Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Use of Chemicals/Drugs
2.2. Study of Animals
2.3. Design of Experiments
2.4. Gastric Ulcer Induction
2.5. Measurement of Gastric Juice Volume and pH
2.6. Morphological and Histological Examination
2.7. Mucin Protein Assessment
2.8. Assessment of Oxidative Stress Markers
2.9. Prostaglandin E2 (PGE2) Assessment
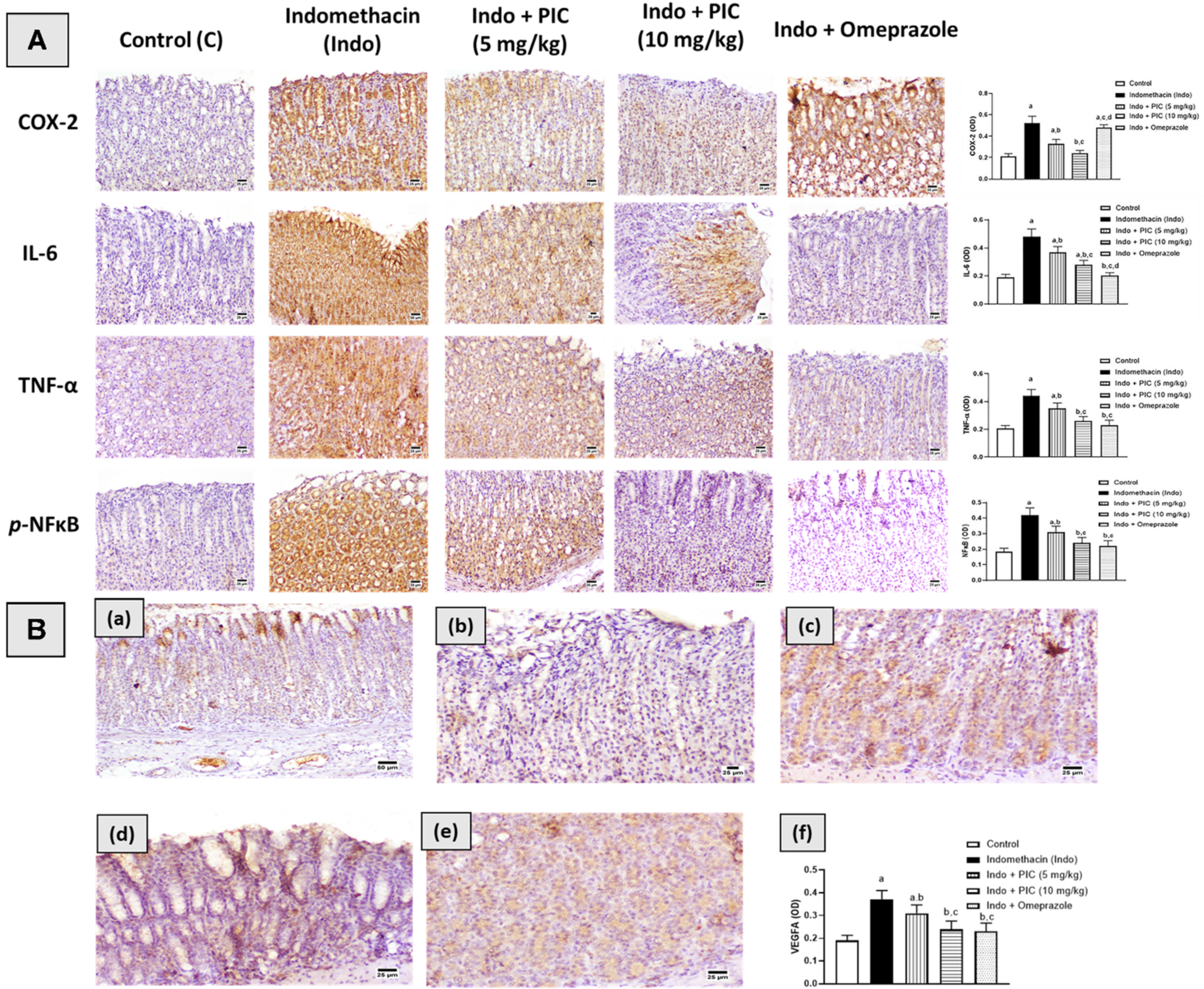
2.10. Immunohistochemical Assessment of Cox-2, IL-6, TNF-α, NF-κβ, and VEGFA
2.11. Quantitative Reverse Transcriptase PCR (qRT-PCR)
2.12. Statistical Analysis
3. Results
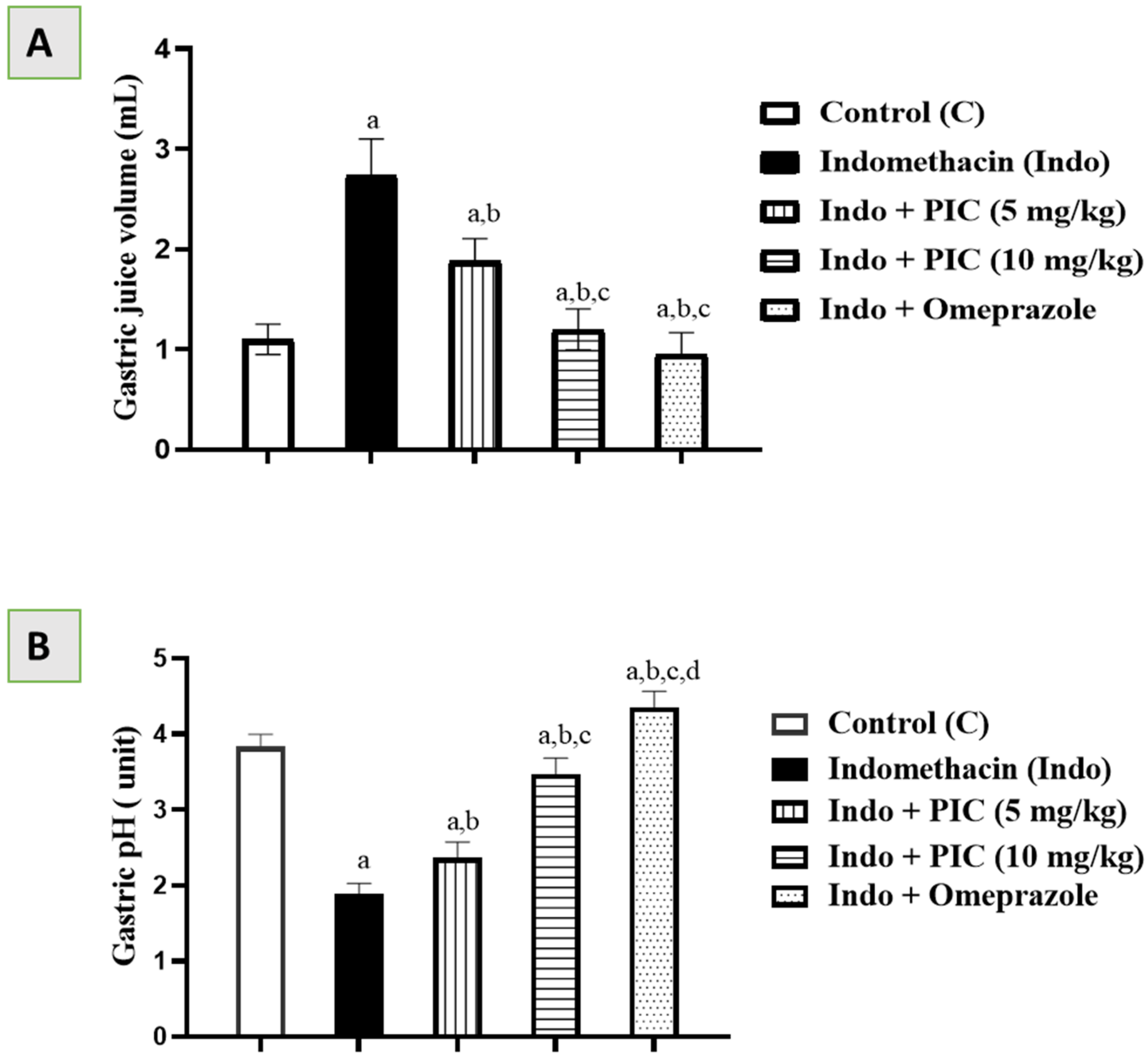
3.1. Effects of PIC on Gastric Juice Volume and pH
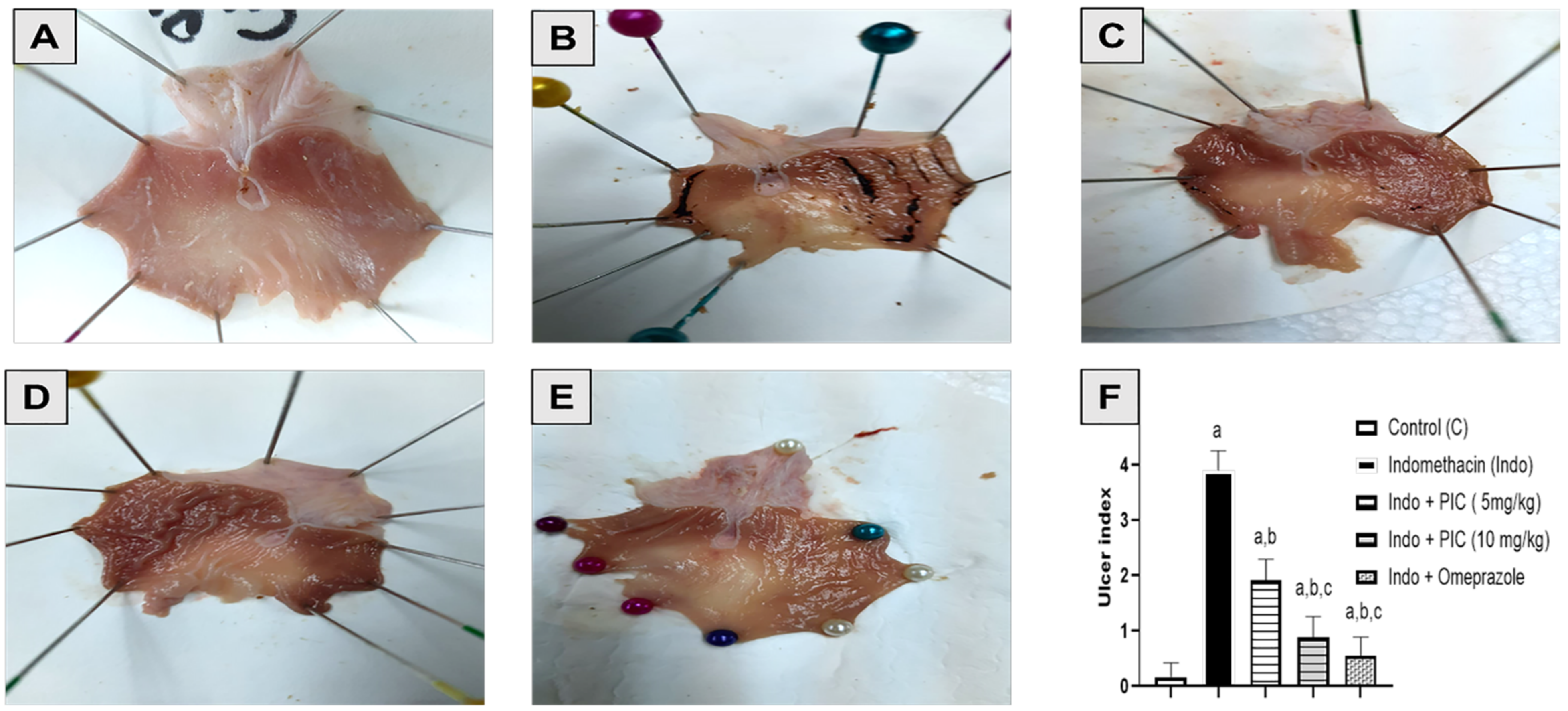
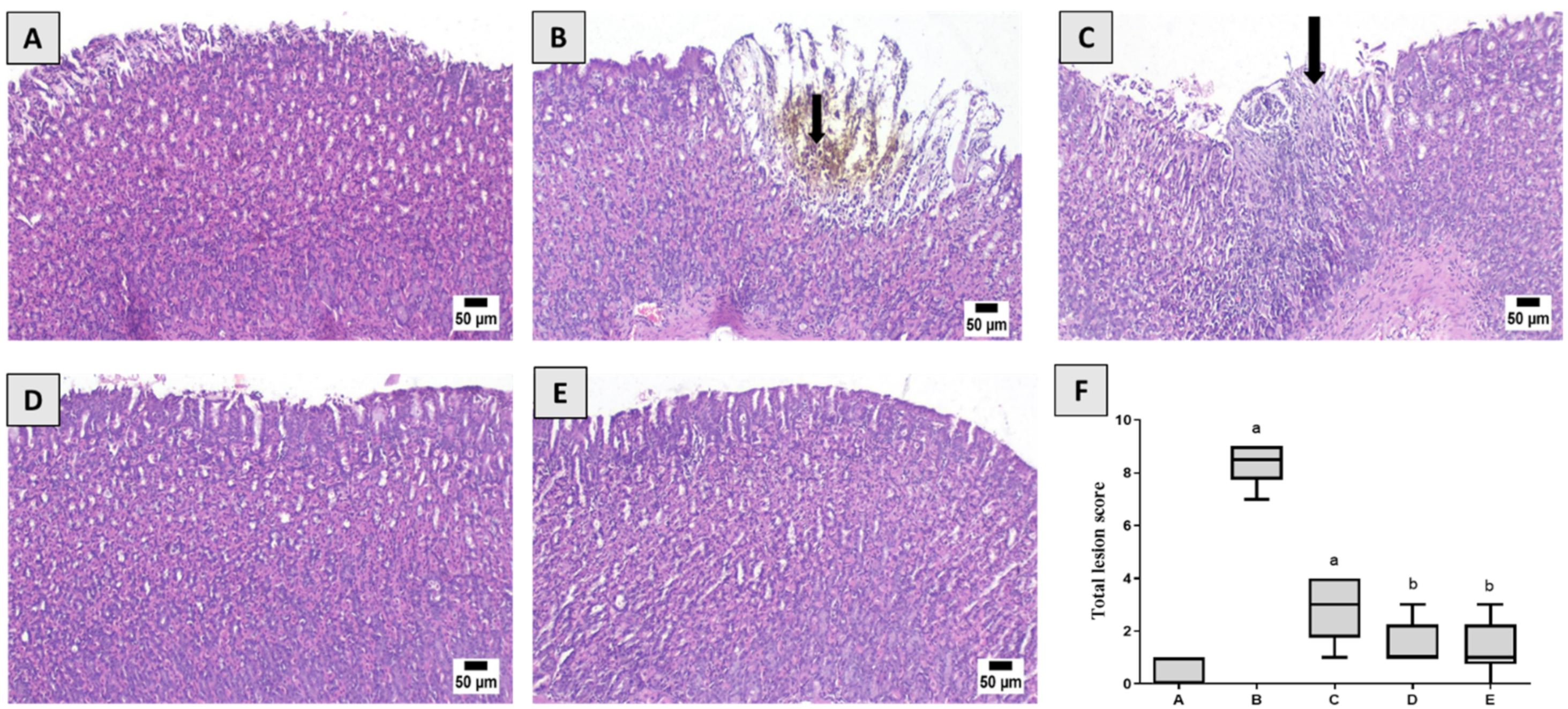
3.2. Effect of PIC Pretreatment on Stomach Morphology and Histopathology
3.3. PIC Affects the Mucin Content
3.4. PIC Affects the Oxidative Stress Denoting Markers
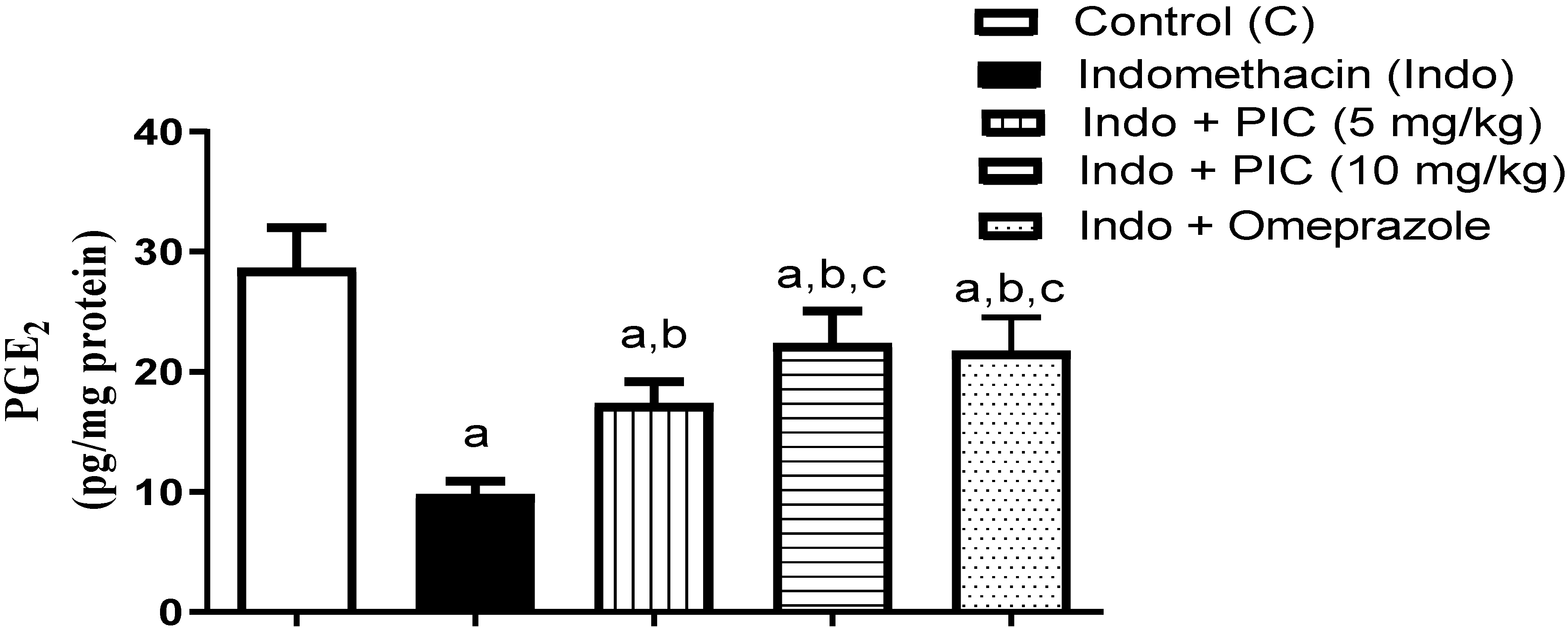
3.5. The Level of Gastric PGE2 Content
3.6. Immunohistochemical Assessment of Cox-2, IL-6, TNF-α, NF-κβ, and VEGFA
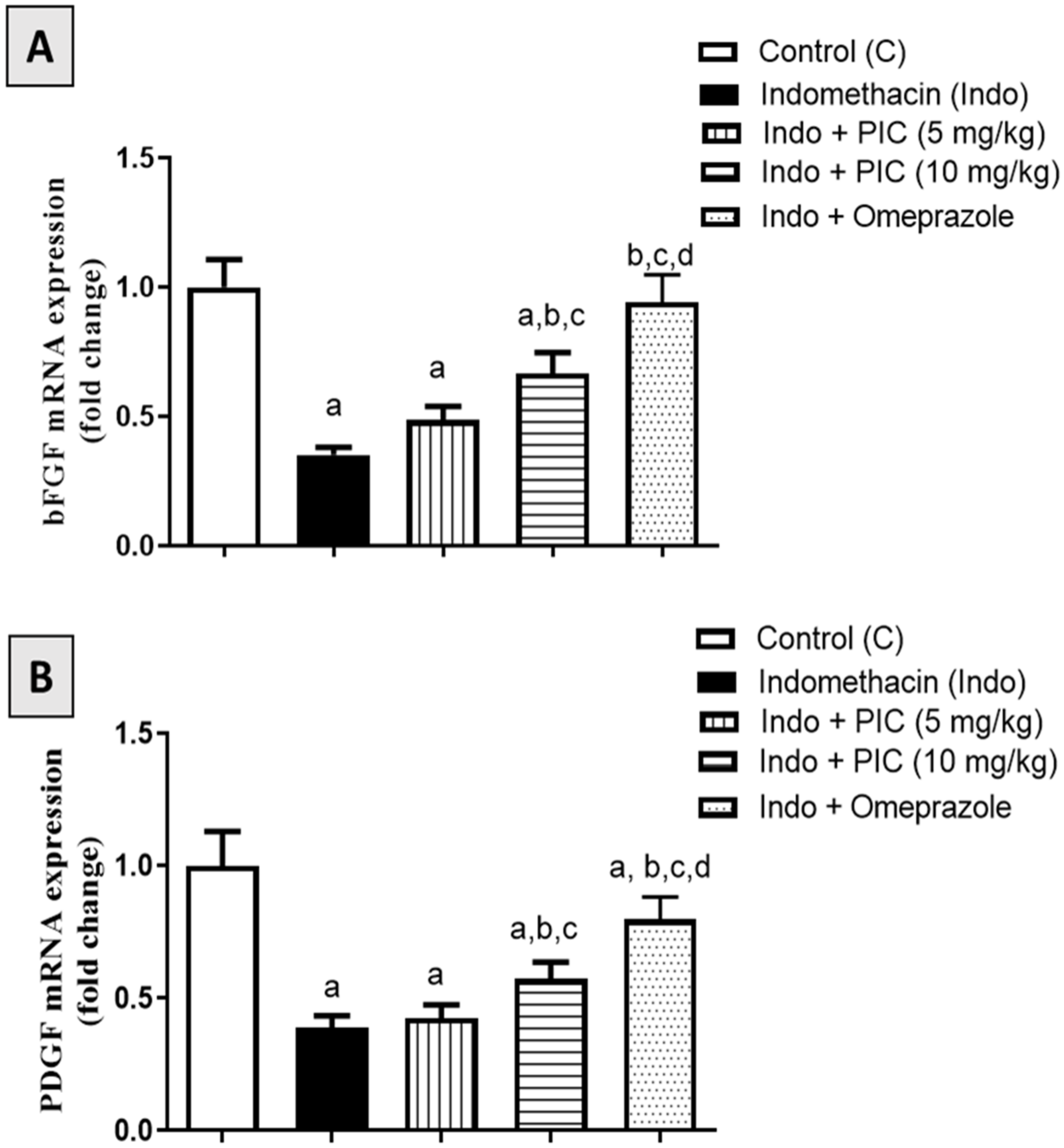
3.7. Effect of PIC on mRNA Expression Levels of bFGF and PDGF Marker Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boligon, A.A.; De Freitas, R.B.; De Brum, T.F.; Waczuk, E.P.; Klimaczewski, C.V.; De Ávila, D.S.; Athayde, M.L.; De Bauermann, L.F. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm. Sin. B 2014, 4, 358. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.M.; Sakr, H.F. Effect of selenium and grape seed extract on indomethacin-induced gastric ulcers in rats. J. Physiol. Biochem. 2013, 69, 527–537. [Google Scholar] [CrossRef]
