Effect of C60 Fullerene on Recovery of Muscle Soleus in Rats after Atrophy Induced by Achillotenotomy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
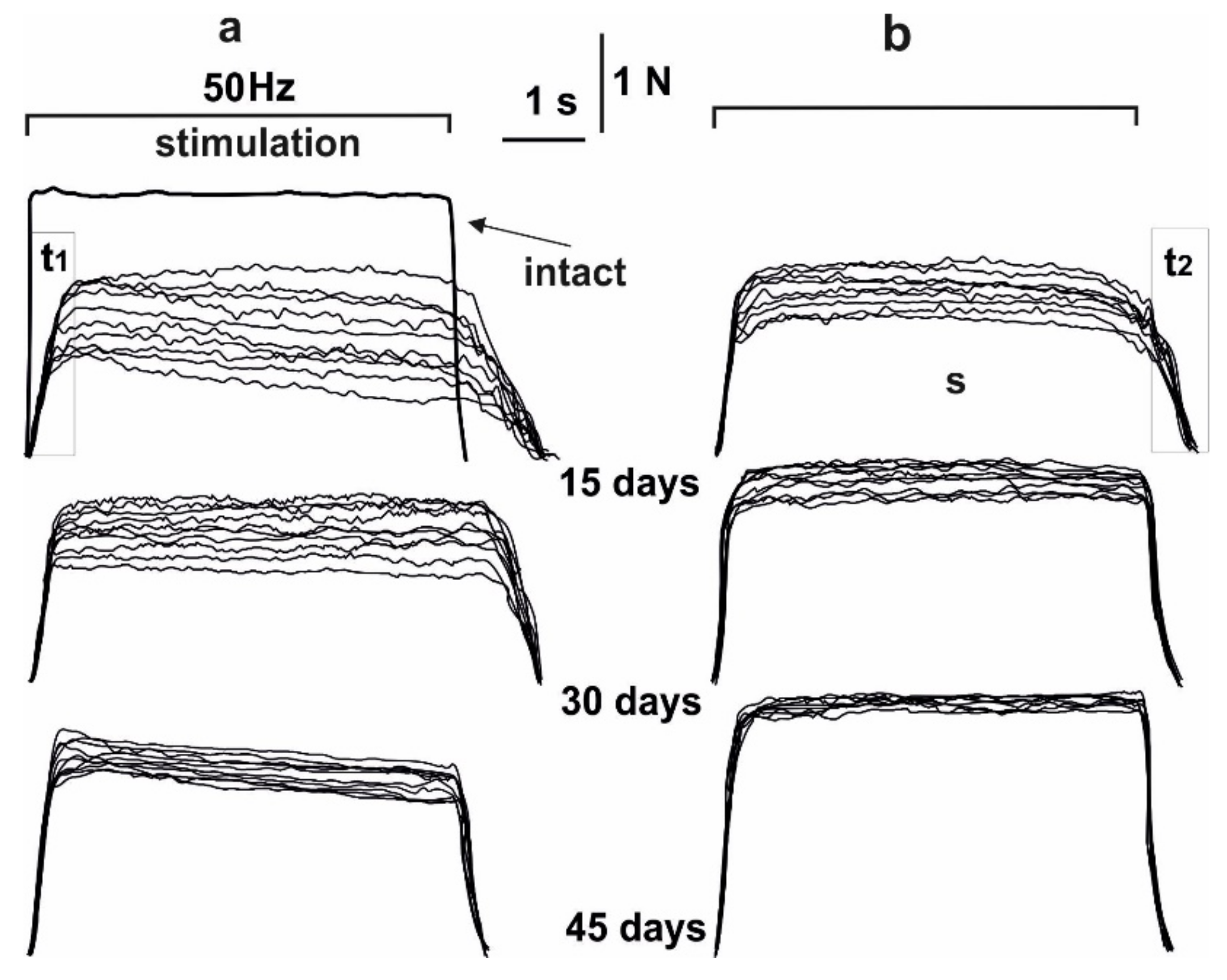
3.1. Dynamics of Muscle Soleus Contraction Force in Rats
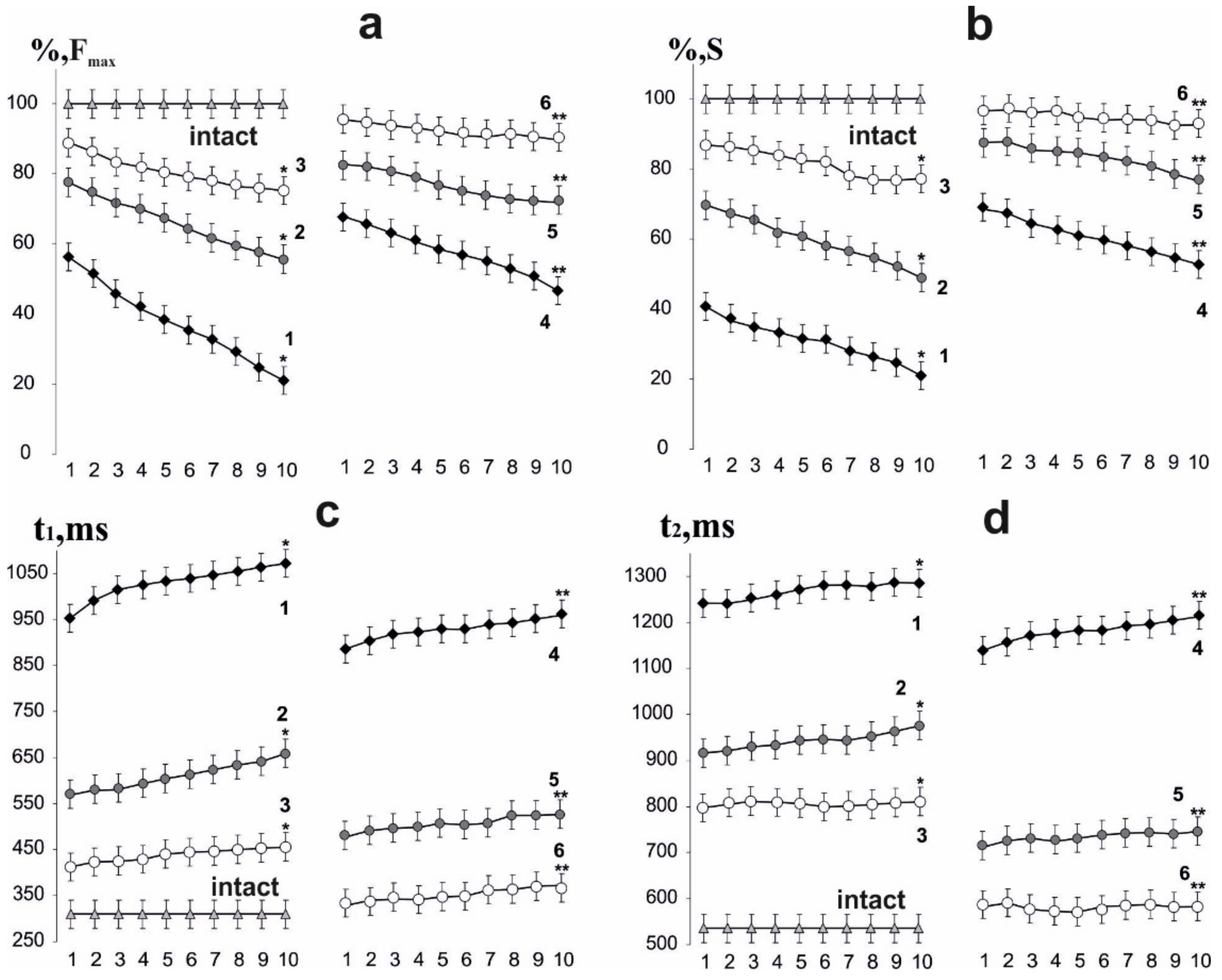
3.2. Estimation of the Time to Reach the Maximum Force Response and Recovery of Muscle Soleus Force Parameters in Rats
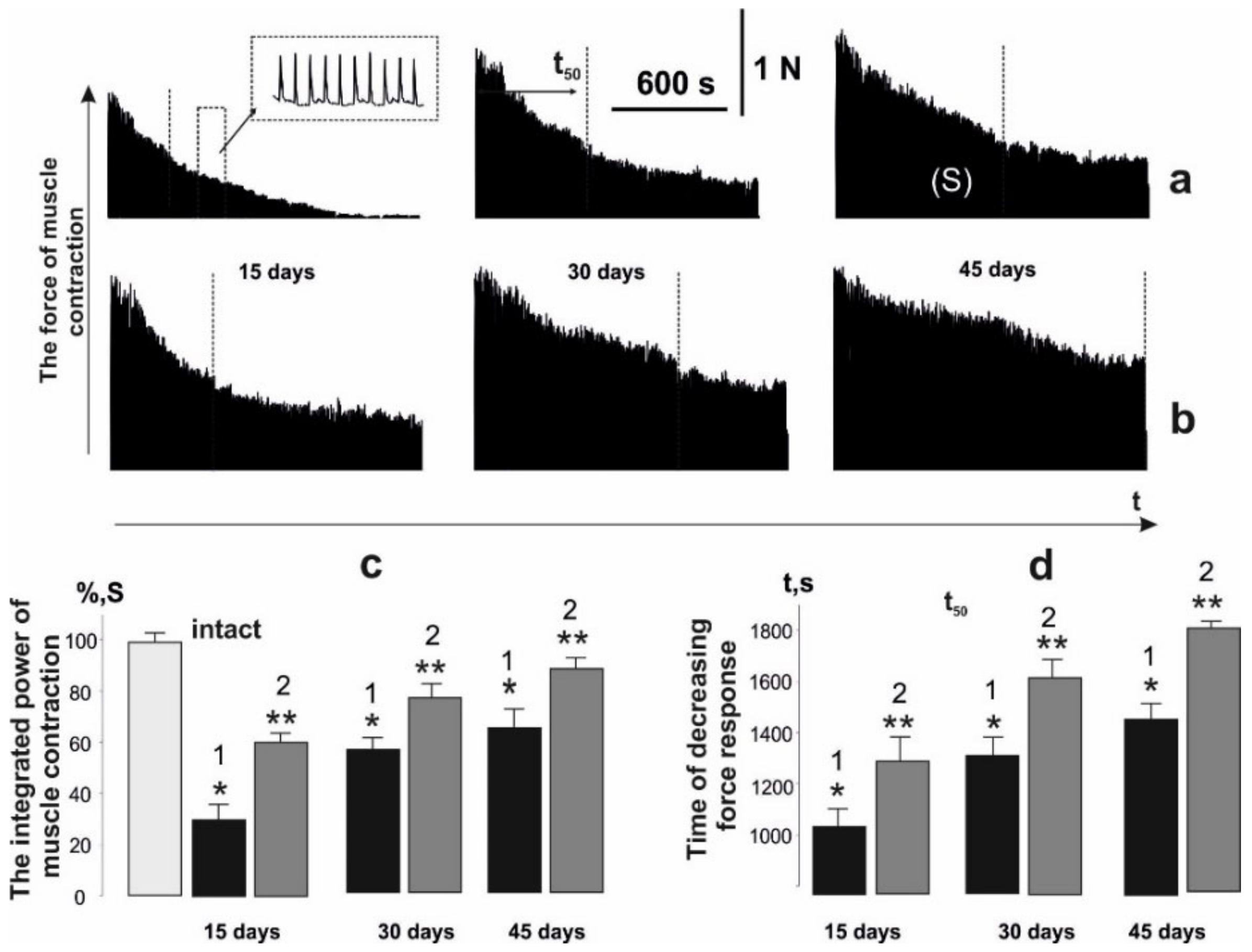
3.3. Analysis of Fatigue Processes in the Muscle Soleus of Rats after AT Using 1 Hz Stimulation
3.4. Analysis of the Occurrence of Smooth Tetanic Contraction of Muscle Soleus in Rats
3.5. Changes in the Body Weights of Animals and the Muscle Soleus, the Value of the Maximum Strength of a Single Tetanic Contraction of an Isolated Muscle, Normalized to the Value of CSA, after AT
3.6. Analysis of Blood Biochemical Parameters in Rats as Markers of Muscle Injury
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohira, Y.; Yoshinaga, T.; Nomura, T.; Kawano, F.; Ishihara, A.; Nonaka, I.; Roy, R.R.; Edgerton, V.R. Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number. Adv. Space Res. 2002, 30, 777–781. [Google Scholar] [CrossRef]
- Nozdrenko, D.N.; Shut, A.N.; Prylutskyy, Y.I. The possible molecular mechanism of the nonlinearity muscle contraction and its experimental substantiation. Biopolym. Cell 2005, 21, 80–83. [Google Scholar] [CrossRef]
- Goldberg, A.L.; Dupont-Versteegden Etlinger, J.D.; Goldspink, D.F.; Jablecki, C. Mechanism of work-induced hypertrophy of skeletal muscle. Med. Sci. Sports 1975, 7, 185–198. [Google Scholar] [PubMed]
- Fluckey, J.D.; Dupont-Versteegden, E.E.; Knox, M.; Gaddy, D.; Tesch, P.A.; Peterson, C.A. Insulin facilitation of muscle protein synthesis following resistance exercise in hindlimb-suspended rats is independent of a rapamycin-sensitive pathway. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1070–E1075. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Cree, M.G.; Hewlings, S.J.; Aarsland, A.; Wolfe, R.R.; Ferrando, A.A. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J. Clin. Endocrinol. Metab. 2006, 91, 4836–4841. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.G.; Dolmans, J.; van Loon, L.J.C. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. Oxf. 2014, 210, 600–611. [Google Scholar] [CrossRef]
- Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. Response of the neuromuscular unit to spaceflight: What has been learned from the rat model. Exerc. Sport Sci. Rev. 1996, 24, 399–425. [Google Scholar] [CrossRef]
- Ebert, S.M.; Al-Zougbi, A.; Bodine, S.C.; Adams, C.M. Skeletal Muscle Atrophy: Discovery of Mechanisms and Potential Therapies. Physiol. Bethesda 2019, 34, 232–239. [Google Scholar] [CrossRef]
- Hodgson, J.A.; Roy, R.R.; Higuchi, N.; Monti, R.J.; Zhong, H.; Grossman, E.; Edgerton, V.R. Does daily activity level determine muscle phenotype? J. Exp. Biol. 2005, 208, 3761–3770. [Google Scholar] [CrossRef]
- Nicholson, G.; Walker, J.; Dawson, Z.; Bissas, A.; Harris, N. Morphological and functional outcomes of operatively treated Achilles tendon ruptures. Phys. Sportsmed. 2020, 48, 290–297. [Google Scholar] [CrossRef]
- Nozdrenko, D.N.; Bogutska, K.I. About molecular mechanisms of fiber muscle contraction at transition to new equilibrium state: Analysis of experimental data using three-componential electrical stimulating signal. Biopolym. Cell 2005, 21, 283–286. [Google Scholar] [CrossRef]
- Barfod, K.W. Achilles tendon rupture; assessment of nonoperative treatment. Dan. Med. J. 2014, 61, B4837. [Google Scholar] [PubMed]
