Contribution of Hypoxic Exercise Testing to Predict High-Altitude Pathology: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Analysis
3. Results
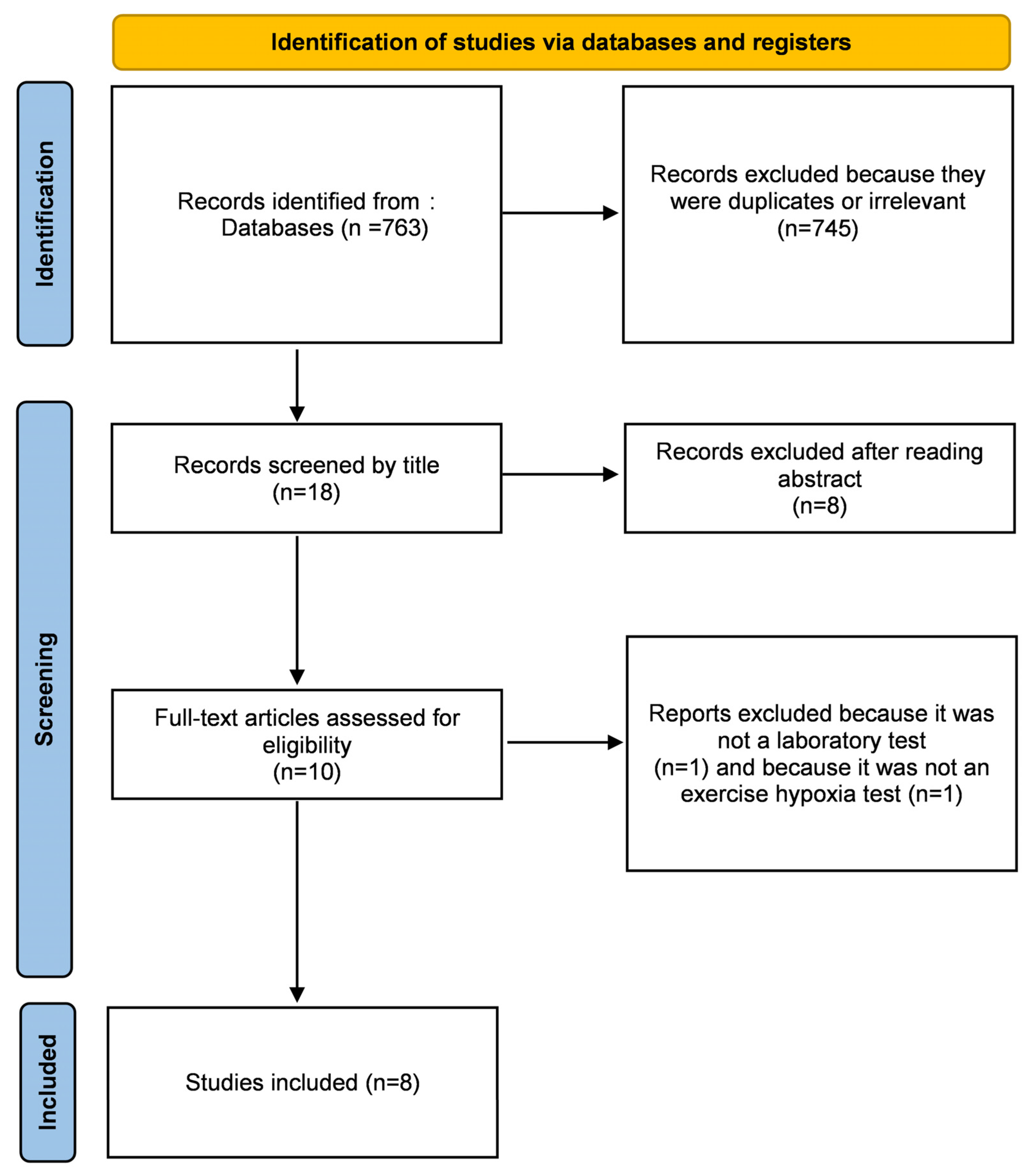
3.1. Study Selection
3.2. Demographic Data
3.3. Diagnosis of HAI
3.4. Methods of Exercise Hypoxia Test
3.5. Contribution of Hypoxic Exercise Test to Predict HAI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Data World Bank International Tourism, Number of Arrivals—Nepal | Data. Available online: https://data.worldbank.org/indicator/ST.INT.ARVL?locations=NP (accessed on 20 January 2022).