- Brooks, P.; Emery, P.; Evans, J.F.; Fenner, H.; Hawkey, C.J.; Patrono, C.; Smolen, J.; Breedveld, F.; Day, R.; Dougados, M.; et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology 1999, 38, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, D.I.; El-Shiekh, R.A.; El-Sayed, M.A.; Khalil, H.M.A.; Mousa, M.R.; Al-Gendy, A.A.; El-Shazly, A.M. Phytochemical characterization and anti-inflammatory potential of Egyptian Murcott mandarin cultivar waste (stem, leaves and peel). Food Funct. 2020, 11, 8214–8236. [Google Scholar] [CrossRef]
- Musumba, C.; Pritchard, D.M.; Pirmohamed, M. Review article: Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment. Pharmacol. Ther. 2009, 30, 517–531. [Google Scholar] [CrossRef]
- Suleyman, H.; Albayrak, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010, 33, 224–234. [Google Scholar] [CrossRef]
- Yadav, S.K.; Adhikary, B.; Chand, S.; Maity, B.; Bandyopadhyay, S.K.; Chattopadhyay, S. Molecular mechanism of indomethacin-induced gastropathy. Free Radic. Biol. Med. 2012, 52, 1175–1187. [Google Scholar] [CrossRef]
- Utsumi, H.; Yasukawa, K.; Soeda, T.; Yamada, K.I.; Shigemi, R.; Yao, T.; Tsuneyoshi, M. Noninvasive mapping of reactive oxygen species by in vivo electron spin resonance spectroscopy in indomethacin-induced gastric ulcers in rats. J. Pharmacol. Exp. Ther. 2006, 317, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarnawski, A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 2005, 50 (Suppl. 1), S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Calabrò, A. Role of growth factors and their receptors in gastric ulcer healing. Microsc. Res. Tech. 2001, 53, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Hung, L.M.; Hsueh, C.H.; Lai, L.P.; Su, M.J. Piceatannol, a derivative of resveratrol, moderately slows INa inactivation and exerts antiarrhythmic action in ischemia-reperfused rat hearts. Br. J. Pharmacol. 