- Heikkinen, J.; Lantto, I.; Piilonen, J.; Flinkkilä, T.; Ohtonen, P.; Siira, P.; Laine, V.; Niinimäki, J.; Pajala, A.; Leppilahti, J. Tendon Length, Calf Muscle Atrophy, and Strength Deficit After Acute Achilles Tendon Rupture: Long-Term Follow-up of Patients in a Previous Study. J. Bone Jt. Surg. Am. 2017, 99, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Enwemeka, C.S.; Spielholz, N.I.; Nelson, A.J. The effect of early functional activities on experimentally tenotomized Achilles tendons in rats. Am. J. Phys. Med. Rehabil. 1988, 67, 264–269. [Google Scholar] [PubMed]
- Geeslin, A.G.; LaPrade, R.F. Surgical treatment of snapping medial hamstring tendons. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1294–1296. [Google Scholar] [CrossRef]
- Malliaropoulos, N.G. Non contact Hamstring injuries in sports. Muscles Ligaments Tendons J. 2013, 2, 309–311. [Google Scholar]
- Brumann, M.; Baumbach, S.F.; Mutschler, W.; Polzer, H. Accelerated rehabilitation following Achilles tendon repair after acute rupture—Development of an evidence-based treatment protocol. Injury 2014, 45, 1782–1790. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, Y.K.; Sharma, S.; Singh, S.N.; Suryakumar, G. High altitude mediated skeletal muscle atrophy: Protective role of curcumin. Biochimie 2019, 156, 138–147. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kubota, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. Glabridin inhibits dexamethasone-induced muscle atrophy. Arch. Biochem. Biophys. 2016, 664, 157–166. [Google Scholar] [CrossRef]
- Gemalmaz, H.C.; Sarıyılmaz, K.; Ozkunt, O.; Gurgen, S.G.; Silay, S. Role of a combination dietary supplement containing mucopolysaccharides, vitamin C, and collagen on tendon healing in rats. Acta Orthop. Traumatol. Turc. 2018, 52, 452–458. [Google Scholar] [CrossRef]
- Eswaran, S.V. Water soluble nanocarbon materials: A panacea for all? Curr. Sci. 2018, 114, 846–1850. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Zay, S.Y.; Zavodovskyi, D.A.; Bogutska, K.I.; Nozdrenko, D.N.; Prylutskyy, Y.I. Prospects of C60 fullerene application as a mean of prevention and correction of ischemic-reperfusion injury in the skeletal muscle tissue. Fiziolohichnyi Zhurnal 2016, 62, 66–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nozdrenko, D.; Matvienko, T.; Vygovska, O.; Bogutska, K.; Motuziuk, O.; Nurishchenko, N.; Prylutskyy, Y.; Scharff, P.; Ritter, U. Protective Effect of Water-Soluble C60 Fullerene Nanoparticles on the Ischemia-Reperfusion Injury of the Muscle Soleus in Rats. Int. J. Mol. Sci. 2021, 22, 6812. [Google Scholar] [CrossRef] [PubMed]
- Nozdrenko, D.; Abramchuk, O.; Prylutska, S.; Vygovska, O.; Soroca, V.; Bogutska, K.; Khrapatyi, S.; Prylutskyy, Y.; Scharff, P.; Ritter, U. Analysis of Biomechanical Parameters of Muscle Soleus Contraction and Blood Biochemical Parameters in Rat with Chronic Glyphosate Intoxication and Therapeutic Use of C60 Fullerene. Int. J. Mol. Sci. 2021, 22, 4977. [Google Scholar] [CrossRef] [PubMed]
- Prylutskyy, Y.I.; Vereshchaka, I.V.; Maznychenko, A.V.; Bulgakova, N.V.; Gonchar, O.O.; Kyzyma, O.A.; Ritter, U.; Scharff, P.; Tomiak, T.; Nozdrenko, D.M.; et al. C60 fullerene as promising therapeutic agent for correcting and preventing skeletal muscle fatigue. J. Nanobiotechnol. 2017, 15, 8. [Google Scholar] [CrossRef]
- Nozdrenko, D.N.; Berehovyi, S.M.; Nikitina, N.S.; Stepanova, L.I.; Beregova, T.V.; Ostapchenko, L.I. The influence of complex drug cocarnit on the nerve conduction velocity in nerve tibialis of rats with diabetic polyneuropathy. Biomed. Res. 2018, 29, 3629–3634. [Google Scholar]
- Nozdrenko, D.; Matvienko, T.; Vygovska, O.; Soroca, V.; Bogutska, K.; Zholos, A.; Scharff, P.; Ritter, U.; Prylutskyy, Y. Post-traumatic recovery of muscle soleus in rats is improved via synergistic effect of C60 fullerene and TRPM8 agonist menthol. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Turov, V.V.; Chehun, V.F.; Krupskaya, T.V.; Barvinchenko, V.N.; Chehun, S.V.; Ugnichenko, A.P.; Prylutskyy, Y.P.; Scharff, P.; Ritter, U. Effect of small addition of C60 fullerenes on the hydrated properties of nanocomposites based on highly dispersed silica and DNA. Chem. Phys. Lett. 2010, 496, 152–156. [Google Scholar] [CrossRef]