- Bärtsch, P.; Swenson, E.R. Acute High-Altitude Illnesses. N. Engl. J. Med. 2013, 369, 1666–1667. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Collet, T.-H.; Locatelli, I.; Cornuz, J.; Kayser, B.; Simel, D.L.; Sartori, C. Does This Patient Have Acute Mountain Sickness?: The Rational Clinical Examination Systematic Review. JAMA 2017, 318, 1810–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richalet, J.P.; Herry, J.P. Médecine de Montagne. Alpinisme et Sports de Montagne; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-2-294-75484-5. [Google Scholar]
- Lawley, J.S.; Levine, B.D.; Williams, M.A.; Malm, J.; Eklund, A.; Polaner, D.M.; Subudhi, A.W.; Hackett, P.H.; Roach, R.C. Cerebral Spinal Fluid Dynamics: Effect of Hypoxia and Implications for High-Altitude Illness. J. Appl. Physiol. 2016, 120, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.H.; Newman, S.; Imray, C.H. The Cerebral Effects of Ascent to High Altitudes. Lancet Neurol. 2009, 8, 175–191. [Google Scholar] [CrossRef]
- Swenson, E.R.; Bärtsch, P. High-Altitude Pulmonary Edema. Compr. Physiol. 2012, 2, 2753–2773. [Google Scholar] [CrossRef]
- Schneider, M.; Bernasch, D.; Weymann, J.; Holle, R.; Bartsch, P. Acute Mountain Sickness: Influence of Susceptibility, Preexposure, and Ascent Rate. Med. Sci. Sports Exerc. 2002, 34, 1886–1891. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R.; Bärtsch, P. Acute High-Altitude Sickness. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2017, 26, 160096. [Google Scholar] [CrossRef]
- Roach, R.C.; Houston, C.S.; Honigman, B.; Nicholas, R.A.; Yaron, M.; Grissom, C.K.; Alexander, J.K.; Hultgren, H.N. How Well Do Older Persons Tolerate Moderate Altitude? West. J. Med. 1995, 162, 32–36. [Google Scholar]
- Karinen, H.M.; Peltonen, J.E.; Kähönen, M.; Tikkanen, H.O. Prediction of Acute Mountain Sickness by Monitoring Arterial Oxygen Saturation during Ascent. High Alt. Med. Biol. 2010, 11, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.R.; Knott, J.R.; Fry, J.P. Oximetry Fails to Predict Acute Mountain Sickness or Summit Success during a Rapid Ascent to 5640 Meters. Wilderness Environ. Med. 2012, 23, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Karinen, H.M.; Uusitalo, A.; Vähä-Ypyä, H.; Kähönen, M.; Peltonen, J.E.; Stein, P.K.; Viik, J.; Tikkanen, H.O. Heart Rate Variability Changes at 2400 m Altitude Predicts Acute Mountain Sickness on Further Ascent at 3000–4300 m Altitudes. Front. Physiol. 2012, 3, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nespoulet, H.; Wuyam, B.; Tamisier, R.; Saunier, C.; Monneret, D.; Remy, J.; Chabre, O.; Pépin, J.-L.; Lévy, P. Altitude Illness Is Related to Low Hypoxic Chemoresponse and Low Oxygenation during Sleep. Eur. Respir. J. 2012, 40, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milledge, J.S.; Beeley, J.M.; Broome, J.; Luff, N.; Pelling, M.; Smith, D. Acute Mountain Sickness Susceptibility, Fitness and Hypoxic Ventilatory Response. Eur. Respir. J. 1991, 4, 1000–1003. [Google Scholar] [PubMed]
- Hohenhaus, E.; Paul, A.; McCullough, R.E.; Kücherer, H.; Bärtsch, P. Ventilatory and Pulmonary Vascular Response to Hypoxia and Susceptibility to High Altitude Pulmonary Oedema. Eur. Respir. J. 1995, 8, 1825–1833. [Google Scholar] [CrossRef]