2009, 157, 381. [Google Scholar] [CrossRef] [Green Version]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.J.; Na, H.K. Therapeutic Potential and Molecular Targets of Piceatannol in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 185–211. [Google Scholar] [CrossRef]
- Tang, Y.L.; Chan, S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother. Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef]
- Kiliç, V. Piceatannol Mediated Modulation of Oxidative Stress and Regeneration in the Liver of Endotoxemic Mice. J. Med. Food 2019, 22, 594–601. [Google Scholar] [CrossRef]
- Cao, Y.; Smith, W.; Yan, L.; Kong, L. Overview of Cellular Mechanisms and Signaling Pathways of Piceatannol. Curr. Stem. Cell Res. Ther. 2020, 15, 4–10. [Google Scholar] [CrossRef]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Cheong, Y.K.; Park, S.H.; Chung, H.T.; Pae, H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015, 12, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.Y.; Moon, D.O.; Lee, K.J.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Piceatannol attenuates lipopolysaccharide-induced NF-kappaB activation and NF-kappaB-related proinflammatory mediators in BV2 microglia. Pharmacol. Res. 2006, 54, 461–467. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Kundu, J.K.; Surh, Y.J. Piceatannol inhibits phorbol ester-induced expression of COX-2 and iNOS in HR-1 hairless mouse skin by blocking the activation of NF-κB and AP-1. Inflamm. Res. 2014, 63, 1013–1021. [Google Scholar] [CrossRef]
