Changes in Envelope Structure and Cell–Cell Communication during Akinete Differentiation and Germination in Filamentous Cyanobacterium Trichormus variabilis ATCC 29413
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Akinete Induction and Germination
2.3. Osmotic-Stress Resistance
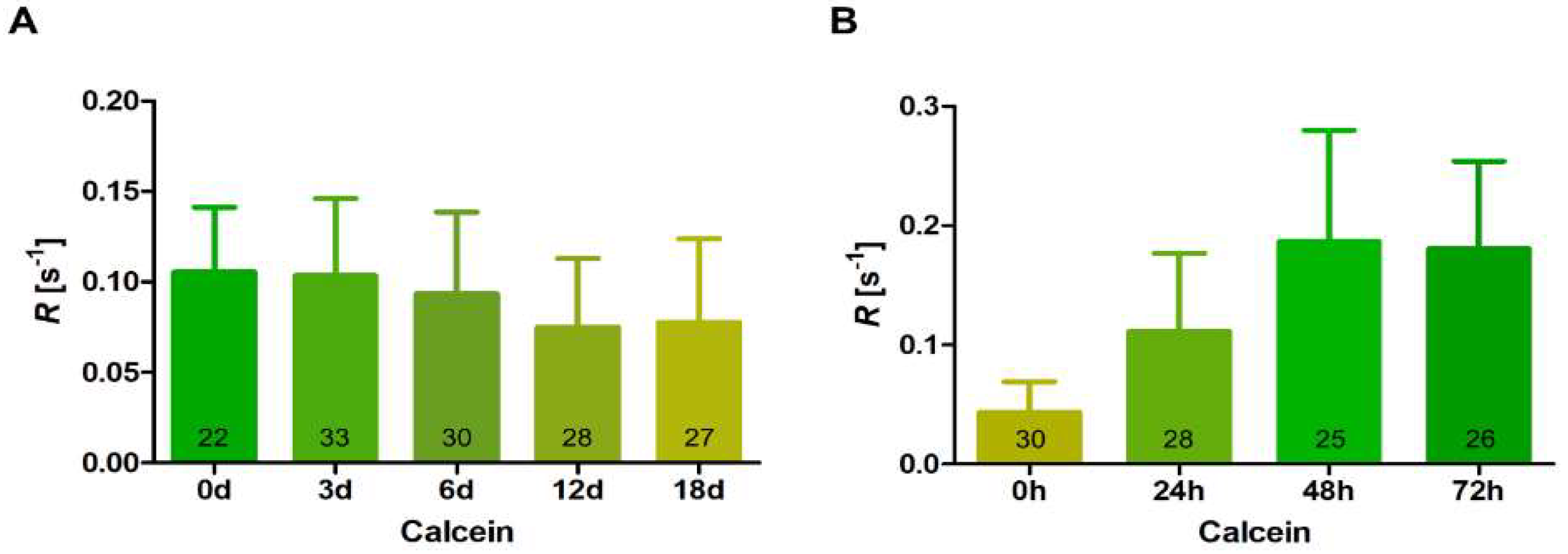
2.4. Fluorescent Recovery after Photobleaching (FRAP) Assay
2.5. EM Preparation and FIB/SEM Imaging
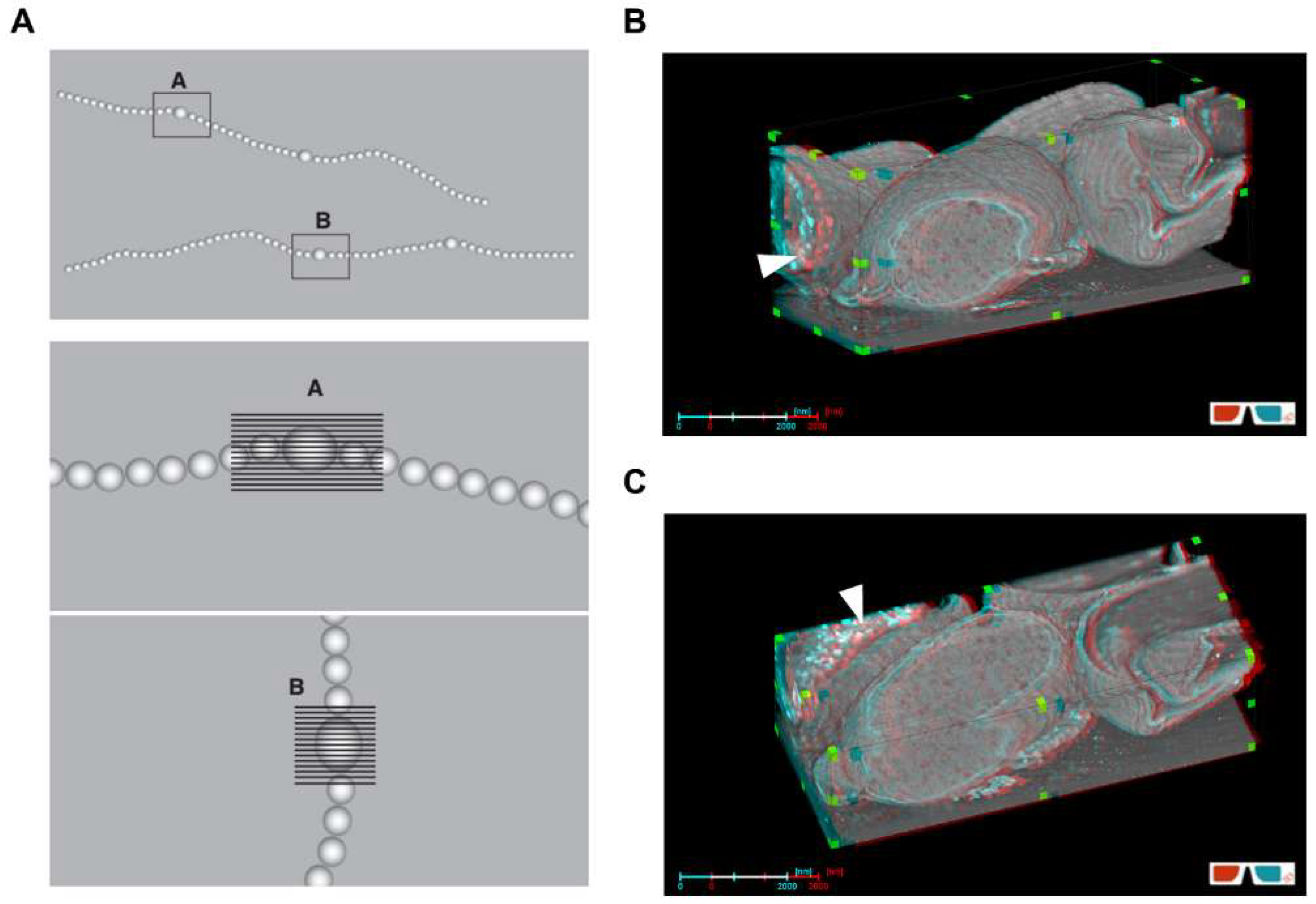
2.6. Three-Dimensional Reconstruction and Visualization
3. Results
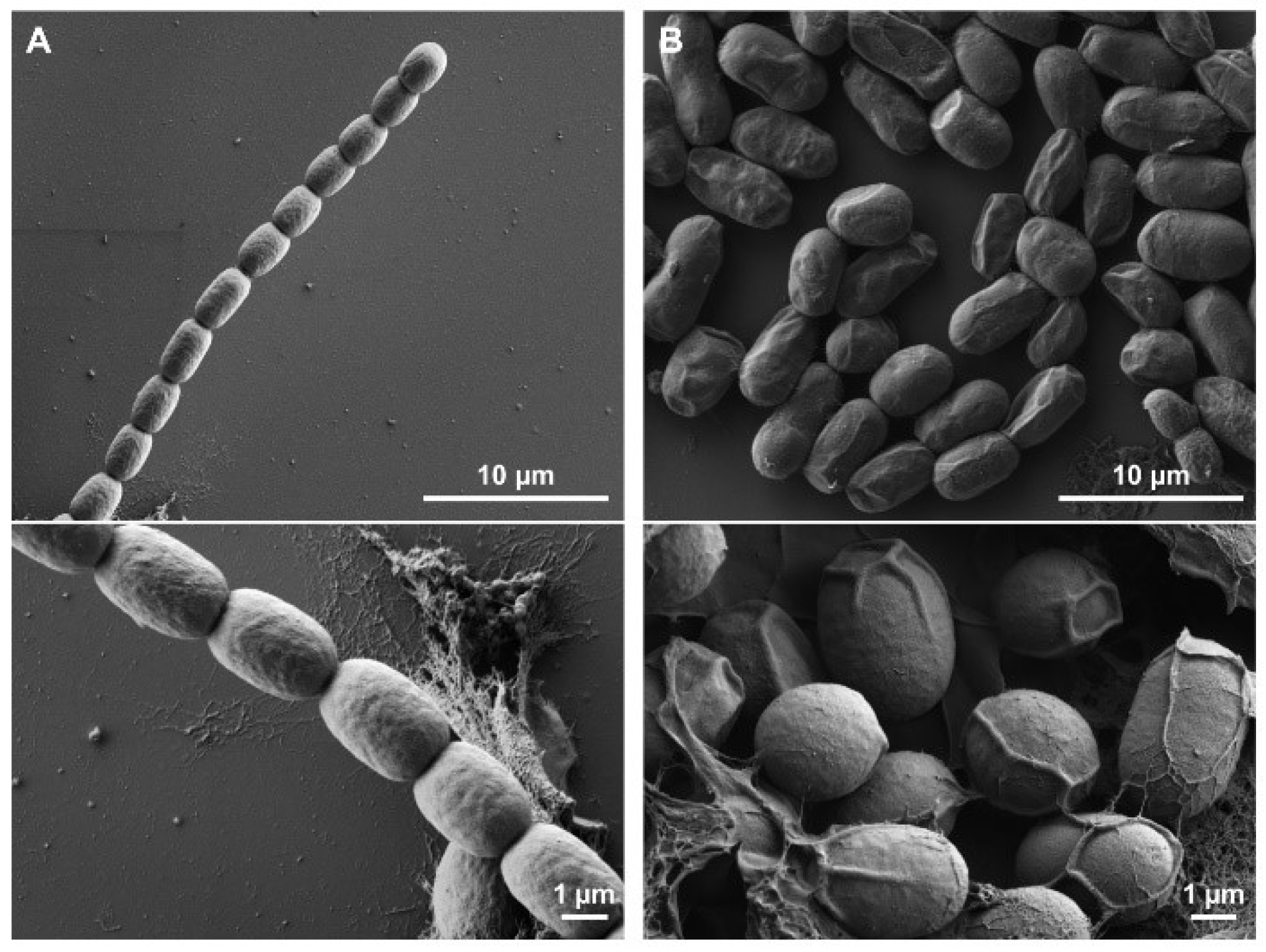
3.1. Morphological Changes Associated with Akinete Formation
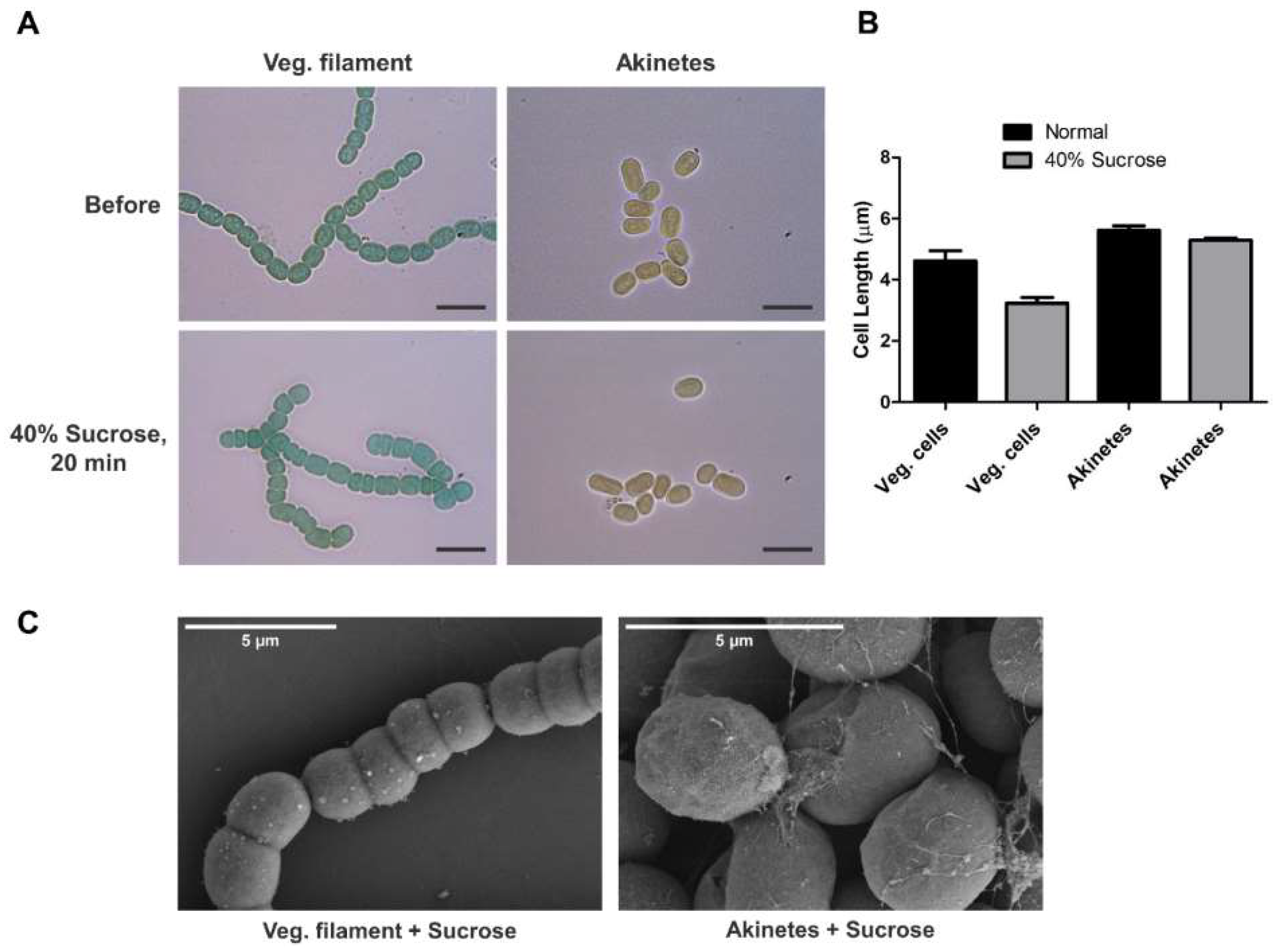
3.2. Tolerance of Akinetes against Sucrose Treatment
3.3. Analysis of Akinete Germination Process by SEM
3.4. Intercellular Communication during Differentiation and Germination of Akinetes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maldener, I.; Summers, M.L.; Sukenik, A. Cellular differentiation in filamentous cyanobacteria. In The Cell Biology of Cyanobacteria; Flores, E., Herrero, A., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 263–291. ISBN 978-1-908230-38-6. [Google Scholar]
- Muro-Pastor, A.M.; Maldener, I. Cyanobacterial heterocysts. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Sukenik, A.; Kaplan-Levy, R.N.; Viner-Mozzini, Y.; Quesada, A.; Hadas, O. Potassium deficiency triggers the development of dormant cells (akinetes) in Aphanizomenon ovalisporum (Nostocales, Cyanoprokaryota). J. Phycol. 2013, 49, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.L.; Rücker, J.; Sukenik, A. Akinetes: Dormant Cells of Cyanobacteria. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 5–27. [Google Scholar]
