The Blood of the HIV-Infected Patients Contains κ-IgG, λ-IgG, and Bispecific κλ-IgG, Which Possess DNase and Amylolytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. HIV-Infected Patients: Clinical Parameters and Sample Collection
2.2. Antibody Purification and Analysis
2.3. ELISA of IgG, κ-IgG, λ-IgG and κλ-IgG
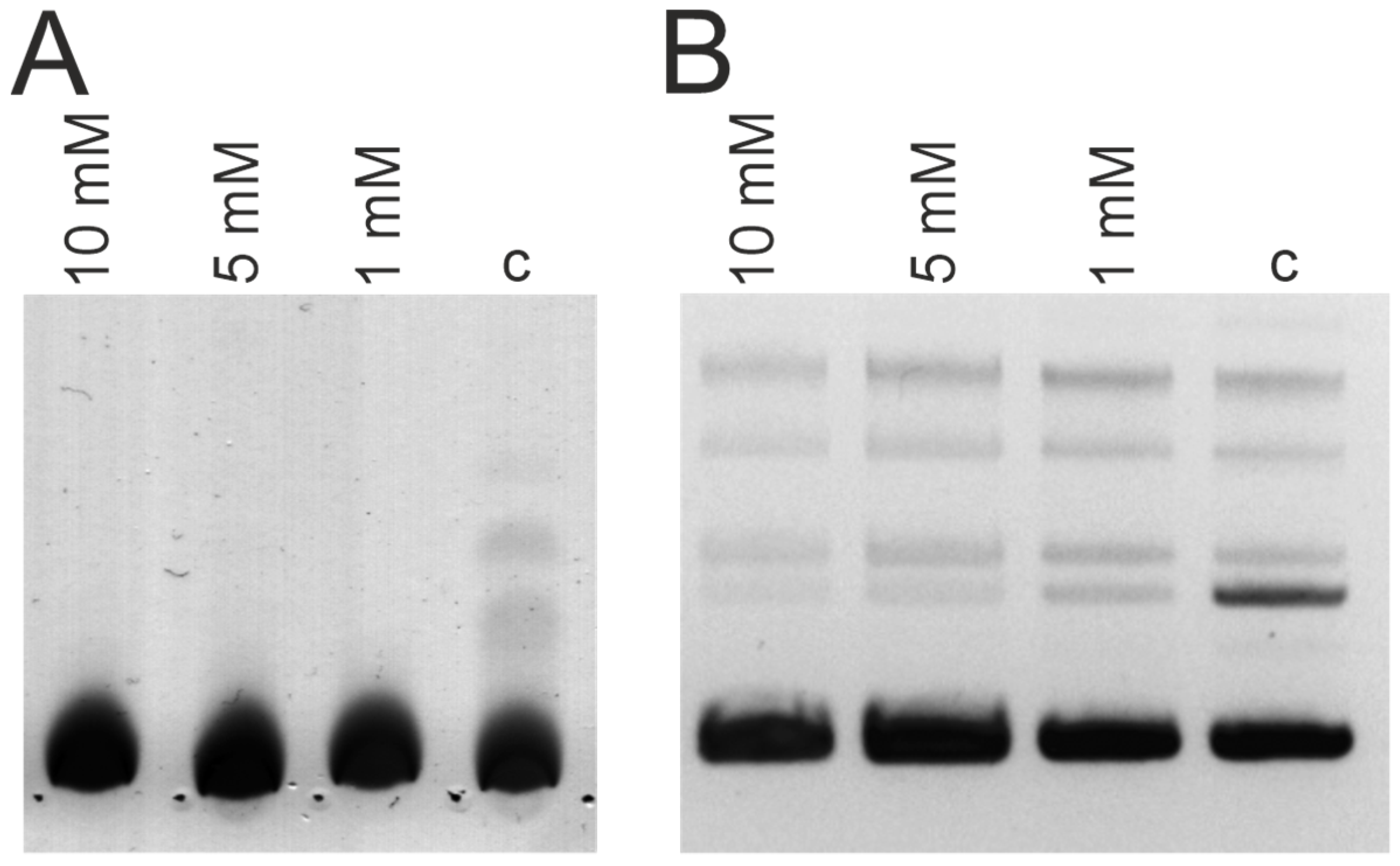
2.4. Analysis of DNase Activity
2.5. Analysis of Amylolytic Activity
2.6. Statistical Analysis
3. Results
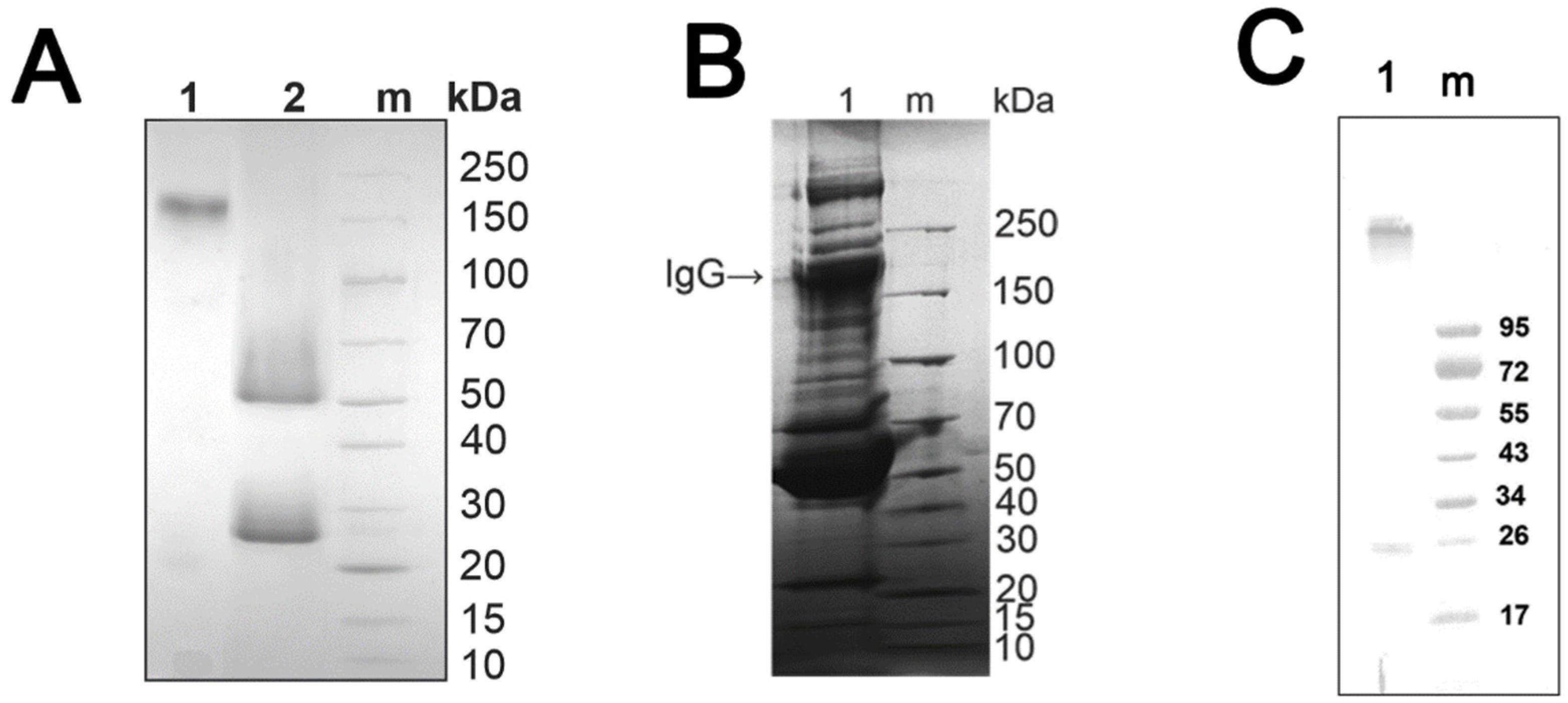
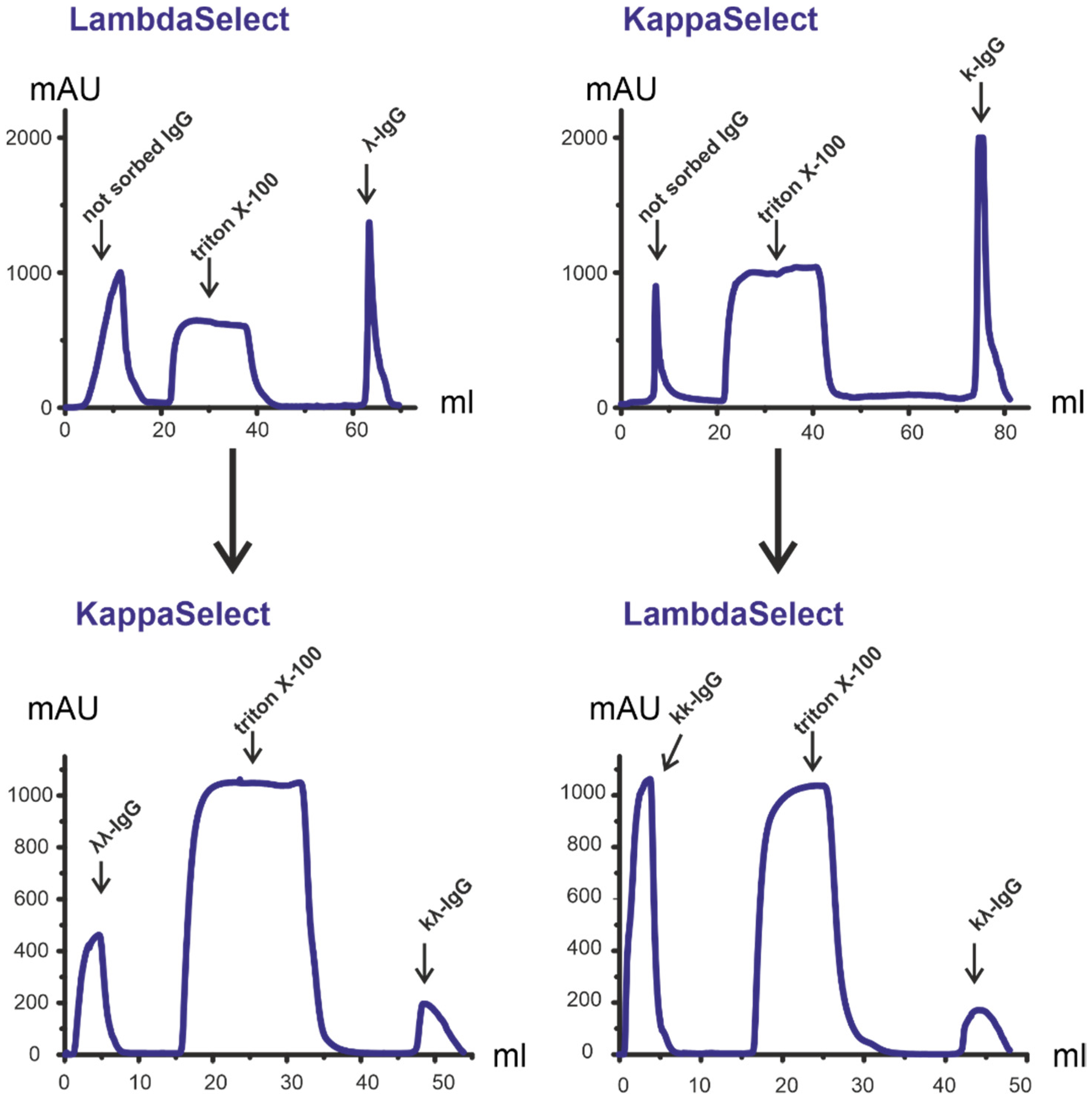
3.1. Characteristics of Patients: Isolation of Antibodies
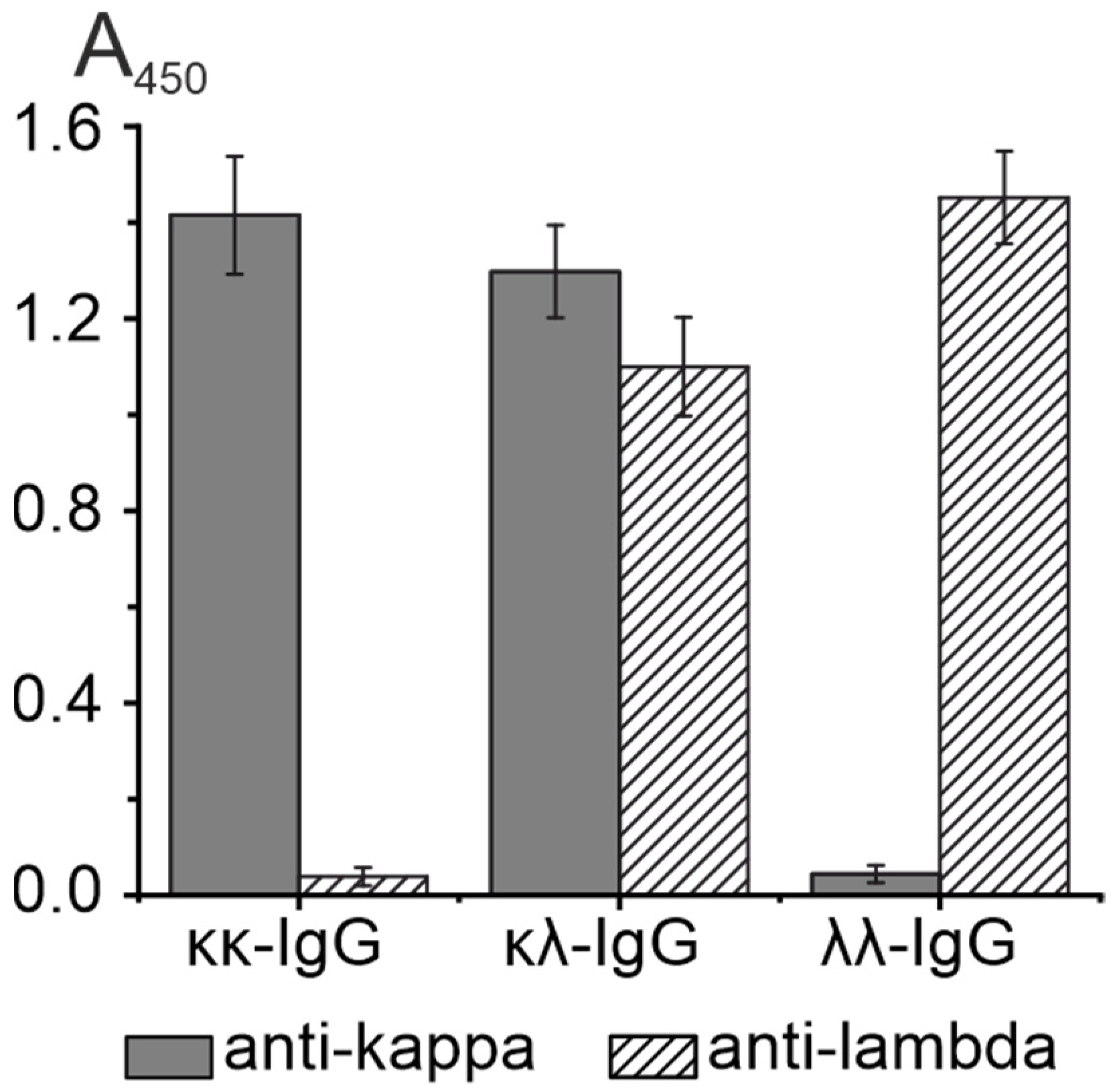
3.2. Isolation and Analysis of κκ-IgG, λλ-IgG and κλ-IgG Subfractions
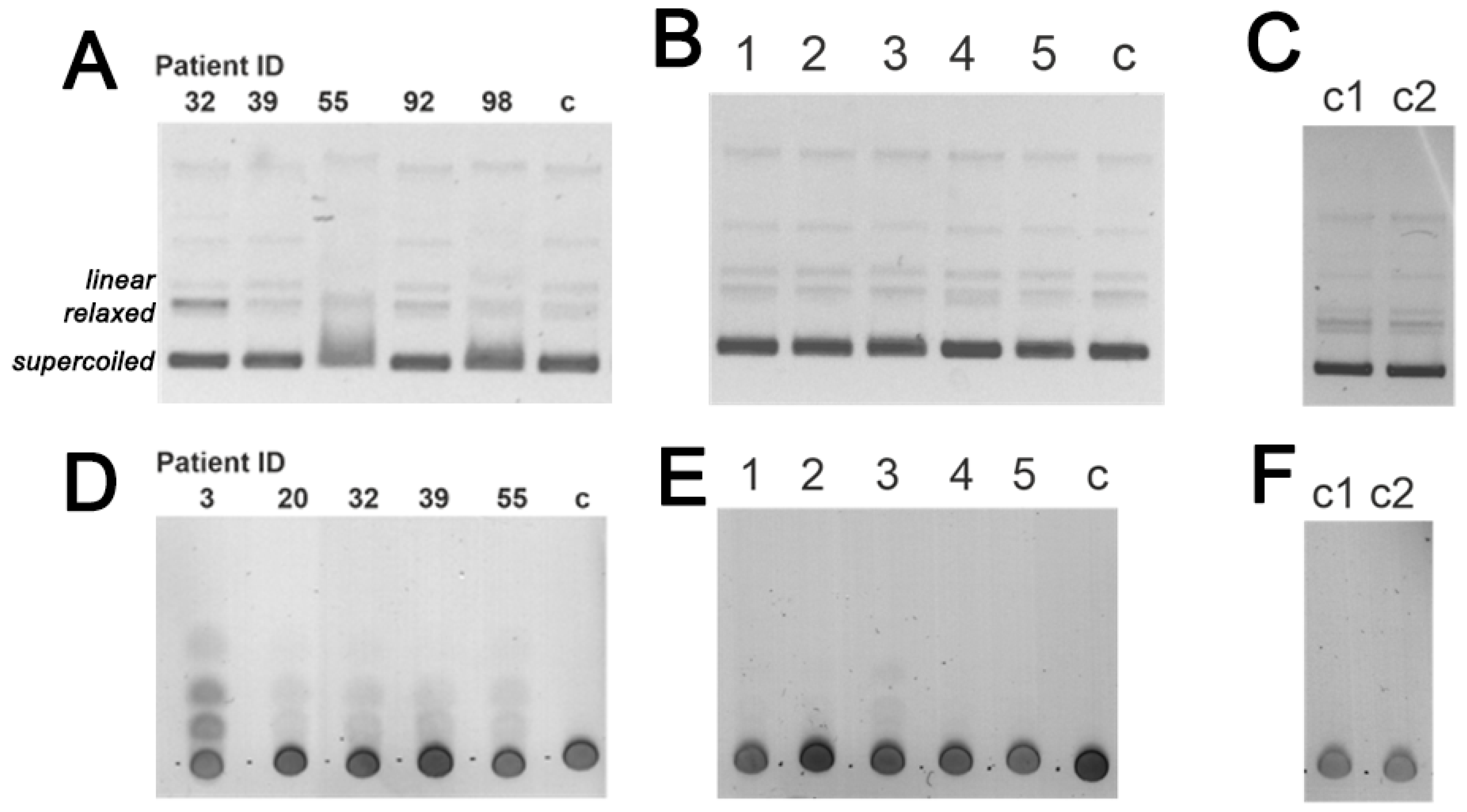
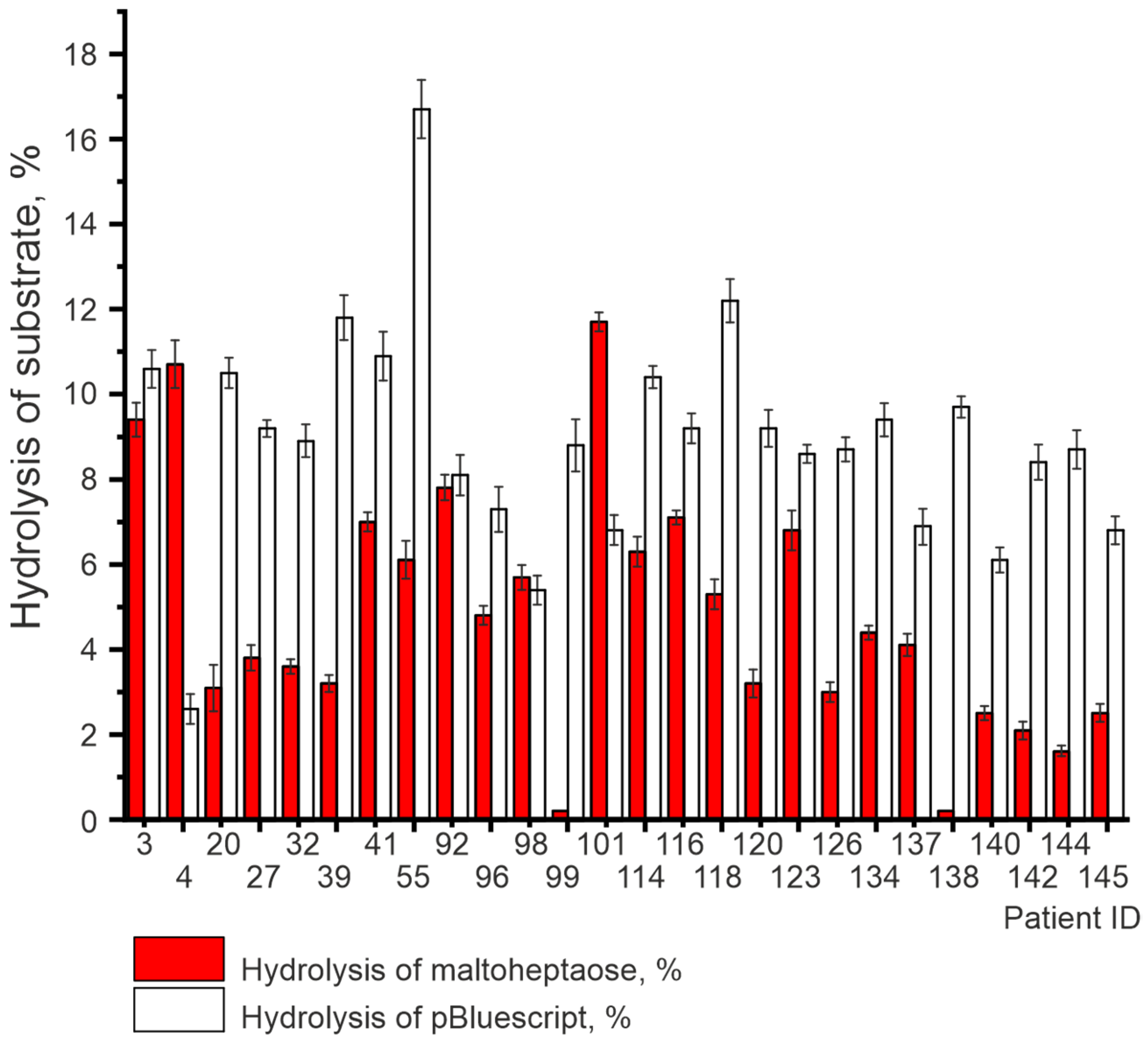
3.3. DNase and Amylolytic Activities
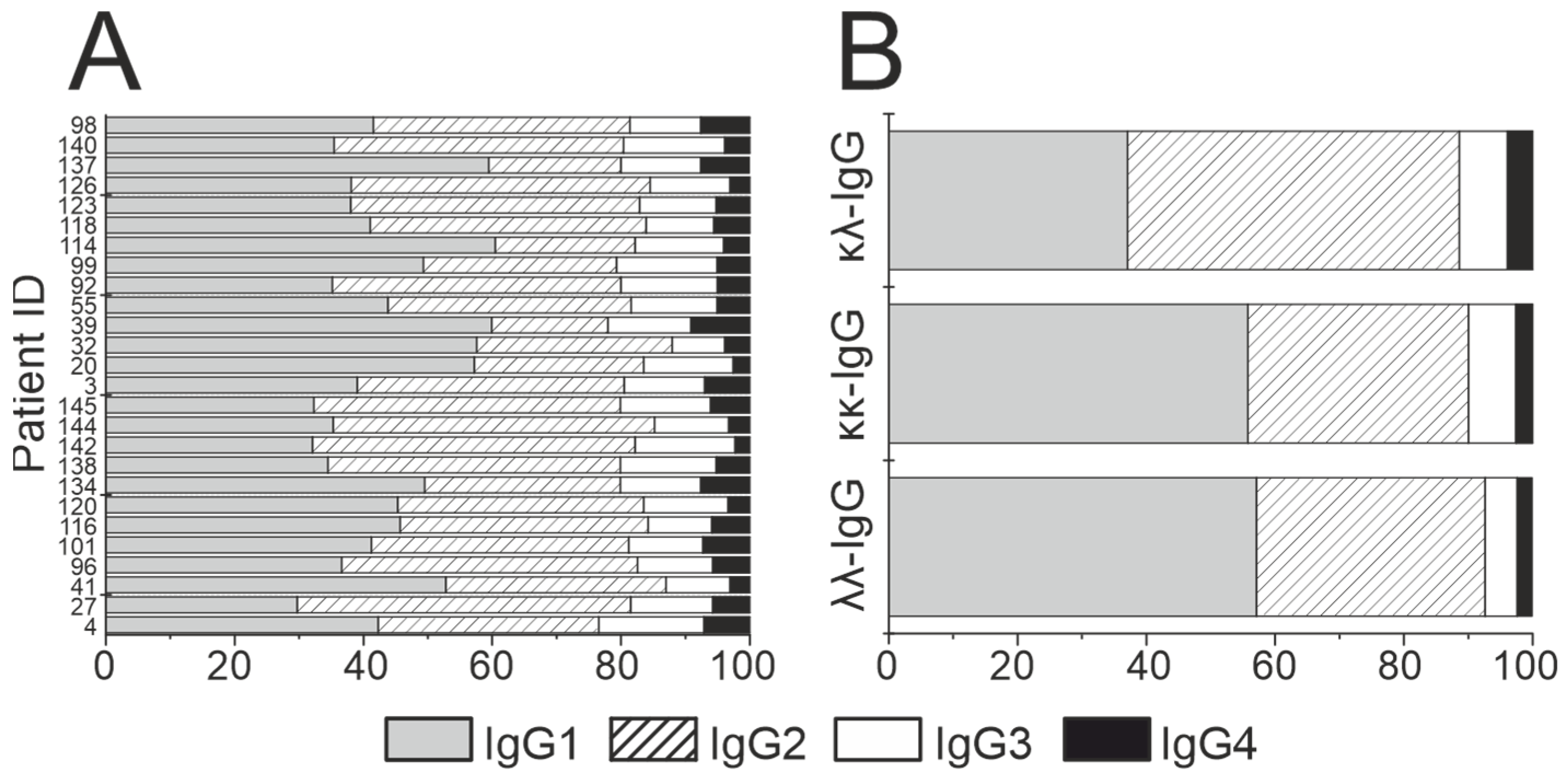
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
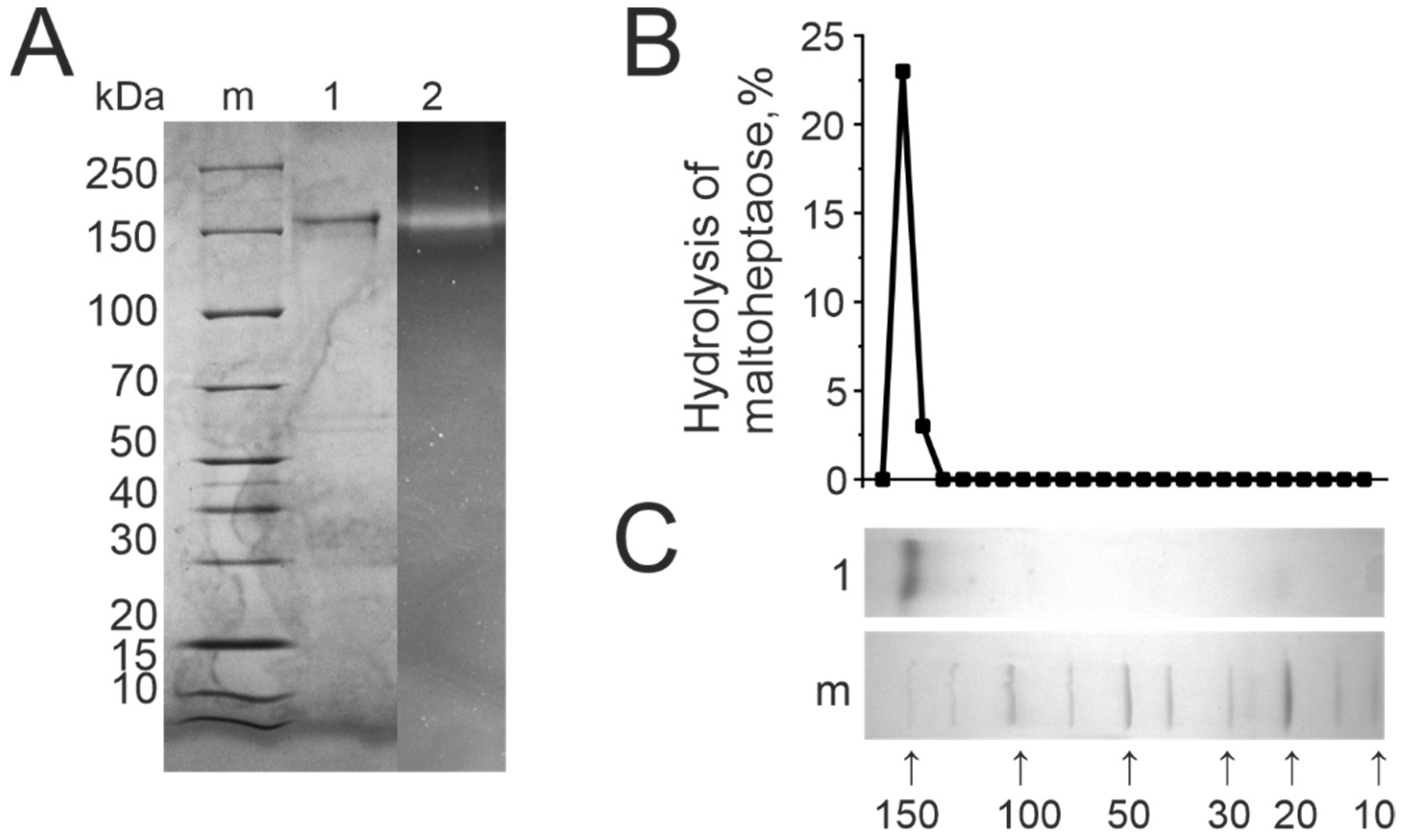
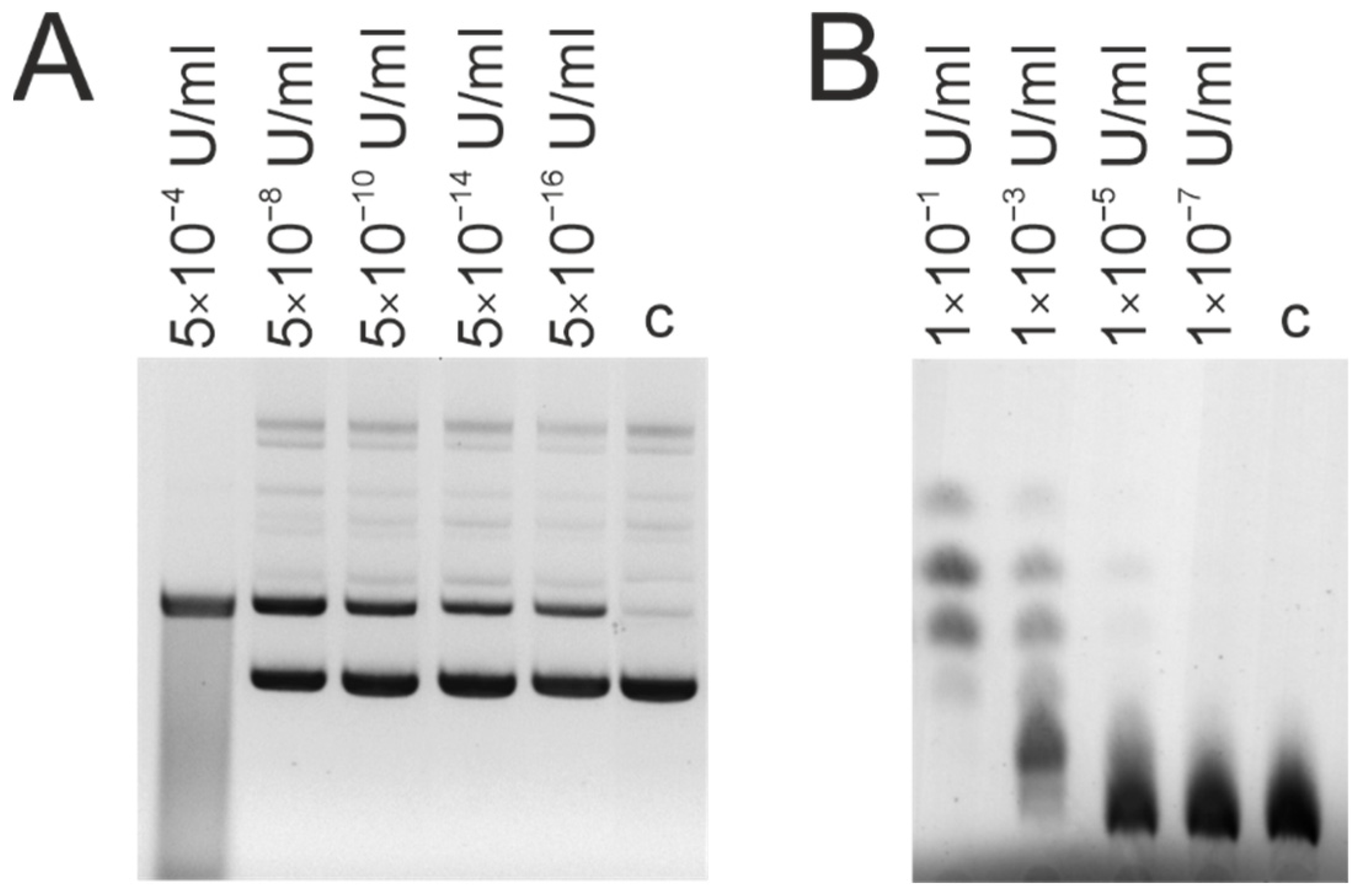
| Patient ID | DNase Activity, U/mg of IgG | Amylolytic Activity, U/mg of IgG |
|---|---|---|
| 3 | 6.6 × 10−16 | 2.2 × 10−8 |
| 20 | 3.3 × 10−16 | 9.2 × 10−9 |
| 32 | 0.8 × 10−16 | 9.9 × 10−9 |
| 39 | 1.1 × 10−15 | 9.3 × 10−9 |
| 55 | 1.1 × 10−13 | 1.4 × 10−8 |
| 92 | 3.5 × 10−17 | 1.7 × 10−8 |
| 98 | 2.8 × 10−18 | 1.3 × 10−8 |
| 99 | 0.7 × 10−16 | 0 |
| 114 | 3.0 × 10−16 | 1.4 × 10−8 |
| 118 | 1.6 × 10−15 | 1.2 × 10−8 |
| 123 | 0.6 × 10−16 | 1.5 × 10−8 |
| 126 | 0.6 × 10−16 | 9.1 × 10−9 |
| 137 | 1.1 × 10−17 | 1.1 × 10−8 |
| 138 | 1.6 × 10−16 | 0 |
| 140 | 0.6 × 10−17 | 8.5 × 10−9 |
| 142 | 4.6 × 10−17 | 8.0 × 10−9 |
| 144 | 0.6 × 10−16 | 7.5 × 10−9 |
| 145 | 1.0 × 10−17 | 8.5 × 10−9 |
| 4 | 2.0 × 10−19 | 2.6 × 10−8 |
| 27 | 1.0 × 10−16 | 1.0 × 10−8 |
| 41 | 4.8 × 10−16 | 1.6 × 10−8 |
| 96 | 1.6 × 10−17 | 1.2 × 10−8 |
| 101 | 1.0 × 10−17 | 3.0 × 10−8 |
| 116 | 1.0 × 10−16 | 1.6 × 10−8 |
| 120 | 1.0 × 10−16 | 9.3 × 10−9 |
| 134 | 1.2 × 10−16 | 1.1 × 10−8 |
References