- Skamrova, G.B.; Laponogov, I.; Buchelnikov, A.S.; Shckorbatov, Y.G.; Prylutska, S.V.; Ritter, U.; Prylutskyy, Y.I.; Evstigneev, M.P. Interceptor effect of C60 fullerene on the in vitro action of aromatic drug molecules. Eur. Biophys. J. 2014, 43, 265–276. [Google Scholar] [CrossRef]
- Theilen, N.T.; Jeremic, N.; Weber, G.J.; Tyagi, S.C. Exercise preconditioning diminishes skeletal muscle atrophy after hindlimb suspension in mice. J. Appl. Physiol. 1985, 125, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60] Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Grebinyk, A.G.; Lynchak, O.V.; Byelinska, I.V.; Cherepanov, V.V.; Tauscher, E.; Matyshevska, O.P.; Prylutskyy, Y.I.; Rybalchenko, V.K.; Ritter, U.; et al. In vitro and in vivo toxicity of pristine C60 fullerene aqueous colloid solution. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 715. [Google Scholar] [CrossRef]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Jozsa, L.; Thöring, J.; Järvinen, M.; Kannus, P.; Lehto, M.; Kvist, M. Quantitative alterations in intramuscular connective tissue following immobilization: An experimental study in the rat calf muscles. Exp. Mol. Pathol. 1988, 49, 267–278. [Google Scholar] [CrossRef]
- Vereshchaka, I.V.; Bulgakova, N.V.; Maznychenko, A.V.; Gonchar, O.O.; Prylutskyy, Y.I.; Ritter, U.; Moska, W.; Tomiak, T.; Nozdrenko, D.M.; Mishchenko, I.V.; et al. C60 Fullerenes Diminish Muscle Fatigue in Rats Comparable to N-acetylcysteine or β-Alanine. Front Physiol. 2018, 9, 517. [Google Scholar] [CrossRef]
- Nozdrenko, D.M.; Korchinska, L.V.; Soroca, V.M. Activity of Ca(2+),Mg(2+)-ATPase of sarcoplasmic reticulum and contraction strength of the frog skeletal muscles under the effect of organophosphorus insecticides. Ukr. Biochem. J. 2015, 87, 63–69. [Google Scholar] [CrossRef]