- Wahdan, S.A.; Azab, S.S.; Elsherbiny, D.A.; El-Demerdash, E. Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-κB inflammatory cascade. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 1331–1345. [Google Scholar] [CrossRef]
- Sathish, R.; Vyawahare, B.; Natarajan, K. Antiulcerogenic activity of Lantana camara leaves on gastric and duodenal ulcers in experimental rats. J. Ethnopharmacol. 2011, 134, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. (Eds.) Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- AlKreathy, H.M.; Alghamdi, M.K.; Esmat, A. Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: Impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric Mucosal Defense and Cytoprotection: Bench to Bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Shaker, E.; Mahmoud, H.; Mnaa, S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn). Food Chem. Toxicol. 2010, 48, 2785–2790. [Google Scholar] [CrossRef]
- Fornai, M.; Antonioli, L.; Colucci, R.; Tuccori, M.; Blandizzi, C. Pathophysiology of Gastric Ulcer Development and Healing: Molecular Mechanisms and Novel Therapeutic Options. Peptic Ulcer Dis. 2011, 113–142. [Google Scholar] [CrossRef] [Green Version]
- Russell, R.I. Non-steroidal anti-inflammatory drugs and gastrointestinal damage—Problems and solutions. Postgrad. Med. J. 2001, 77, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Maity, P.; Bindu, S.; Dey, S.; Goyal, M.; Alam, A.; Pal, C.; Mitra, K.; Bandyopadhyay, U. Indomethacin, a non-steroidal anti-inflammatory drug, develops gastropathy by inducing reactive oxygen species-mediated mitochondrial pathology and associated apoptosis in gastric mucosa: A novel role of mitochondrial aconitase oxidation. J. Biol. Chem. 2009, 284, 3058–3068. [Google Scholar] [CrossRef] [Green Version]