- Grünig, E.; Mereles, D.; Hildebrandt, W.; Swenson, E.R.; Kübler, W.; Kuecherer, H.; Bärtsch, P. Stress Doppler Echocardiography for Identification of Susceptibility to High Altitude Pulmonary Edema. J. Am. Coll. Cardiol. 2000, 35, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Dehnert, C.; Grünig, E.; Mereles, D.; von Lennep, N.; Bärtsch, P. Identification of Individuals Susceptible to High-Altitude Pulmonary Oedema at Low Altitude. Eur. Respir. J. 2005, 25, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Holmström, P.; Mulder, E.; Sundström, A.L.; Limbu, P.; Schagatay, E. The Magnitude of Diving Bradycardia During Apnea at Low-Altitude Reveals Tolerance to High Altitude Hypoxia. Front. Physiol. 2019, 10, 1075. [Google Scholar] [CrossRef] [Green Version]
- Roach, R.C.; Maes, D.; Sandoval, D.; Robergs, R.A.; Icenogle, M.; Hinghofer-Szalkay, H.; Lium, D.; Loeppky, J.A. Exercise Exacerbates Acute Mountain Sickness at Simulated High Altitude. J. Appl. Physiol. 2000, 88, 581–585. [Google Scholar] [CrossRef]
- Pfoh, J.R.; Steinback, C.D.; Vanden Berg, E.R.; Bruce, C.D.; Day, T.A. Assessing Chemoreflexes and Oxygenation in the Context of Acute Hypoxia: Implications for Field Studies. Respir. Physiol. Neurobiol. 2017, 246, 67–75. [Google Scholar] [CrossRef]
- Woorons, X.; Mollard, P.; Pichon, A.; Lamberto, C.; Duvallet, A.; Richalet, J.-P. Moderate Exercise in Hypoxia Induces a Greater Arterial Desaturation in Trained than Untrained Men. Scand. J. Med. Sci. Sports 2007, 17, 431–436. [Google Scholar] [CrossRef]
- Mollard, P.; Woorons, X.; Letournel, M.; Lamberto, C.; Favret, F.; Pichon, A.; Beaudry, M.; Richalet, J.-P. Determinants of Maximal Oxygen Uptake in Moderate Acute Hypoxia in Endurance Athletes. Eur. J. Appl. Physiol. 2007, 100, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P.; La Jean, D. Consultation de Médecine de Montagne. In Médecine de Montagne. Alpinisme et Sports de Montagne; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–308. ISBN 978-2-294-75484-5. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. Lond. Engl. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE Working Group GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, A.B.; Levett, D.Z.H.; Mitchell, K.; Aveling, W.; Hurlbut, D.; Gilbert-Kawai, E.; Hennis, P.J.; Mythen, M.G.; Grocott, M.P.W.; Martin, D.S.; et al. Physiological Responses during Ascent to High Altitude and the Incidence of Acute Mountain Sickness. Physiol. Rep. 2021, 9, e14809. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Wells, S.; Furness, S.; Strid, J.; Arroll, B.; Milne, R. Handbook for the Preparation of Explicit Evidence-Based Clinical Practice Guidelines; New Zealand Guidelines Group: Wellington, New Zealand, 2001. [Google Scholar]
- Richalet, J.-P.; Pillard, F.; LE Moal, D.; Rivière, D.; Oriol, P.; Poussel, M.; Chenuel, B.; Doutreleau, S.; Vergès, S.; Demanez, S.; et al. Validation of a Score for the Detection of Subjects with High Risk for Severe High-Altitude Illness. Med. Sci. Sports Exerc. 2021, 53, 1294–1302. [Google Scholar] [CrossRef]
- Kammerer, T.; Faihs, V.; Hulde, N.; Bayer, A.; Hübner, M.; Brettner, F.; Karlen, W.; Kröpfl, J.M.; Rehm, M.; Spengler, C.; et al. Changes of Hemodynamic and Cerebral Oxygenation after Exercise in Normobaric and Hypobaric Hypoxia: Associations with Acute Mountain Sickness. Ann. Occup. Environ. Med. 2018, 30, 66. [Google Scholar] [CrossRef]