- Grottel, K.; Celichowski, J. Division of motor units in medial gastrocnemius muscle of the rat in the light of variability of their principal properties. Acta Neurobiol. Exp. 1990, 50, 571–587. [Google Scholar]
- Malavaki, C.J.; Sakkas, G.K.; Mitrou, G.I.; Kalyva, A.; Stefanidis, I.; Myburgh, K.H.; Karatzaferi, C. Skeletal muscle atrophy: Disease-induced mechanisms may mask disuse atrophy. J. Muscle Res. Cell Motil. 2015, 36, 405–421. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef]
- Halenova, T.I.; Vareniuk, I.M.; Roslova, N.M.; Dzerzhynsky, M.E.; Savchuk, O.M.; Ostapchenko, L.I.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P. Hepatoprotective effect of orally applied water-soluble pristine C60 fullerene against CCl4-induced acute liver injury in rats. RSC Adv. 2016, 6, 100046–100055. [Google Scholar] [CrossRef]
- Gonchar, O.O.; Maznychenko, A.V.; Bulgakova, N.V.; Vereshchaka, I.V.; Tomiak, T.; Ritter, U.; Prylutskyy, Y.I.; Mankovska, I.M.; Kostyukov, A.I. C60 fullerene Prevents Restraint Stress-induced Oxidative Disorders in Rat Tissues: Possible Involvement of the Nrf2/ARE-Antioxidant Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 2518676. [Google Scholar] [CrossRef] [PubMed]
- Halenova, T.; Raksha, N.; Vovk, T.; Savchuk, O.; Ostapchenko, L.; Prylutskyy, Y.; Kyzyma, O.; Ritter, U.; Scharff, P. Effect of C60 fullerene nanoparticles on the diet-induced obesity in rats. Int. J. Obes. 2018, 42, 1987–1998. [Google Scholar] [CrossRef]
- Lepley, L.K.; Davi, S.M.; Burland, J.P.; Lepley, A.S. Muscle Atrophy After ACL Injury: Implications for Clinical Practice. Sports Health 2020, 12, 579–586. [Google Scholar] [CrossRef] [PubMed]



| Group | Rat Weight, g | Soleus Weight, mg | Soleus Weight/Rat weight | P0, mN | P0/CSA, N/cm2 |
|---|---|---|---|---|---|
| intact | 205 ± 8 | 102.4 ± 1.5 | 0.49 ± 0.011 | 882.4 ± 14.3 | 23.4 ± 1.2 |