- Sukenik, A.; Rücker, J.; Maldener, I. Dormant cells (akinetes) of filamentous cyanobacteria demonstrate a great variability in morphology, physiology, and ecological function. In Cyanobacteria: From Basic Science to Applications; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–77. ISBN 9780128146682. [Google Scholar]
- Garg, R.; Maldener, I. The formation of spore-like akinetes: A survival strategy of filamentous cyanobacteria. Microb. Physiol. 2021, 31, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Fay, P. Viability of akinetes of the planktonic cyanobacterium Anabaena circinalis. Proc. R. Soc. London Ser. B. Biol. Sci. 1988, 234, 283–301. [Google Scholar]
- Adams, D.G.; Duggan, P.S. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 1999, 144, 3–33. [Google Scholar] [CrossRef]
- Perez, R.; Forchhammer, K.; Salerno, G.; Maldener, I. Clear differences in metabolic and morphological adaptations of akinetes of two Nostocales living in different habitats. Microbiol. 2016, 162, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Cardemil, L.; Wolk, C.P. Polysaccharides from the envelopes of heterocysts and spores of the blue-green algae Anabaena variabilis and Cylindrospermum licheniforme. J. Phycol. 1981, 17, 234–240. [Google Scholar] [CrossRef]
- Nichols, J.M.; Adams, D.G. Akinetes. In The Biology of Cyanobacteria; Carr, N.G., Whitton, B.A., Eds.; Blackwell: Oxford, UK, 1982; pp. 387–412. [Google Scholar]
- Sutherland, J.M.; Stewart, W.D.P.; Herdman, M. Akinetes of the cyanobacterium Nostoc PCC 7524: Morphological changes during synchronous germination. Arch. Microbiol. 1985, 142, 269–274. [Google Scholar] [CrossRef]
- Sarma, T.A.; Ahuja, G.; Khattar, J.I.S. Nutrient stress causes akinete differentiation in cyanobacterium Anabaena torulosa with concomitant increase in nitrogen reserve substances. Folia Microbiol. 2004, 49, 557–561. [Google Scholar] [CrossRef]
- Simon, R.D. Inclusion bodies in the cyanobacteria: Cyanophycin, polyphosphate, polyhedral bodies. Cyanobacteria 1987, 199–225. [Google Scholar]