- Tastekin, E.; Ayvaz, S.; Usta, U.; Aydogdu, N.; Cancilar, E.; Oz Puyan, F. Indomethacin-induced gastric damage in rats and the protective effect of donkey milk. Arch. Med. Sci. 2018, 14, 671. [Google Scholar] [CrossRef]
- Kershaw, J.; Kim, K.H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, F.P.; Puty, B.; Nogueira, L.S.; Mitre, G.P.; Dos Santos, S.M.; Teixeira, B.J.B.; Kataoka, M.S.D.S.; Martins, M.D.; Barboza, C.A.G.; Monteiro, M.C.; et al. Piceatannol Increases Antioxidant Defense and Reduces Cell Death in Human Periodontal Ligament Fibroblast under Oxidative Stress. Antioxidants 2020, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Guha, P.; Dey, A.; Chatterjee, A.; Chattopadhyay, S.; Bandyopadhyay, S.K. Pro-ulcer effects of resveratrol in mice with indomethacin-induced gastric ulcers are reversed by L-arginine. Br. J. Pharmacol. 2010, 159, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Sun, Y.; Yi, J.; Zhou, L.; Cai, S. Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2022, 284, 114752. [Google Scholar] [CrossRef] [PubMed]
- Sabiu, S.; Garuba, T.; Sunmonu, T.; Ajani, E.; Sulyman, A.; Nurain, I.; Balogun, A. Indomethacin-induced gastric ulceration in rats: Protective roles of Spondias mombin and Ficus exasperata. Toxicol. Rep. 2015, 2, 261. [Google Scholar] [CrossRef] [Green Version]
- Olaleye, S.B.; Farombi, E.O. Attenuation of indomethacin- and HCl/ethanol-induced oxidative gastric mucosa damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seed. Phytother. Res. 2006, 20, 14–20. [Google Scholar] [CrossRef]
- Takeuchi, K.; Tanaka, A.; Hayashi, Y.; Kubo, Y. Functional mechanism underlying COX-2 expression following administration of indomethacin in rat stomachs: Importance of gastric hypermotility. Dig. Dis. Sci. 2004, 49, 180–187. [Google Scholar] [CrossRef]