- Weiss, R. How does HIV cause AIDS? Science 1993, 260, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Douek, D.C.; Roederer, M.; Koup, R.A. Emerging Concepts in the Immunopathogenesis of AIDS. Annu. Rev. Med. 2009, 60, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Kurtz, S.E.; Blauvelt, A.; Shimada, S. The role of Langerhans cells in the sexual transmission of HIV. J. Dermatol. Sci. 2005, 40, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Hämmerle, S.; Rothenaigner, I.; Wolff, H.; Bell, J.E.; Brack-Werner, R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005, 111, 194–213. [Google Scholar] [CrossRef]
- Holmes, C.B.; Losina, E.; Walensky, R.P.; Yazdanpanah, Y.; Freedberg, K.A. Review of Human Immunodeficiency Virus Type 1–Related Opportunistic Infections in Sub-Saharan Africa. Clin. Infect. Dis. 2003, 36, 652–662. [Google Scholar] [CrossRef]
- Alimonti, J.B.; Ball, T.B.; Fowke, K.R. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 2003, 84, 1649–1661. [Google Scholar] [CrossRef]
- Guss, D.A. The acquired immune deficiency syndrome: An overview for the emergency physician, Part 2. J. Emerg. Med. 1994, 12, 491–497. [Google Scholar] [CrossRef]
- Ternynck, T.; Falanga, P.B.; Unterklrscher, C.; Gregolre, J.; da Silva, L.P.; Avrameas, S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. Int. Immunol. 1991, 3, 29–37. [Google Scholar] [CrossRef]
- Hentati, B.; Sato, M.N.; Payelle-Brogard, B.; Avrameas, S.; Ternynck, T. Beneficial effect of polyclonal immunoglobulins from malaria-infected BALB/c mice on the lupus-like syndrome of (NZB × NZW)F1 mice. Eur. J. Immunol. 1994, 24, 8–15. [Google Scholar] [CrossRef]
- Barzilai, O.; Ram, M.; Shoenfeld, Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007, 19, 636–643. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Shoenfeld, Y. HIV and autoimmunity. Autoimmun. Rev. 2002, 1, 329–337. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Sizyakina, L.P.; Buneva, V.N.; Nevinsky, G.A. DNA-hydrolyzing IgG antibodies from the blood of patients with acquired immune deficiency syndrome. Mol. Biol. 2006, 40, 770–777. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Buneva, V.N.; Nevinsky, G.A. Autoantibodies in HIV-infected patients: Cross site-specific hydrolysis of H1 histone and myelin basic protein. BioFactors 2019, 45, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies from the sera of HIV-infected patients efficiently hydrolyze all human histones. J. Mol. Recognit. 