| 15 days | 231 ± 6 * | 63.4 ± 1.8 * | 0.27 ± 0.032 * | 432.5 ± 16.1 * | 14.4 ± 2.5 * |
| 30 days | 243 ± 4 * | 73.4 ± 1.2 * | 0.30 ± 0.015 * | 676.5 ± 11.6 * | 17.6 ± 7.3 * |
| 45 days | 250 ± 6 * | 86.4 ± 1.5 * | 0.34 ± 0.018 * | 693.3 ± 14.1 * | 18.6 ± 4.4 * |
| 15 days + C60 fullerene | 244 ± 5 ** | 79.4 ± 1.2 ** | 0.32 ± 0.015 ** | 602.5 ± 12.2 ** | 18.1 ± 1.2 ** |
| 30 days + C60 fullerene | 254 ± 2 ** | 89.4 ± 1.3 ** | 0.35 ± 0.023 ** | 711.5 ± 22.5 ** | 19.2 ± 1.1 ** |
| 45 days + C60 fullerene | 269 ± 7 ** | 105.4 ± 1.9 ** | 0.39 ± 0.054 ** | 782.5 ± 16.3 ** | 20.3 ± 1.2 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozdrenko, D.; Prylutska, S.; Bogutska, K.; Nurishchenko, N.Y.; Abramchuk, O.; Motuziuk, O.; Prylutskyy, Y.; Scharff, P.; Ritter, U. Effect of C60 Fullerene on Recovery of Muscle Soleus in Rats after Atrophy Induced by Achillotenotomy. Life 2022, 12, 332. https://doi.org/10.3390/life12030332
Nozdrenko D, Prylutska S, Bogutska K, Nurishchenko NY, Abramchuk O, Motuziuk O, Prylutskyy Y, Scharff P, Ritter U. Effect of C60 Fullerene on Recovery of Muscle Soleus in Rats after Atrophy Induced by Achillotenotomy. Life. 2022; 12(3):332. https://doi.org/10.3390/life12030332
Chicago/Turabian StyleNozdrenko, Dmytro, Svitlana Prylutska, Kateryna Bogutska, Natalia Y. Nurishchenko, Olga Abramchuk, Olexandr Motuziuk, Yuriy Prylutskyy, Peter Scharff, and Uwe Ritter. 2022. "Effect of C60 Fullerene on Recovery of Muscle Soleus in Rats after Atrophy Induced by Achillotenotomy" Life 12, no. 3: 332. https://doi.org/10.3390/life12030332
APA StyleNozdrenko, D., Prylutska, S., Bogutska, K., Nurishchenko, N. Y., Abramchuk, O., Motuziuk, O., Prylutskyy, Y., Scharff, P., & Ritter, U. (2022). Effect of C60 Fullerene on Recovery of Muscle Soleus in Rats after Atrophy Induced by Achillotenotomy. Life, 12(3), 332. https://doi.org/10.3390/life12030332