- Gentile, L.B.; Queiroz-Hazarbassanov, N.; Massoco, C.D.O.; Fecchio, D. Modulation of cytokines production by indomethacin acute dose during the evolution of Ehrlich Ascites tumor in mice. Mediat. Inflamm. 2015, 2015, 1–8, 924028. [Google Scholar] [CrossRef] [Green Version]
- Halter, F.; Tarnawski, A.S.; Schmassmann, A.; Peskar, B.M. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: Controversial issues and perspectives. Gut 2001, 49, 443–453. [Google Scholar] [CrossRef]
- Mizuno, H.; Sakamoto, C.; Matsuda, K.; Wada, K.; Uchida, T.; Noguchi, H.; Akamatsu, T.; Kasuga, M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology 1997, 112, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Sharkey, K.A.; Asfaha, S.; Macnaughton, W.K.; Wallace, J.L. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment. Pharmacol. Ther. 1997, 11, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, Y.; Wada, K.; Nakamoto, K.; Kawasaki, H.; Hasegawa, J. Levels of cyclooxygenase-1 and -2 mRNA expression at various stages of acute gastric injury induced by ischemia-reperfusion in rats. Arch. Biochem. Biophys. 1998, 352, 153–157. [Google Scholar] [CrossRef]
- Takeuchi, K.; Ueshima, K.; Hironaka, Y.; Fujioka, Y.; Matsumoto, J.; Okabe, S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion 1991, 49, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Niida, H.; Takeuchi, K.; Okabe, S. Role of prostaglandin deficiency in pathogenetic mechanism of gastric lesions induced by indomethacin in rats. Dig. Dis. Sci. 1989, 34, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Lonnroth, C.; Svaninger, G.; Gelin, J.; Cahlin, C.; Iresjo, B.M.; Cvetkovska, E.; Edstrom, S.; Andersson, M.; Svanberg, E.; Lundholm, K. Effects related to indomethacin prolonged survival and decreased tumor-growth in a mouse-tumor model with cytokine dependent cancer cachexia. Int. J. Oncol. 