- Thiel, T.; Wolk, C.P. Metabolic activities of isolated akinetes of the cyanobacterium Nostoc spongiaeforme. J. Bacteriol. 1983, 156, 369–374. [Google Scholar] [CrossRef]
- Sutherland, J.M.; Herdman, M.; Stewart, W.D.P. Akinetes of the cyanobacterium Nostoc PCC 7524: Macromolecular composition, structure and control of differentiation. J. Gen. Microbiol. 1979, 115, 273–287. [Google Scholar] [CrossRef]
- Yamamoto, Y. Effect of some physical and chemical factors on the germination of akinetes of Anabaena cylindrica. J. Gen. Appl. Microbiol. 1976, 22, 311–323. [Google Scholar] [CrossRef]
- Rai, A.K.; Pandey, G.P. Influence of environmental stress on the germination of Anabaena vaginicola akinetes. Ann. Bot. 1981, 48, 361–370. [Google Scholar] [CrossRef]
- Huber, A.L. Factors affecting the germination of akinetes of Nodularia spumigena (Cyanobacteriaceae). Appl. Environ. Microbiol. 1985, 49, 73–78. [Google Scholar] [CrossRef]
- Van Dok, W.; Hart, B.T. Akinete germination in Anabaena circinalis (Cyanophyta). J. Phycol. 1997, 33, 12–17. [Google Scholar]
- Braune, W. Structural aspects of akinete germination in the cyanobacterium Anabaena variabilis. Arch. Microbiol. 1980, 126, 257–261. [Google Scholar] [CrossRef]
- Perez, R.; Wörmer, L.; Sass, P.; Maldener, I. A highly asynchronous developmental program triggered during germination of dormant akinetes of filamentous diazotrophic cyanobacteria. FEMS Microbiol. Ecol. 2018, 94, 1–11. [Google Scholar] [CrossRef]
- Gambacorta, A.; Pagnotta, E.; Romano, I.; Sodano, G.; Trincone, A. Heterocyst glycolipids from nitrogen-fixing cyanobacteria other than Nostocaceae. Phytochemistry 1998, 48, 801–805. [Google Scholar] [CrossRef]
- Wörmer, L.; Cirés, S.; Velázquez, D.; Quesada, A.; Hinrichs, K.U. Cyanobacterial heterocyst glycolipids in cultures and environmental samples: Diversity and biomarker potential. Limnol. Oceanogr. 2012, 57, 1775–1788. [Google Scholar] [CrossRef]