2016, 29, 346–362. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H1 histone from the sera of HIV-infected patients recognize and catalyze site-specific degradation of this histone. J. Mol. Recognit. 2017, 30, e2588. [Google Scholar] [CrossRef] [PubMed]
- Baranova, S.V.; Dmitrienok, P.S.; Ivanisenko, N.V.; Buneva, V.N.; Nevinsky, G.A. Antibodies to H2a and H2b histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. Mol. BioSyst. 2017, 13, 1090–1101. [Google Scholar] [CrossRef]
- Baranova, S.V.; Dmitrenok, P.S.; Zubkova, A.D.; Ivanisenko, N.V.; Odintsova, E.S.; Buneva, V.N.; Nevinsky, G.A. Antibodies against H3 and H4 histones from the sera of HIV-infected patients catalyze site-specific degradation of these histones. J. Mol. Recognit. 2018, 31, e2703. [Google Scholar] [CrossRef]
- Baranova, S.V.; Buneva, V.N.; Kharitonova, M.A.; Sizyakina, L.P.; Calmels, C.; Andreola, M.-L.; Parissi, V.; Nevinsky, G. HIV-1 integrase-hydrolyzing antibodies from sera of HIV-infected patients. Biochimie 2009, 91, 1081–1086. [Google Scholar] [CrossRef]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Rasskazov, V.A.; Calmels, C.; Parissi, V.; Andreola, M.-L.; Buneva, V.N.; Zakharova, O.D.; Nevinsky, G. a Antibodies to HIV integrase catalyze site-specific degradation of their antigen. Int. Immunol. 2011, 23, 601–612. [Google Scholar] [CrossRef][Green Version]
- Odintsova, E.S.; Baranova, S.V.; Dmitrenok, P.S.; Calmels, C.; Parissi, V.; Andreola, M.-L.; Buneva, V.N.; Nevinsky, G.A. Anti-integrase abzymes from the sera of HIV-infected patients specifically hydrolyze integrase but nonspecifically cleave short oligopeptides. J. Mol. Recognit. 2012, 25, 193–207. [Google Scholar] [CrossRef]
- Sizyakina, L.P.; Buneva, V.N.; Nevinsky, G.A.; Odintsova, E.S.; Kharitonova, M.A.; Baranovskii, A.G.; Sizyakina, L.P.; Buneva, V.N.; Nevinsky, G.A. Proteolytic activity of IgG antibodies from blood of acquired immunodeficiency syndrome patients. Biochemistry 2006, 71, 251–261. [Google Scholar] [CrossRef]
