2-Fluorofucose Attenuates Hydrogen Peroxide-Induced Oxidative Stress in HepG2 Cells via Nrf2/keap1 and NF-κB Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture and Treatments
2.3. siRNA Transfection
2.4. Cell Viability Assay
2.5. Fluorescence Probe-Dihydroethidium (DHE) Staining
2.6. Lactate Dehydrogenase Assay
2.7. Measurement of MDA Levels
2.8. Enzyme Activity Assay
2.9. Mitochondrial Membrane Potential (MMP) Assay
2.10. Immunofluorescence
2.11. Western Blot and Lectin Blot Assay
2.12. Statistical Analysis
3. Results
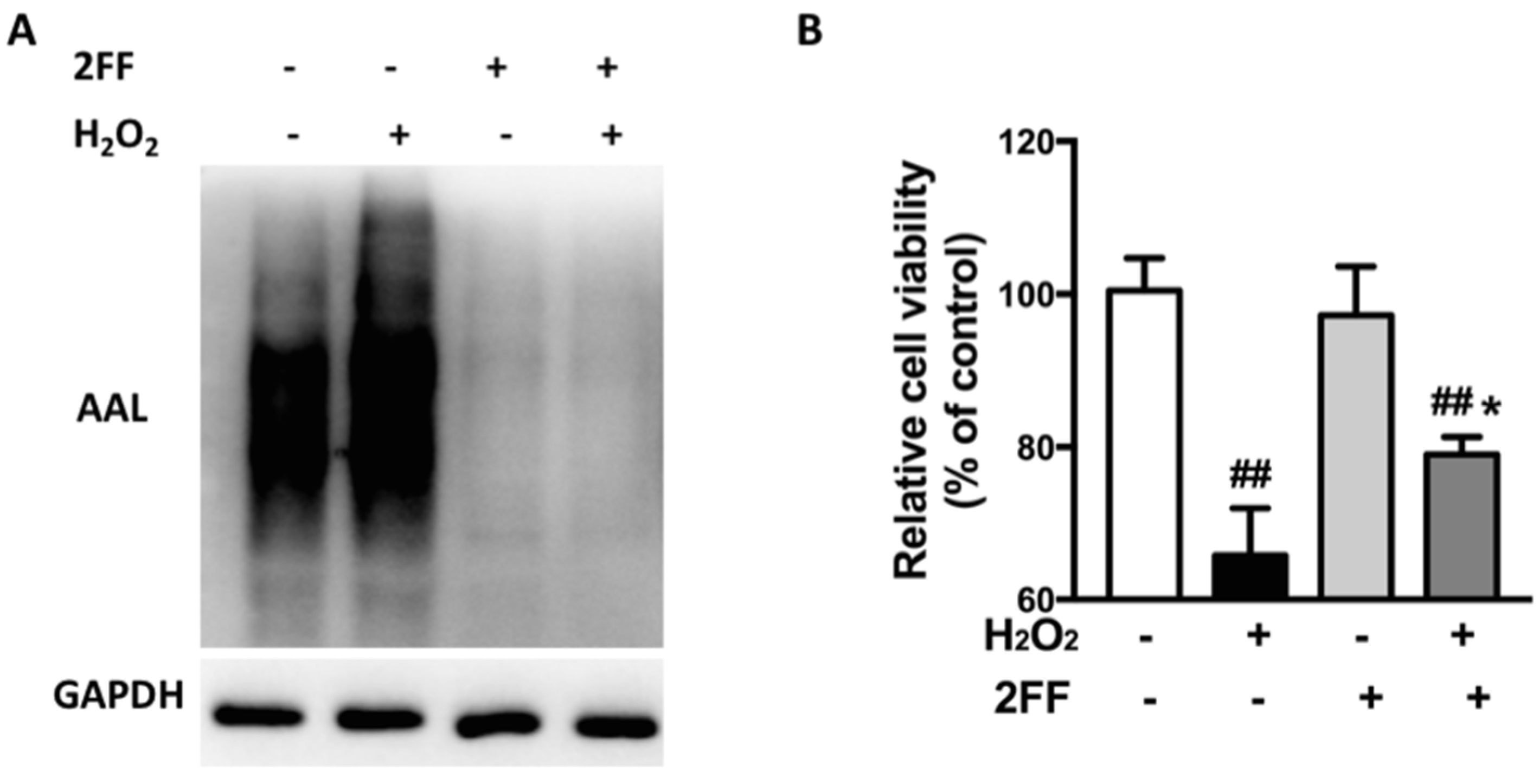
3.1. 2FF Improved Cell Viability in H2O2-Treated HepG2 Cells
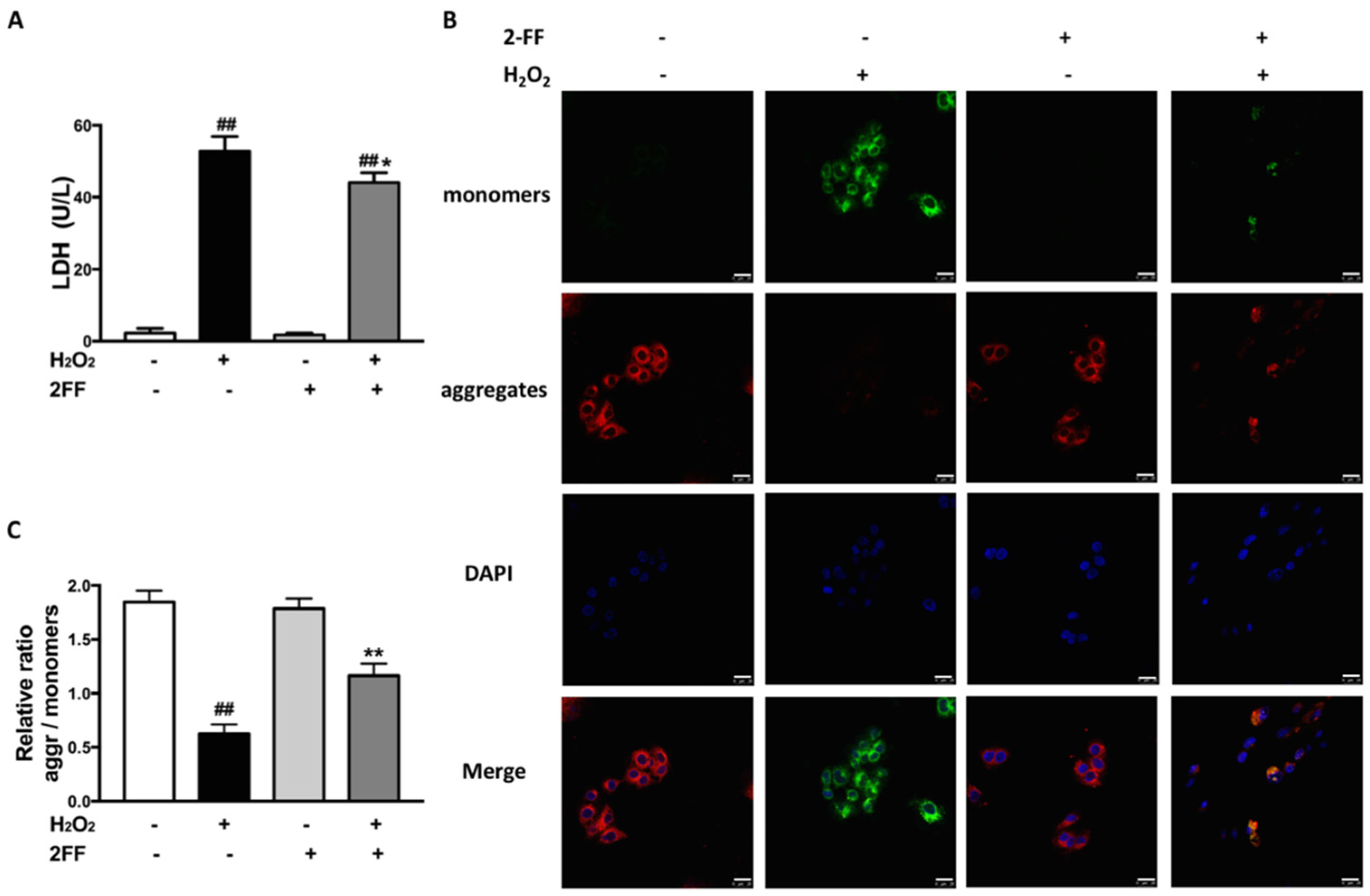
3.2. 2FF Alleviated Cell Damage and Loss of MMP in H2O2-Treated HepG2 Cells
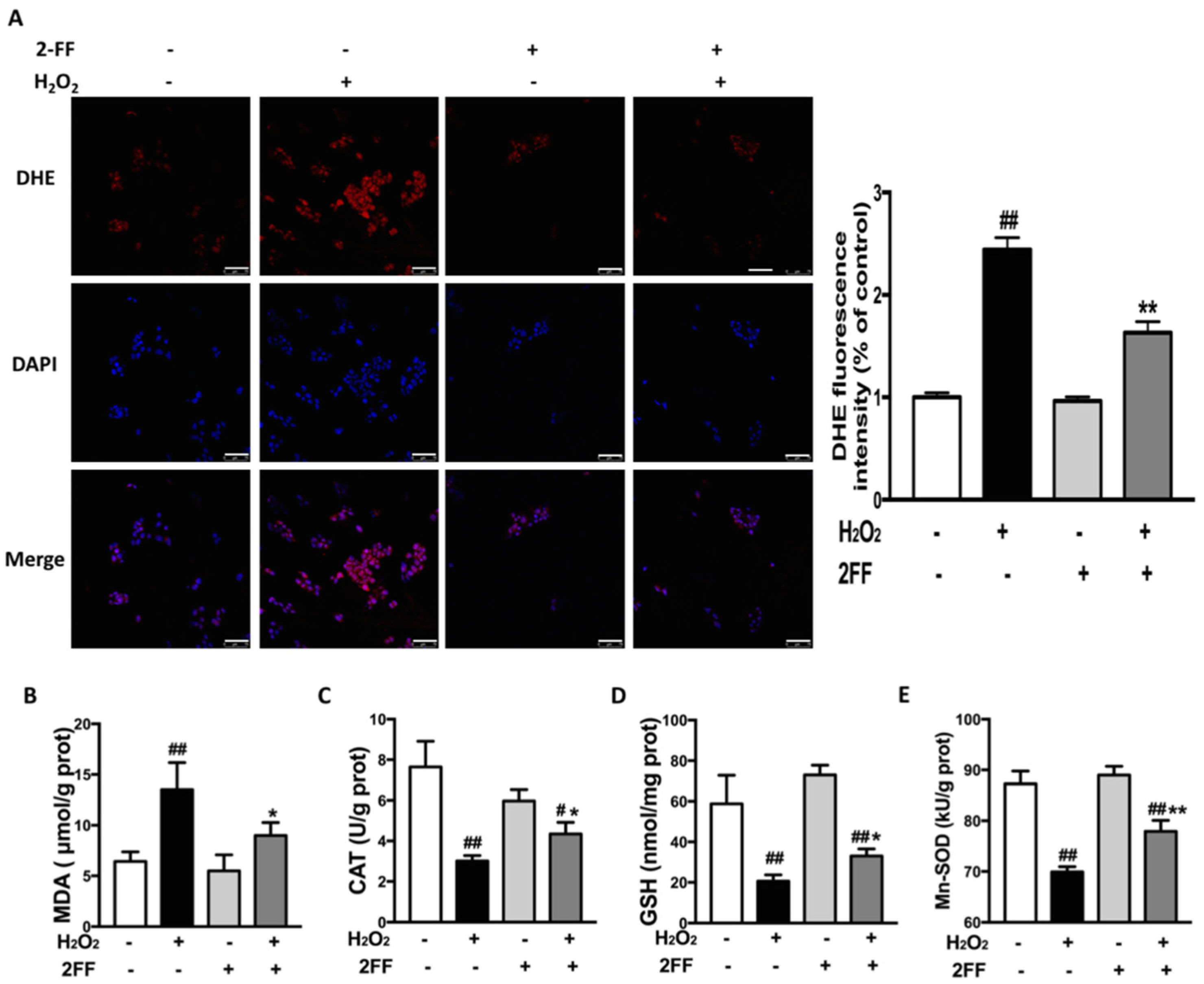
3.3. 2FF Alleviated H2O2-Induced ROS Accumulation in HepG2 Cells
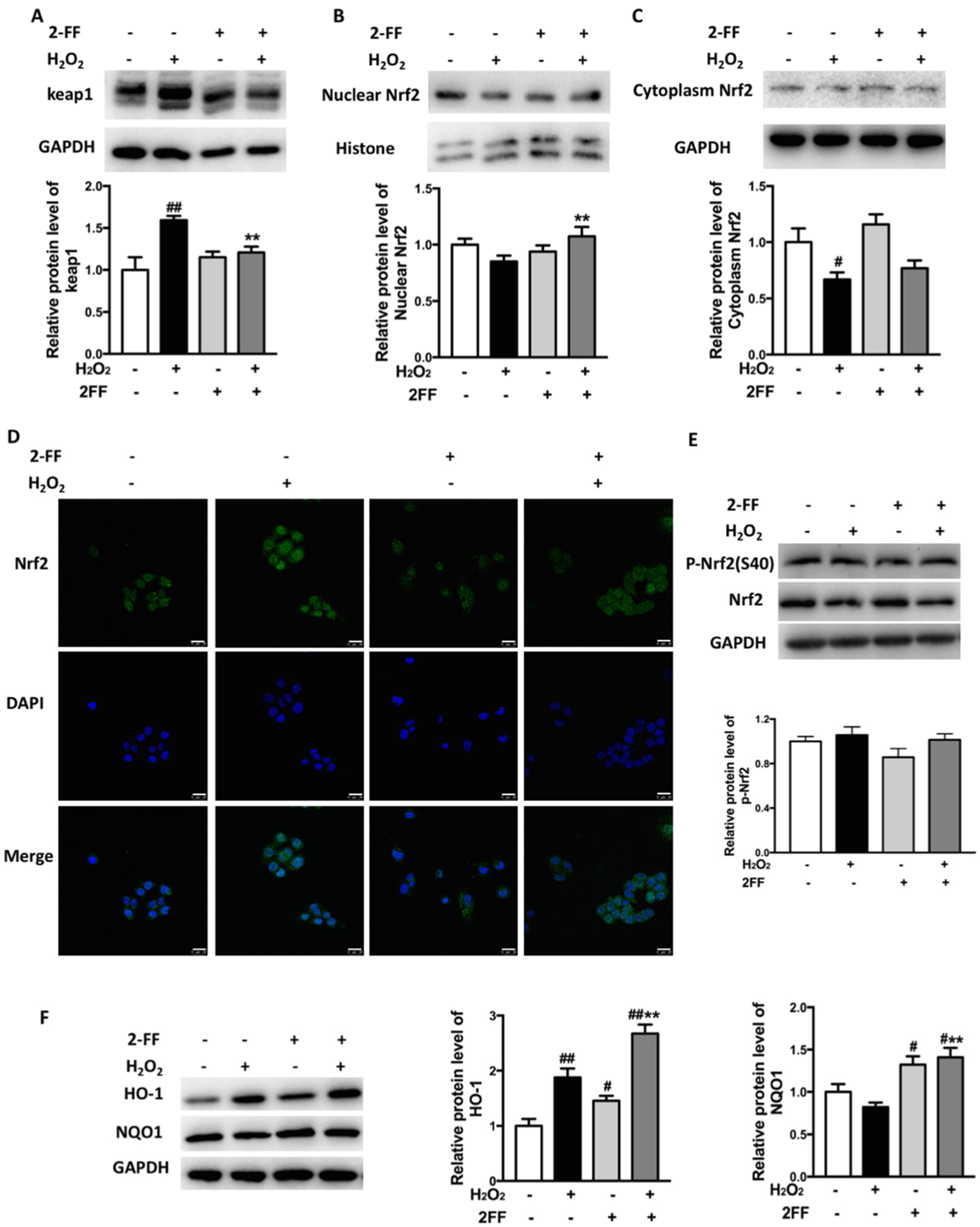
3.4. Inhibition of Fucosylation Influenced Nrf2/keap1 Signaling Pathway in H2O2-Treated HepG2 Cells