- Bauersachs, T.; Mudimu, O.; Schulz, R.; Schwark, L. Distribution of long chain heterocyst glycolipids in N2-fixing cyanobacteria of the order Stigonematales. Phytochemistry 2014, 98, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Soriente, A.; Gambacorta, A.; Trincone, A.; Sili, C.; Vincenzini, M.; Sodano, G. Heterocyst glycolipids of the cyanobacterium Cyanospira rippkae. Phytochemistry 1993, 33, 393–396. [Google Scholar] [CrossRef]
- Garg, R.; Maldener, I. The dual role of the glycolipid envelope in different cell types of the multicellular cyanobacterium Anabaena variabilis ATCC 29413. Front. Microbiol. 2021, 12, 645028. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y. Effect of desiccation on the germination of akinetes of Anabaena cylindrica. Plant Cell Physiol. 1975, 16, 749–752. [Google Scholar]
- Sili, C.; Ena, A.; Materassi, R.; Vincenzini, M. Germination of desiccated aged akinetes of alkaliphilic cyanobacteria. Arch. Microbiol. 1994, 162, 20–25. [Google Scholar] [CrossRef]
- Hori, K.; Okamoto, J.; Tanji, Y.; Unno, H. Formation, sedimentation and germination properties of Anabaena akinetes. Biochem. Eng. J. 2003, 14, 67–73. [Google Scholar] [CrossRef]
- Kaplan, F.; Lewis, L.A.; Herburger, K.; Holzinger, A. Osmotic stress in Arctic and Antarctic strains of the green alga Zygnema (Zygnematales, Streptophyta): Effects on photosynthesis and ultrastructure. Micron 2013, 44, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Pichrtová, M.; Kulichová, J.; Holzinger, A. Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae, Streptophyta) from polar habitats. PLoS ONE 2014, 9, e113137. [Google Scholar] [CrossRef] [PubMed]
