|---|---|
| The intensity of anxiety on HADS-M * | 7 | 9 | 15,943.00 | −3.80 | 0.00 |
| The intensity of depression on HADS-M * | 6 | 7 | 18,078.50 | −2.02 | 0.04 |
| The intensity of aggression on HADS-M * | 3 | 3 | 20,104.00 | 0.33 | 0.74 |
| The Intensity of Anxiety (HADS—M) * | Without ADs N (%) | ADs N (%) | Total | p |
|---|---|---|---|---|
| 0–7 points | 82 (43.16%) | 97 (41.99%) | 179 | p = 0.04 |
| 8–10 points | 58 (30.53%) | 50 (21.65%) | 108 | |
| >10 points | 50 (26.32%) | 84 (36.36%) | 134 | |
| Total | 190 | 231 | 421 |
| The Intensity of Anxiety (HADS—M) * | No hypnotic N (%) | Hypnotic N (%) | Total | p |
|---|---|---|---|---|
| 0–7 points | 176 (43.67%) | 3 (16.67%) | 179 | p = 0.01 |
| 8–10 points | 105 (26.05%) | 3 (16.67%) | 108 | |
| >10 points | 122 (30.27%) | 12 (66.67%) | 134 | |
| Total | 403 | 18 | 421 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolacz, M.; Kosson, D.; Puchalska-Kowalczyk, E.; Mikaszewska-Sokolewicz, M.; Lisowska, B.; Malec-Milewska, M. Analysis of Antidepressant, Benzodiazepine Anxiolytic, and Hypnotic Use When Treating Depression, Anxiety, and Aggression in Pain Clinic Patients Treated for Neuropathic Pain. Life 2022, 12, 433. https://doi.org/10.3390/life12030433
Kolacz M, Kosson D, Puchalska-Kowalczyk E, Mikaszewska-Sokolewicz M, Lisowska B, Malec-Milewska M. Analysis of Antidepressant, Benzodiazepine Anxiolytic, and Hypnotic Use When Treating Depression, Anxiety, and Aggression in Pain Clinic Patients Treated for Neuropathic Pain. Life. 2022; 12(3):433. https://doi.org/10.3390/life12030433
Chicago/Turabian StyleKolacz, Marcin, Dariusz Kosson, Ewa Puchalska-Kowalczyk, Malgorzata Mikaszewska-Sokolewicz, Barbara Lisowska, and Malgorzata Malec-Milewska. 2022. "Analysis of Antidepressant, Benzodiazepine Anxiolytic, and Hypnotic Use When Treating Depression, Anxiety, and Aggression in Pain Clinic Patients Treated for Neuropathic Pain" Life 12, no. 3: 433. https://doi.org/10.3390/life12030433
APA StyleKolacz, M., Kosson, D., Puchalska-Kowalczyk, E., Mikaszewska-Sokolewicz, M., Lisowska, B., & Malec-Milewska, M. (2022). Analysis of Antidepressant, Benzodiazepine Anxiolytic, and Hypnotic Use When Treating Depression, Anxiety, and Aggression in Pain Clinic Patients Treated for Neuropathic Pain. Life, 12(3), 433. https://doi.org/10.3390/life12030433