3.5. Knockdown of Nrf2 Eliminated the Protective Effect of 2FF against H2O2-Induced Oxidative Injury in HepG2 Cells
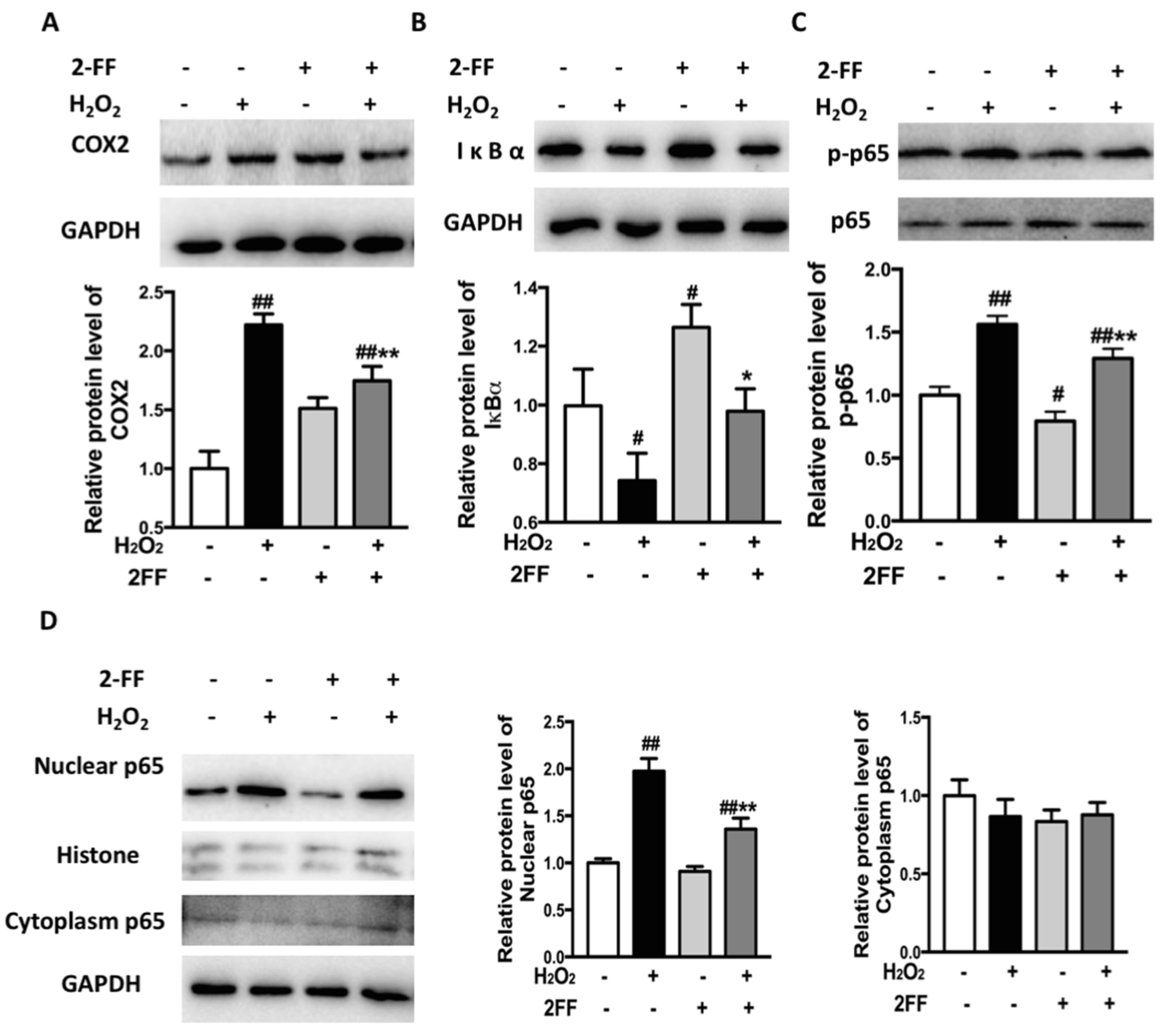
3.6. Inhibition of Fucosylation Affected the Expression of Inflammation-Associated Proteins in H2O2-Treated HepG2 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, T.F.N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Farid, O.A.; Rizk, H.A.; Shehata, A.M. Hydrogen peroxide modulates redox status, energy metabolism, and gene expression in a dose- and time-dependent manner in rat liver. J. Biochem. Mol. Toxicol. 2018, 32, e22199. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: Evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Haltiwanger, R.S.; Lowe, J.B. Role of glycosylation in development. Annu. Rev. Biochem. 2004, 73, 491–537. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Ferrara, C.; Grau, S.; Jager, C.; Sondermann, P.; Brunker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Okeley, N.M.; Alley, S.C.; Anderson, M.E.; Boursalian, T.E.; Burke, P.J.; Emmerton, K.M.; Jeffrey, S.C.; Klussman, K.; Law, C.