- Nevinsky, G.A. Natural Catalytic Antibodies in Norm and in HIV-Infected Patients. In Understanding HIV/AIDS Management and Care-Pandemic Approaches in the 21st Century; InTech: London, UK, 2011. [Google Scholar]
- Dubrovskaya, V.V.; Andryushkova, A.S.; Kuznetsova, I.A.; Toporkova, L.B.; Buneva, V.N.; Orlovskaya, I.A.; Nevinsky, G.A. DNA-hydrolyzing antibodies from sera of autoimmune-prone MRL/MpJ-lpr mice. Biochemistry 2003, 68, 1081–1088. [Google Scholar] [CrossRef]
- Andryushkova, A.A.; Kuznetsova, I.A.; Bineva, V.N.; Toporkova, L.B.; Sakhno, L.V.; Tikhonova, M.A.; Chernykh, E.R.; Orlovskaya, I.A.; Nevinsky, G.A. Formation of different abzymes in autoimmune-prone MRL-lpr/lpr mice is associated with changes in colony formation of haematopoietic progenitors. J. Cell. Mol. Med. 2007, 11, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Systemic Lupus Erythematosus and DNA Degradation and Elimination Defects. Front. Immunol. 2019, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Nevinsky, G.A. Catalytic Antibodies in Norm and Systemic Lupus Erythematosus. In Lupus; InTech: London, UK, 2017. [Google Scholar]
- Shoenfeld, Y.; Ben-Yehuda, O.; Messinger, Y.; Bentwitch, Z.; Rauch, J.; Isenberg, D.I.; Gadoth Gadoth, N. Autoimmune diseases other than lupus share common anti-DNA idiotypes. Immunol. Lett. 1988, 17, 285–291. [Google Scholar] [CrossRef]
- Blanco, F.; Kalsi, J.; Isenberg, D.A. Analysis of antibodies to RNA in patients with systemic lupus erythematosus and other autoimmune rheumatic diseases. Clin. Exp. Immunol. 2008, 86, 66–70. [Google Scholar] [CrossRef]
- Sato, T.; Uchiumi, T.; Arakawa, M.; Kominiami, R. Serological association of lupus autoantibodies to a limited functional domain of 28S ribosomal RNA and to the ribosomal proteins bound to the domain. Clin. Exp. Immunol. 2008, 98, 35–39. [Google Scholar] [CrossRef]
- Buneva, V.N.; Nevinsky, G.A. Exceptional Diversity of Catalytic Antibodies with Varying Activity in the Blood of Autoimmune and Viral Disease Patients. Mol. Biol. 1997, 51, 969–984. [Google Scholar] [CrossRef]
- Saveliev, A.N.; Ivanen, D.R.; Kulminskaya, A.A.; Ershova, N.A.; Kanyshkova, T.G.; Buneva, V.N.; Mogelnitskii, A.S.; Doronin, B.M.; Favorova, O.O.; Nevinsky, G.A.; et al. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol. Lett. 2003, 86, 291–297. [Google Scholar] [CrossRef]