- Richardson, A.; Twomey, R.; Watt, P.; Maxwell, N. Physiological Responses to Graded Acute Normobaric Hypoxia Using an Intermittent Walking Protocol. Wilderness Environ. Med. 2008, 19, 252–260. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Larmignat, P.; Poitrine, E.; Letournel, M.; Canouï-Poitrine, F. Physiological Risk Factors for Severe High-Altitude Illness: A Prospective Cohort Study. Am. J. Respir. Crit. Care Med. 2012, 185, 192–198. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Lhuissier, F.; Jean, D. Ventilatory Response to Hypoxia and Tolerance to High Altitude in Women: Influence of Menstrual Cycle, Oral Contraception, and Menopause. High Alt. Med. Biol. 2020, 21, 12–19. [Google Scholar] [CrossRef]
- Coustet, B.; Lhuissier, F.J.; Vincent, R.; Richalet, J.-P. Electrocardiographic Changes during Exercise in Acute Hypoxia and Susceptibility to Severe High-Altitude Illnesses. Circulation 2015, 131, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Canouï-Poitrine, F.; Veerabudun, K.; Larmignat, P.; Letournel, M.; Bastuji-Garin, S.; Richalet, J.-P. Risk Prediction Score for Severe High Altitude Illness: A Cohort Study. PLoS ONE 2014, 9, e100642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackett, P.H.; Rennie, D.; Levine, H.D. The Incidence, Importance, and Prophylaxis of Acute Mountain Sickness. Lancet Lond. Engl. 1976, 2, 1149–1155. [Google Scholar] [CrossRef]
- Maggiorini, M.; Bühler, B.; Walter, M.; Oelz, O. Prevalence of Acute Mountain Sickness in the Swiss Alps. BMJ 1990, 301, 853–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bärtsch, P.; Luks, A.M.; MacInnis, M.J.; Baillie, J.K. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt. Med. Biol. 2018, 19, 4–6. [Google Scholar] [CrossRef]
- Savourey, G.; Guinet, A.; Besnard, Y.; Garcia, N.; Hanniquet, A.M.; Bittel, J. Evaluation of the Lake Louise Acute Mountain Sickness Scoring System in a Hypobaric Chamber. Aviat. Space Environ. Med. 1995, 66, 963–967. [Google Scholar]
- Winkler, L.; Lhuissier, F.J.; Richalet, J.-P. Systemic Blood Pressure at Exercise in Hypoxia in Hypertensive and Normotensive Patients. J. Hypertens. 2017, 35, 2402–2410. [Google Scholar] [CrossRef]
- Richalet, J.; Lhuissier, F.-J.; Larmignat, P.; Canouï-Poitrine, F. -P. Évaluation de La Tolérance à l’hypoxie et Susceptibilité Aux Pathologies de Haute Altitude. Sci. Sports 2015, 30, 355–363. [Google Scholar] [CrossRef]
- Burtscher, M.; Flatz, M.; Faulhaber, M. Prediction of Susceptibility to Acute Mountain Sickness by SaO2 Values during Short-Term Exposure to Hypoxia. High Alt. Med. Biol. 2004, 5, 335–340. [Google Scholar] [CrossRef]
- O’Connor, T.; Dubowitz, G.; Bickler, P.E. Pulse Oximetry in the Diagnosis of Acute Mountain Sickness. High Alt. Med. Biol. 2004, 5, 341–348. [Google Scholar] [CrossRef]
- Behan, M.; Wenninger, J.M. Sex Steroidal Hormones and Respiratory Control. Respir. Physiol. Neurobiol. 2008, 164, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Takano, N. Changes of Ventilation and Ventilatory Response to Hypoxia during the Menstrual Cycle. Pflugers Arch. 1984, 402, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Schoene, R.B.; Robertson, H.T.; Pierson, D.J.; Peterson, A.P. Respiratory Drives and Exercise in Menstrual Cycles of Athletic and Nonathletic Women. J. Appl. Physiol. 1981, 50, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Regensteiner, J.G.; McCullough, R.G.; McCullough, R.E.; Pickett, C.K.; Moore, L.G. Combined Effects of Female Hormones and Exercise on Hypoxic Ventilatory Response. Respir. Physiol. 1990, 82, 107–114. [Google Scholar] [CrossRef]