-L.; Sussman, D.; et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA 2013, 110, 5404–5409. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.A.; Sliva, M.; Ramalho, J.S.; Pen, C.; Martins, M.; Pascoal, C.; Amaral, C.; Serrano, I.; Oliveira, M.J.; Sackstein, R.; et al. Inhibiton of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation. Mol. Oncol. 2018, 12, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fukuda, T.; Hang, Q.; Hou, S.; Isaji, T.; Kameyama, A.; Gu, J. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci. Rep. 2017, 7, 11563. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Abdulla, F.; Nguyen, P.; Nguyen, M.; Okeley, N.M.; Benjamin, D.R.; Senter, P.S.; Vercellotti, G.M. The fucosylation inhibitor, 2-fluorofucose, inhibits vaso-occlusion, leukocyte-endothelium interactions and NF-kB activation in transgenic sickle mice. PLoS ONE 2015, 10, e0117772. [Google Scholar] [CrossRef][Green Version]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Luo, H.; Xu, M.; Meng, G.; Zhang, W. Ca(2+)/calmodulin-dependent protein kinase II regulation by inhibitor 1 of protein phosphatase 1 alleviates necroptosis in high glucose-induced cardiomyocytes injury. Biochem. Pharmacol. 2019, 163, 194–205. [Google Scholar] [CrossRef]
- Song, S.; Ding, Y.; Dai, G.-L.; Zhang, Y.; Xu, M.-T.; Shen, J.-R.; Chen, T.-T.; Chen, Y.; Meng, G.-L. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 2021, 42, 230–241. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Li, X.M.; Huang, D.; Yu, Q.; Yang, J.; Yao, J. Neuroligin-3 protects retinal cells from H2O2-induced cell death via activation of Nrf2 signaling. Biochem. Biophys. Res. Commun. 2018, 502, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, C.F.; Ping, N.N.; Sun, Y.Y.; Wang, Z.; Zhao, G.X.; Yuan, S.H.; Zibrila, A.I.; Soong, L.; Liu, J.J. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22467. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chang, S.F.; Chau, S.F.; Chiu, S.C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Ananta, P.; Britta, E.V.; Rainer, B.; Stephan, I. Signaling to heme oxygenase-1 and anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar]
- Derek, L.; Iris, M.J.X.; David, K.C.C.; Josef, L.; Aki, P.W.T.; Macus, H.R.B.; Yuen, V.W.H.; Chan, C.Y.K.; Lai, R.K.H.; Chin, D.W.C.; et al. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology 2019, 69, 1768–1786. [Google Scholar]
- Perillo, B.; di Donato, M.; Pezone, A.; di Zazzo, E.; Giovanelli, P.; Galasso, G.; Castoria, C.; Migliacio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Coriat, R.; Nicco, C.; Chereau, C.; Mir, O.; Alexandre, J.; Ropert, S.; Weill, B.; Chaussade, S.; Goldwasser, F.; Batteux, F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 2284–2293. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, M.; Fan, X.; Shi, J.; Jing, S.; Xu, X.; Wang, Y. 2-Fluorofucose Attenuates Hydrogen Peroxide-Induced Oxidative Stress in HepG2 Cells via Nrf2/keap1 and NF-κB Signaling Pathways. Life 2022, 12, 406. https://doi.org/10.3390/life12030406
Tu M, Fan X, Shi J, Jing S, Xu X, Wang Y. 2-Fluorofucose Attenuates Hydrogen Peroxide-Induced Oxidative Stress in HepG2 Cells via Nrf2/keap1 and NF-κB Signaling Pathways. Life. 2022; 12(3):406. https://doi.org/10.3390/life12030406
Chicago/Turabian StyleTu, Mengjue, Xingshuo Fan, Jianan Shi, Shengnan Jing, Xiaole Xu, and Yuqin Wang. 2022. "2-Fluorofucose Attenuates Hydrogen Peroxide-Induced Oxidative Stress in HepG2 Cells via Nrf2/keap1 and NF-κB Signaling Pathways" Life 12, no. 3: 406. https://doi.org/10.3390/life12030406
APA StyleTu, M., Fan, X., Shi, J., Jing, S., Xu, X., & Wang, Y. (2022). 2-Fluorofucose Attenuates Hydrogen Peroxide-Induced Oxidative Stress in HepG2 Cells via Nrf2/keap1 and NF-κB Signaling Pathways. Life, 12(3), 406. https://doi.org/10.3390/life12030406