1995, 7, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Mucha, K.; Foroncewicz, B.; Koziak, K.; Czarkowska-Paczek, B.; Paczek, L. The effects of indomethacin on angiogenic factors mRNA expression in renal cortex of healthy rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2007, 58, 165–178. [Google Scholar]
- Poonam, D.; Vinay, C.S.; Gautam, P. Cyclo-oxygenase-2 expression and prostaglandin E2 production in experimental chronic gastric ulcer healing. Eur. J. Pharmacol. 2005, 519, 277–284. [Google Scholar] [CrossRef]
- Okabe, S.; Miyake, H.; Awane, Y. Cytoprotective effects of NC-1300 and omeprazole on Hcl. ethanol-induced gastric lesions in rats. Jpn. J. Pharmacol. 1986, 42, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, O.; Okada, Y.; Okabe, S. Effects of a proton pump inhibitor, omeprazole, on gastric secretion and gastric and duodenal ulcers or erosions in rats. Dig. Dis. Sci. 1984, 29, 394–401. [Google Scholar] [CrossRef]
- Robinson, M.; Horn, J. Clinical pharmacology of proton pump inhibitors: What the practising physician needs to know. Drugs 2003, 63, 2739–2754. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Mitstui, M.; Zamami, Y.; Niimura, T.; Izawa-Ishizawa, Y.; Goda, M.; Chuma, M.; Fukunaga, K.; Shibata, T.; Ishida, S.; et al. Investigation of drugs affecting hypertension in bevacizumab-treated patients and examination of the impact on the therapeutic effect. Cancer Med. 