- Doronin, V.B.; Parkhomenko, T.A.; Castellazzi, M.; Cesnik, E.; Buneva, V.N.; Granieri, E.; Nevinsky, G.A. Comparison of Antibodies with Amylase Activity from Cerebrospinal Fluid and Serum of Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0154688. [Google Scholar] [CrossRef]
- Savel’ev, A.N.; Kanyshkova, T.G.; Kulminskaya, A.A.; Buneva, V.N.; Eneyskaya, E.V.; Filatov, M.V.; Nevinsky, G.A.; Neustroev, K.N. Amylolytic activity of IgG and sIgA immunoglobulins from human milk. Clin. Chim. Acta 2001, 314, 141–152. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Smirnova, L.P.; Parkhomenko, T.A.; Dmitrenok, P.S.; Krotenko, N.M.; Fattakhov, N.S.; Bokhan, N.A.; Semke, A.V.; Ivanova, S.A.; Buneva, V.N.; et al. DNA-hydrolysing activity of IgG antibodies from the sera of patients with schizophrenia. Open Biol. 2015, 5, 150064. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Blood-Derived RNA- and microRNA-Hydrolyzing IgG Antibodies in Schizophrenia Patients. Biochemistry 2018, 83, 507–526. [Google Scholar] [CrossRef]
- Bezuglova, A.M.; Konenkova, L.P.; Doronin, B.M.; Buneva, V.N.; Nevinsky, G.A. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J. Mol. Recognit. 2011, 24, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, S.E.; Lekchnov, E.A.; Prince, V.V.; Buneva, V.N.; Nevinsky, G.A. Half molecular exchange of IgGs in the blood of healthy humans: Chimeric lambda-kappa-immunoglobulins containing HL fragments of antibodies of different subclasses (IgG1–IgG4). Mol. BioSyst. 2016, 12, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Parkhomenko, T.A.; Buneva, V.N.; Tyshkevich, O.B.; Generalov, I.I.; Doronin, B.M.; Nevinsky, G.A. DNA-hydrolyzing activity of IgG antibodies from the sera of patients with tick-borne encephalitis. Biochimie 2010, 92, 545–554. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Dmitrenok, P.S.; Konenkova, L.P.; Buneva, V.N.; Nevinsky, G.A. Multiple Sites of the Cleavage of 21- and 25-Mer Encephalytogenic Oligopeptides Corresponding to Human Myelin Basic Protein ( MBP ) by Specific Anti-MBP Antibodies from Patients with Systemic Lupus Erythematosus. PLoS ONE 2013, 8, e51600. [Google Scholar] [CrossRef]