- Trumhová, K.; Holzinger, A.; Obwegeser, S.; Neuner, G.; Pichrtová, M. The conjugating green alga Zygnema sp. (Zygnematophyceae) from the arctic shows high frost tolerance in mature cells (pre-akinetes). Protoplasma 2019, 256, 1681–1694. [Google Scholar] [CrossRef]
- Livingstone, D.; Jaworski, G.H.M. The viability of akinetes of blue-green algae recovered from the sediments of rostherne mere. Br. Phycol. J. 1980, 15, 357–364. [Google Scholar] [CrossRef]
- Kimura, S.; Tomita-Yokotani, K.; Igarashi, Y.; Sato, S.; Katoh, H.; Abe, T.; Sonoike, K.; Ohmori, M. The Heat tolerance of dry colonies of a terrestrial cyanobacterium, Nostoc sp. HK-01. Biol. Sci. Sp. 2015, 29, 12–18. [Google Scholar] [CrossRef][Green Version]
- Kimura, S.; Ong, M.; Ichikawa, S.; Tomita-Yokotani, K. Compatible solutes in the akinetes of the terrestrial cyanobacterium Nostoc sp. HK-01 contribute to its heat tolerance. Am. J. Plant Sci. 2017, 08, 2695–2711. [Google Scholar] [CrossRef][Green Version]
- Mullineaux, C.W.; Mariscal, V.; Nenninger, A.; Khanum, H.; Herrero, A.; Flores, E.; Adams, D.G. Mechanism of intercellular molecular exchange in heterocyst-forming cyanobacteria. EMBO J. 2008, 27, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Morión, M.; Mullineaux, C.W.; Flores, E. Molecular diffusion through cyanobacterial septal junctions. MBio 2017, 8, e01756-16. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, A.K.; Maldener, I. Cell–cell communication through septal junctions in filamentous cyanobacteria. Curr. Opin. Microbiol. 2021, 61, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.L.; Kieninger, A.K.; Maldener, I.; Forchhammer, K.; Pilhofer, M. Structure and function of a bacterial gap junction analog. Cell 2019, 178, 374–384.e15. [Google Scholar] [CrossRef] [PubMed]