- Macnutt, M.J.; De Souza, M.J.; Tomczak, S.E.; Homer, J.L.; Sheel, A.W. Resting and Exercise Ventilatory Chemosensitivity across the Menstrual Cycle. J. Appl. Physiol. 2012, 112, 737–747. [Google Scholar] [CrossRef]
- Dombovy, M.L.; Bonekat, H.W.; Williams, T.J.; Staats, B.A. Exercise Performance and Ventilatory Response in the Menstrual Cycle. Med. Sci. Sports Exerc. 1987, 19, 111–117. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Lhuissier, F.J. Aging, Tolerance to High Altitude, and Cardiorespiratory Response to Hypoxia. High Alt. Med. Biol. 2015, 16, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.E.; McKenzie, D.C.; Milsom, W.K.; Sheel, A.W. Effects of Two Protocols of Intermittent Hypoxia on Human Ventilatory, Cardiovascular and Cerebral Responses to Hypoxia. J. Physiol. 2005, 567, 689–699. [Google Scholar] [CrossRef]
- Millet, G.P.; Faiss, R.; Pialoux, V. Point: Hypobaric Hypoxia Induces Different Physiological Responses from Normobaric Hypoxia. J. Appl. Physiol. 2012, 112, 1783–1784. [Google Scholar] [CrossRef]
- Bärtsch, P. Con: Hypoxic Cardiopulmonary Exercise Testing Identifies Subjects at Risk for Severe High Altitude Illnesses. High Alt. Med. Biol. 2014, 15, 318–320. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Canoui-Poitrine, F. Pro: Hypoxic Cardiopulmonary Exercise Testing Identifies Subjects at Risk for Severe High Altitude Illnesses. High Alt. Med. Biol. 2014, 15, 315–317. [Google Scholar] [CrossRef]
- Pla, R.; Brocherie, F.; Le Garrec, S.; Richalet, J.-P. Effectiveness of the Hypoxic Exercise Test to Predict Altitude Illness and Performance at Moderate Altitude in High-Level Swimmers. Physiol. Rep. 2020, 8, e14390. [Google Scholar] [CrossRef] [PubMed]
| Studies | Design | Level of Evidence | Limitations in Study Design or Execution | Inconsistency of Results | Directness | Imprecision | Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Richardson et al. (2008) | Cohort study | II | - | - | Direct | + | Low |
| Richalet et al. (2012) | Cohort study | II | + | - | Direct | + | Moderate |
| Canouï-Poitrine et al. (2014) | Cohort study | II | + | - | Direct | - | Moderate |
| Coustet et al. (2015) | Cohort study | II | + | - | Direct | - | Moderate |
| Winkler et al. (2017) | Cohort study | II | + | - | Direct | - | Moderate |
| Kammerer et al. (2018) | Cohort study | II | - | - | Direct | + | Low |
| Richalet et al. (2019) | Cohort study | II | + | - | Direct | - | Moderate |
| Richalet et al. (2020) | Cohort study | II | + | - | Direct | - | Moderate |
| Studies | Total Patients (n) | Mean Age (Years) | Gender (M/F) | Altitude Reached (m) | Type of Complications | Number of Complications |
|---|---|---|---|---|---|---|
| Richardson et al. (2008) | 12 | 27 ± 7 | 12/0 | NA | AMS | Not mentioned |
| Richalet et al. (2012) | 1326 | 44.7 (13.8) | 784/542 | 5079 (1038) | SHAI | 318 (24%) |
| Canouï-Poitrine et al. (2014) | 1017 | 44.3 (14.3) | 639/378 | Not mentioned | SHAI | Not mentioned |
| Coustet et al. (2015) | 113 | 49.3 (14.7) | 62/51 | 5275 (959) | SHAI | 22 (19%) |
| Winkler et al. (2017) | 182 | 59.3 (9.6) | Not mentioned | 4861 (828) | sAMS | 40 (22%) |
| Kammerer et al. (2018) | 7 | 36.3 (4) | 4/3 | 3883 (0) | AMS | Not mentioned |
| Richalet et al. (2019) | 260 | 50.0 (14.9) | 0/260 | 5011 (802) | sAMS | 67 (26%) |
| Richalet et al. (2020) | 641 | 50.4 (14.2) | 349/292 | 5202 (766) | SHAI | 150 (23%) |
| Total | 3558 | 46.9 (13.9) | 1850/1526 | 5092 (923) | - | 597 (24%) |
| Studies | Hypoxia Strategy | Diagnosis of HAP | Main Outcomes |