- Andreev, S.L.; Buneva, V.N.; Nevinsky, G.A. How human IgGs against DNA recognize oligonucleotides and DNA. J. Mol. Recognit. 2016, 29, 596–610. [Google Scholar] [CrossRef]
- Gashnikova, N.M.; Astakhova, E.M.; Gashnikova, M.P.; Bocharov, E.F.; Petrova, S.V.; Pun’ko, O.A.; Popkov, A.V.; Totmenin, A.V. HIV-1 Epidemiology, Genetic Diversity, and Primary Drug Resistance in the Tyumen Oblast, Russia. BioMed Res. Int. 2016, 2016, 2496280. [Google Scholar] [CrossRef]
- Maksimenko, L.V.; Totmenin, A.V.; Gashnikova, M.P.; Astakhova, E.M.; Skudarnov, S.E.; Ostapova, T.S.; Yaschenko, S.V.; Meshkov, I.O.; Bocharov, E.F.; Maksyutov, R.A.; et al. Genetic Diversity of HIV-1 in Krasnoyarsk Krai: Area with High Levels of HIV-1 Recombination in Russia. BioMed Res. Int. 2020, 2020, 9057541. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. Human milk IgGs contain various combinations of different antigen-binding sites resulting in multiple variants of their bispecificity. PLoS ONE 2012, 7, e42942. [Google Scholar] [CrossRef] [PubMed]
- Lekchnov, E.A.; Sedykh, S.E.; Dmitrenok, P.S.; Buneva, V.N.; Nevinsky, G.A. Human placenta: Relative content of antibodies of different classes and subclasses (IgG1–IgG4) containing lambda- and kappa-light chains and chimeric lambda-kappa-immunoglobulins. Int. Immunol. 2015, 27, 297–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sedykh, M.A.; Buneva, V.N.; Nevinsky, G.A. Polyreactivity of natural antibodies: Exchange by HL-fragments. Biochemistry 2013, 78, 1305–1320. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Buneva, V.N.; Nevinsky, G.A. Human Milk Lactoferrin and Antibodies: Catalytic Activities, Complexes, and Other Features. In Milk Proteins-From Structure to Biological Properties and Health Aspects; InTech: London, UK, 2016; pp. 51–80. [Google Scholar]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of immunoglobulin G4 Fab-arm exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef]