- Lehner, J.; Berendt, S.; Dörsam, B.; Pérez, R.; Forchhammer, K.; Maldener, I. Prokaryotic multicellularity: A nanopore array for bacterial cell communication. FASEB J. 2013, 27, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Nürnberg, D.J.; Mariscal, V.; Bornikoel, J.; Nieves-Morión, M.; Krauß, N.; Herrero, A.; Maldener, I.; Flores, E.; Mullineaux, C.W. Intercellular diffusion of a fluorescent sucrose analog via the septal junctions in a filamentous cyanobacterium. MBio 2015, 6, e02109-14. [Google Scholar] [CrossRef]
- Kieninger, A.K.; Forchhammer, K.; Maldener, I. A nanopore array in the septal peptidoglycan hosts gated septal junctions for cell-cell communication in multicellular cyanobacteria. Int. J. Med. Microbiol. 2019, 309, 151303. [Google Scholar] [CrossRef]
- Bornikoel, J.; Carrión, A.; Fan, Q.; Flores, E.; Forchhammer, K.; Mariscal, V.; Mullineaux, C.W.; Perez, R.; Silber, N.; Peter Wolk, C.; et al. Role of two cell wall amidases in septal junction and nanopore formation in the multicellular cyanobacterium Anabaena sp. PCC 7120. Front. Cell. Infect. Microbiol. 2017, 7, 386. [Google Scholar] [CrossRef]
- Currier, T.C.; Wolk, C.P. Characteristics of Anabaena variabilis influencing plaque formation by cyanophage N-1. J. Bacteriol. 1979, 139, 88–92. [Google Scholar] [CrossRef]
- Thiel, T.; Pratte, B.S.; Zhong, J.; Goodwin, L.; Copeland, A.; Lucas, S.; Han, C.; Pitluck, S.; Land, M.L.; Kyrpides, N.C.; et al. Complete genome sequence of Anabaena variabilis ATCC 29413. Stand. Genom. Sci. 2014, 9, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Merino-Puerto, V.; Schwarz, H.; Maldener, I.; Mariscal, V.; Mullineaux, C.W.; Herrero, A.; Flores, E. FraC/FraD-dependent intercellular molecular exchange in the filaments of a heterocyst-forming cyanobacterium, Anabaena sp. Mol. Microbiol. 