|---|---|---|---|
| Richardson et al. (2008) | 125 min walking HET | LLS and ESQ measured in normobaric hypoxia | Decrease in SpO2 correlated to an increase in the AMS score (p < 0.05). Core temperature, heart rate, ‟physiological strain”, perceived thirst and decrease in body mass positively correlated (r = 0.681 and 0.667 for LLS and ESQ) to the AMS score (p < 0.05). |
| Richalet et al. (2012) | 20 min HET on cycloergometer | HS, HAPE and HACE determined by questionnaire filled out by patients during their altitude stay | HVRe < 0.78 L/min/kg, ΔSae ≥ 22%, and HCRe < 0.84 beats/min/% were associated to SHAI in multivariate analysis, in NAU (OR were respectively 6.68 [3.83–11.63], 2.50 [1.52–4.11], 2.12 [1.37–2.89]). HVRe < 0.78 L/min/kg independently associated to SHAI in AU (OR 3.89 [1.74–8.73]). |
| Canouï-Poitrine et al. (2014) | 20 min HET on cycloergometer | HS, HAPE and HACE determined by questionnaire filled out by patients during their altitude stay | HVRe, ΔSae and HCRe, AUC increased by 7% (to 0.91) in the PRE group and 17% (to 0.89) in the ABS group |
| Coustet et al. (2015) | 20 min HET on cycloergometer | sAMS, HAPE and HACE determined by questionnaire filled out by patients during their altitude stay | No ECG characteristics predicted the risk of SHAI |
| Winkler et al. (2017) | 20 min HET on cycloergometer | LLS, HAPE and HACE determined by questionnaire filled out by patients during their altitude stay | BP variation during HET is not a useful predictor of intolerance to high altitude |
| Kammerer et al. (2018) | 120 min HET with alternate walking and cycling | LLS measured in hypobaric condition | rScO2-decrease after exercise in normobaric hypoxia correlated to AMS (r = −0.971; p < 0.01) |
| Richalet et al. (2019) | 20 min HET on cycloergometer | LLS, HAPE and HACE determined by questionnaire filled out by patients during their altitude stay | HVRe was higher in the luteal phase than in the follicular phase (0.89 ± 0.37 vs. 0.75 ± 0.27 L/min/kg). Oral contraception and hormonal treatment had no effect on response to hypoxia |
| Richalet et al. (2020) | 20 min HET on cycloergometer | LLS, HAPE and HACE determined by questionnaire filled out by patients during their altitude stay | Elaboration of a decision tree, thanks to HET, with a negative predictive value of 81% to detect subjects who will suffer from SHAI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georges, T.; Menu, P.; Le Blanc, C.; Ferreol, S.; Dauty, M.; Fouasson-Chailloux, A. Contribution of Hypoxic Exercise Testing to Predict High-Altitude Pathology: A Systematic Review. Life 2022, 12, 377. https://doi.org/10.3390/life12030377
Georges T, Menu P, Le Blanc C, Ferreol S, Dauty M, Fouasson-Chailloux A. Contribution of Hypoxic Exercise Testing to Predict High-Altitude Pathology: A Systematic Review. Life. 2022; 12(3):377. https://doi.org/10.3390/life12030377
Chicago/Turabian StyleGeorges, Thomas, Pierre Menu, Camille Le Blanc, Sophie Ferreol, Marc Dauty, and Alban Fouasson-Chailloux. 2022. "Contribution of Hypoxic Exercise Testing to Predict High-Altitude Pathology: A Systematic Review" Life 12, no. 3: 377. https://doi.org/10.3390/life12030377
APA StyleGeorges, T., Menu, P., Le Blanc, C., Ferreol, S., Dauty, M., & Fouasson-Chailloux, A. (2022). Contribution of Hypoxic Exercise Testing to Predict High-Altitude Pathology: A Systematic Review. Life, 12(3), 377. https://doi.org/10.3390/life12030377