- Zettervall, O.; Link, H. Electrophoretic distribution of kappa and lambda immunoglobulin light chain determinants in serum and cerebrospinal fluid in multiple sclerosis. Clin. Exp. Immunol. 1970, 7, 365–372. [Google Scholar] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Schuurman, J. IgG4 breaking the rules. Immunology 2002, 105, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zaksas, N.; Gluhcheva, Y.; Sedykh, S.; Madzharova, M.; Atanassova, N.; Nevinsky, G. Effect of CoCl(2) treatment on major and trace elements metabolism and protein concentration in mice. J. Trace Elem. Med. Biol. 2013, 27, 27–30. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of Antibodies. Microbiol. Spectr. 2014, 2, 1–17. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G.A. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Devel. Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Nevinsky, G.A.; Buneva, V.N. Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. Int. J. Mol. Sci. 2020, 21, 5392. [Google Scholar] [CrossRef] [PubMed]
- Doores, K.J. The HIV glycan shield as a target for broadly neutralizing antibodies. FEBS J. 2015, 282, 4679–4691. [Google Scholar] [CrossRef] [PubMed]
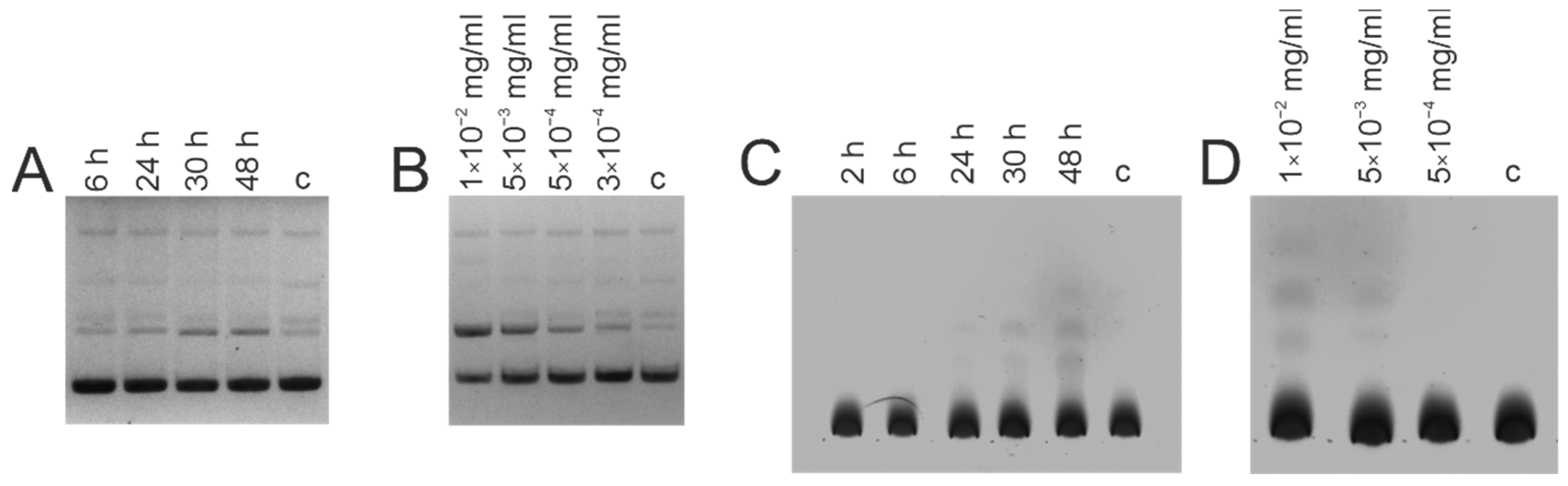
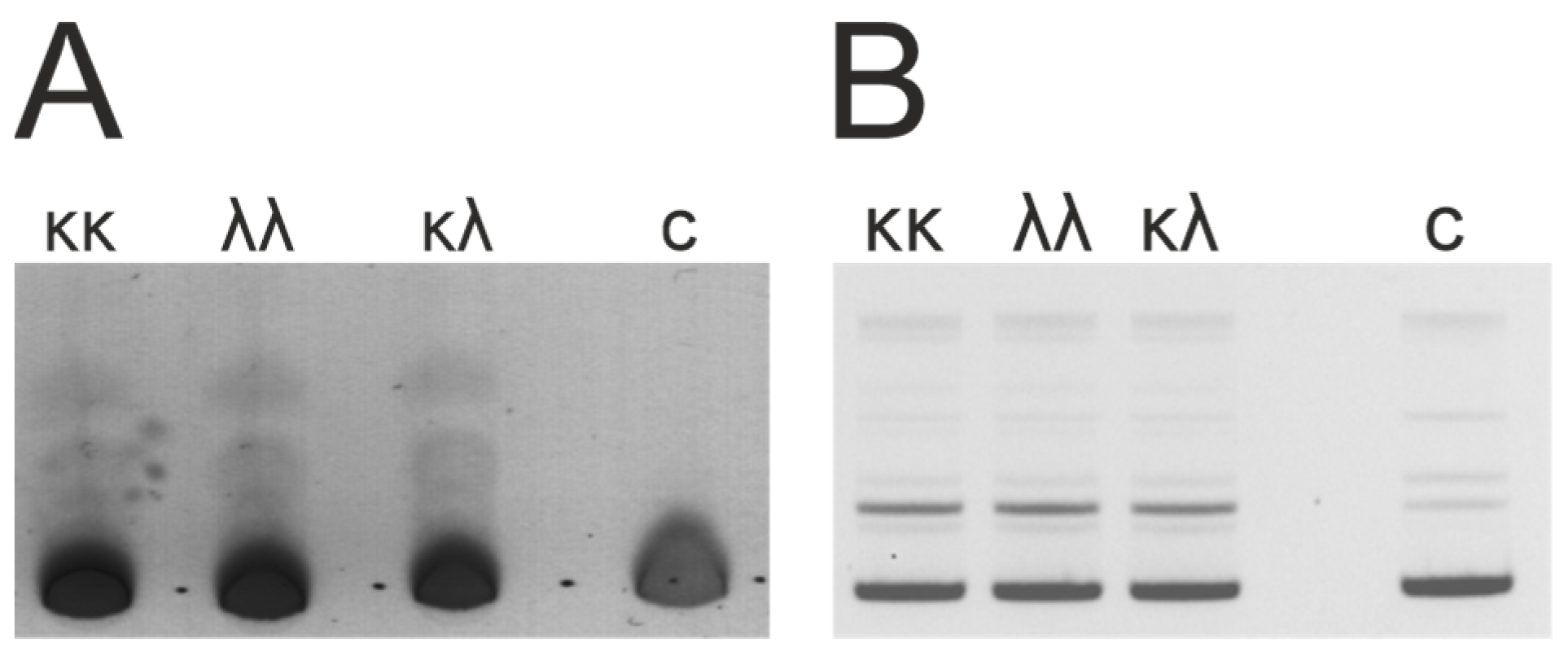
| Patient ID | Age | Sex | HIV Subtype | Route of Infection | Year of Infection (Supposed) | Viral Load (Copies per mL) | CD4 Count (Cells per mL) | Stage of HIV 3 |
|---|---|---|---|---|---|---|---|---|
| 3 | 31 | male | A6 | PWID 1 | 2017 | 27,700 | 556 | 3 6 |
| 4 | 43 | male | CRF63_02A | PWID | 2017 | 69,200 | 568 | 3 |
| 20 | 38 | female | A6 | Heterosexual with PWID | 2016 | 75,000 | 825 | 3 |
| 27 | 45 | female | A6 | Het 2 | 2017 | 31,600 | 558 | 2B 5 |
| 32 | 38 | female | A6 | Het | 2017 | 41,000 | 553 | 3 |
| 39 | 35 | male | A6 | PWID | 2016 | 47,800 | 603 | 3 |
| 41 | 36 | female | A6 | Het | 2017 | 84,600 | 710 | 3 |
| 55 | 34 | female | URF_63/A | PWID | 2015 | 10,500 | 559 | 3 |
| 92 | 27 | female | URF_63/A | Het | 2017 | 13,100 | 540 | 3 |
| 96 | 33 | male | A6 | PWID | 2015 | 54,700 | 576 | 3 |
| 98 | 35 | female | A6 | PWID | 2013 | 25,700 | 597 | 3 |
| 99 | 50 | male | A6 | PWID | 2008 | 32,900 | 520 | 3 |
| 101 | 32 | female | CRF63_02A | PWID | 2015 | 120,000 | 532 | 3 |