2011, 82, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Luckner, M.; Wanner, G. Precise and economic FIB/SEM for CLEM: With 2 nm voxels through mitosis. Histochem. Cell Biol. 2018, 150, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Wanner, G.; Schäfer, T.; Lütz-Meindl, U. 3-D analysis of dictyosomes and multivesicular bodies in the green alga Micrasterias denticulata by FIB/SEM tomography. J. Struct. Biol. 2013, 184, 203–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brunt, J.; Cross, K.L.; Peck, M.W. Apertures in the Clostridium sporogenes spore coat and exosporium align to facilitate emergence of the vegetative cell. Food Microbiol. 2015, 51, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, S.; Flores, E. Pentapeptide-repeat, cytoplasmic-membrane protein HglK influences the septal junctions in the heterocystous cyanobacterium Anabaena. Mol. Microbiol. 2020, 113, 794–806. [Google Scholar] [CrossRef]
- Argueta, C.; Summers, M.L. Characterization of a model system for the study of Nostoc punctiforme akinetes. Arch. Microbiol. 2005, 183, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.N.; Morton, R.; Kulkarni, G.; Summers, M.L.; Newman, D.K. Hopanoids play a role in stress tolerance and nutrient storage in the cyanobacterium Nostoc punctiforme. Geobiology 2017, 15, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Leggett, M.J.; Mcdonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef]
- Li, Y.; Butzin, X.Y.; Davis, A.; Setlow, B.; Korza, G.; Üstok, F.I.; Christie, G.; Setlow, P.; Hao, B. Activity and regulation of various forms of cwlJ, SleB, and YpeB proteins in degrading cortex peptidoglycan of spores of Bacillus species in vitro and during spore germination. J. Bacteriol. 2013, 195, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Yamane, K.; Sekiguchi, J. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 1998, 180, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, R.; Hattori, A.; Miyata, S.; Kudoh, S.; Makino, S. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to L-alanine-mediated germination. J. Bacteriol. 1996, 178, 6059–6063. [Google Scholar] [CrossRef][Green Version]