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Swamy, A.H.M.V.; Sajjan, M.; Thippeswamy, A.H.M.; Koti, B.C.; Sadiq, A.J. Influence of Proton Pump Inhibitors on Dexamethasone-Induced Gastric Mucosal Damage in Rats. Indian J. Pharm. Sci. 2011, 73, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Primer (5′ to 3′) | |
|---|---|
| PDGF | F: ATTGGCAATGAGCGGTTCCGC |
| R: CTCCTGCTTGCTGATCCACATC | |
| BFGF | F: TGGCATTCTCAGGTTCTGGCCATT |
| R: TGGCATTCTCAGGTTCTGGCCATT | |
| β-Actin | F: TCCGTCGCCGGTCCACACCC |
| R: TCACCAACTGGGACGATATG |
| MDA (nmol/mg Protein) | GSH (µg/mg Protein) | SOD (U/mg Protein) | CAT (U/mg Protein) | |
|---|---|---|---|---|
| Control (C) | 0.72 ± 0.09 | 0.44 ± 0.05 | 39.92 ± 4.21 | 0.88 ± 0.10 |
| Indomethacin (Indo) | 4.11 a ± 0.45 | 0.13 a ± 0.02 | 17.84 a ± 2.10 | 0.48 a ± 0.06 |
| Indo + PIC (5 mg/kg) | 2.82 a,b ± 0.31 | 0.19 a,b ± 0.02 | 24.80 a,b ± 2.86 | 0.68 a,b ± 0.08 |
| Indo + PIC (10 mg/kg) | 2.32 a,b,c ± 0.28 | 0.21a,b ± 0.02 | 29.57 a,b± 3.31 | 0.77 b ± 0.09 |
| Indo + Omeprazole | 1.70 a,b,c,d + 0.19 | 0.23 a,b ± 0.03 | 33.57 a,b,c ± 3.94 | 0.71 a,b ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, R.A.; Eid, B.G. Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats. Life 2022, 12, 356. https://doi.org/10.3390/life12030356
Shaik RA, Eid BG. Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats. Life. 2022; 12(3):356. https://doi.org/10.3390/life12030356
Chicago/Turabian StyleShaik, Rasheed A., and Basma G. Eid. 2022. "Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats" Life 12, no. 3: 356. https://doi.org/10.3390/life12030356
APA StyleShaik, R. A., & Eid, B. G. (2022). Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats. Life, 12(3), 356. https://doi.org/10.3390/life12030356