| 114 | 36 | male | A6 | PWID | 2007 | 87,000 | 597 | 3 |
| 116 | 40 | male | A6 | PWID | 2016 | 90,500 | 694 | 3 |
| 118 | 47 | male | A6 | PWID | 2014 | 85,400 | 805 | 3 |
| 120 | 33 | female | CRF63_02A | PWID | 2014 | 75,000 | 600 | 3 |
| 123 | 28 | male | A6 | Het | 2017 | 64,500 | 522 | 3 |
| 126 | 39 | female | URF_63/A | Het | 2014 | 97,800 | 501 | 3 |
| 134 | 29 | male | A6 | Het | 2015 | no data | 962 | 3 |
| 137 | 32 | male | URF_63/A | PWID | 2013 | 175,000 | 518 | 4A 7 |
| 138 | 39 | female | A6 | Het | 2016 | 150,000 | 540 | 3 |
| 140 | 44 | male | A6 | PWID | 2008 | 78,400 | 703 | 3 |
| 142 | 32 | female | A6 | Het | 2016 | 19,000 | 636 | 3 |
| 144 | 30 | male | A6 | Het | 2016 | 87,400 | 530 | 3 |
| 145 | 33 | female | A6 | Heterosexual with PWID | 2017 | 34,800 | 790 | 2A 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, A.; Sedykh, S.; Maksimenko, L.; Sedykh, T.; Skudarnov, S.; Ostapova, T.; Yaschenko, S.; Gashnikova, N.; Nevinsky, G. The Blood of the HIV-Infected Patients Contains κ-IgG, λ-IgG, and Bispecific κλ-IgG, Which Possess DNase and Amylolytic Activity. Life 2022, 12, 304. https://doi.org/10.3390/life12020304
Timofeeva A, Sedykh S, Maksimenko L, Sedykh T, Skudarnov S, Ostapova T, Yaschenko S, Gashnikova N, Nevinsky G. The Blood of the HIV-Infected Patients Contains κ-IgG, λ-IgG, and Bispecific κλ-IgG, Which Possess DNase and Amylolytic Activity. Life. 2022; 12(2):304. https://doi.org/10.3390/life12020304
Chicago/Turabian StyleTimofeeva, Anna, Sergey Sedykh, Lada Maksimenko, Tatyana Sedykh, Sergey Skudarnov, Tatyana Ostapova, Svetlana Yaschenko, Natalya Gashnikova, and Georgy Nevinsky. 2022. "The Blood of the HIV-Infected Patients Contains κ-IgG, λ-IgG, and Bispecific κλ-IgG, Which Possess DNase and Amylolytic Activity" Life 12, no. 2: 304. https://doi.org/10.3390/life12020304
APA StyleTimofeeva, A., Sedykh, S., Maksimenko, L., Sedykh, T., Skudarnov, S., Ostapova, T., Yaschenko, S., Gashnikova, N., & Nevinsky, G. (2022). The Blood of the HIV-Infected Patients Contains κ-IgG, λ-IgG, and Bispecific κλ-IgG, Which Possess DNase and Amylolytic Activity. Life, 12(2), 304. https://doi.org/10.3390/life12020304