- Nürnberg, D.J.; Mariscal, V.; Parker, J.; Mastroianni, G.; Flores, E.; Mullineaux, C.W. Branching and intercellular communication in the Section V cyanobacterium Mastigocladus laminosus, a complex multicellular prokaryote. Mol. Microbiol. 2014, 91, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Mullineaux, C.W. Motility in cyanobacteria: Polysaccharide tracks and Type IV pilus motors. Mol. Microbiol. 2015, 98, 998–1001. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, R.; Luckner, M.; Berger, J.; Hipp, K.; Wanner, G.; Forchhammer, K.; Maldener, I. Changes in Envelope Structure and Cell–Cell Communication during Akinete Differentiation and Germination in Filamentous Cyanobacterium Trichormus variabilis ATCC 29413. Life 2022, 12, 429. https://doi.org/10.3390/life12030429
Garg R, Luckner M, Berger J, Hipp K, Wanner G, Forchhammer K, Maldener I. Changes in Envelope Structure and Cell–Cell Communication during Akinete Differentiation and Germination in Filamentous Cyanobacterium Trichormus variabilis ATCC 29413. Life. 2022; 12(3):429. https://doi.org/10.3390/life12030429
Chicago/Turabian StyleGarg, Ritu, Manja Luckner, Jürgen Berger, Katharina Hipp, Gerhard Wanner, Karl Forchhammer, and Iris Maldener. 2022. "Changes in Envelope Structure and Cell–Cell Communication during Akinete Differentiation and Germination in Filamentous Cyanobacterium Trichormus variabilis ATCC 29413" Life 12, no. 3: 429. https://doi.org/10.3390/life12030429
APA StyleGarg, R., Luckner, M., Berger, J., Hipp, K., Wanner, G., Forchhammer, K., & Maldener, I. (2022). Changes in Envelope Structure and Cell–Cell Communication during Akinete Differentiation and Germination in Filamentous Cyanobacterium Trichormus variabilis ATCC 29413. Life, 12(3), 429. https://doi.org/10.3390/life12030429






