Study of Hypersensitivity to Malassezia furfur in Patients with Atopic Dermatitis with Head and Neck Pattern: Is It Useful as a Biomarker and Therapeutic Indicator in These Patients?
Abstract
:1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdács, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A Multicenter Study on the Prevalence of Clinical Patterns and Clinical Phenotypes in Adult Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Jaros, J.; Hendricks, A.J.; Shi, V.Y.; Lio, P.A. A Practical Approach to Recalcitrant Face and Neck Dermatitis in Atopic Dermatitis. Dermatitis 2020, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ribero, S.; Puglisi, B.; Giura, M.T.; Viola, R.; Siliquini, N.; Quaglino, P.; Ortoncelli, M. Head and neck severity index is associated to a significant worsening of quality of life in atopic dermatitis patients. Exp. Dermatol. 2021, 30, 1717–1718. [Google Scholar] [CrossRef] [PubMed]
- Bayrou, O.; Pecquet, C.; Flahault, A.; Artigou, C.; Abuaf, N.; Leynadier, F. Head and neck atopic dermatitis and Malassezia-furfur-specific IgE antibodies. Dermatology 2005, 211, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Tanaka, T.; Tajima, M.; Tsuboi, R.; Kato, H.; Nishikawa, A.; Sugita, T. Anti-Malassezia-Specific IgE Antibodies Production in Japanese Patients with Head and Neck Atopic Dermatitis: Relationship between the Level of Specific IgE Antibody and the Colonization Frequency of Cutaneous Malassezia Species and Clinical Severity. J. Allergy 2011, 2011, 645670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzecry, V.; Pravettoni, V.; Segatto, G.; Marzano, A.V.; Ferrucci, S. Type 2 Inflammation: Atopic Dermatitis, Asthma, and Hypereosinophilia Successfully Treated With Dupilumab. J. Investig. Allergol. Clin. Immunol. 2021, 31, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.F.; Ong, R.Y.; Common, J.E. Skin microbiome of atopic dermatitis. Allergol. Int. 2021, 71, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, R.; Wang, R. Dysbiosis of skin mycobiome in atopic dermatitis. Mycoses 2021. [Google Scholar] [CrossRef] [PubMed]
- Aspres, N.; Anderson, C. Malassezia yeasts in the pathogenesis of atopic dermatitis. Australas. J. Dermatol. 2004, 45, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Buentke, E.; Zargari, A.; Heffler, L.C.; Avila-Cariño, J.; Savolainen, J.; Scheynius, A. Uptake of the yeast Malassezia furfur and its allergenic components by human immature CD1a+ dendritic cells. Clin. Exp. Allergy 2000, 30, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Buentke, E.; Heffler, L.C.; Wallin, R.P.A.; Löfman, C.; Ljunggren, H.G.; Scheynius, A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin. Exp. Allergy 2001, 31, 1583–1593. [Google Scholar] [CrossRef]
- Jaulent, L.; Staumont-Sallé, D.; Tauber, M.; Paul, C.; Aubert, H.; Marchetti, A.; Sassolas, B.; Valois, A.; Nicolas, J.; Nosbaum, A.; et al. De novo psoriasis in atopic dermatitis patients treated with dupilumab: A retrospective cohort. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e296–e297. [Google Scholar] [CrossRef]
- Tao, R.; Wang, R.; Wan, Z.; Song, Y.; Wu, Y.; Li, R. Ketoconazole 2% Cream Alters the Skin Fungal Microbiome in Seborrheic Dermatitis: A Cohort Study. Clin. Exp. Dermatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kaffenberger, B.H.; Mathis, J.; Zirwas, M.J. A retrospective descriptive study of oral azole antifungal agents in patients with patch test–negative head and neck predominant atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 480–483. [Google Scholar] [CrossRef] [PubMed]
- De Beer, F.S.A.; Bakker, D.S.; Haeck, I.; Ariens, L.; van der Schaft, J.; van Dijk, M.R.; de Bruin-Weller, M.S. Dupilumab facial redness: Positive effect of itraconazole. JAAD Case Rep. 2019, 5, 888–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okiyama, N.; Nakamura, Y.; Ishitsuka, Y.; Inoue, S.; Kubota, N.; Saito, A.; Watanabe, R.; Fujisawa, Y.; Igawa, K. Successful topical treatment with ketoconazole for facial rashes refractory to dupilumab in patients with atopic dermatitis: Case reports. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e474–e476. [Google Scholar] [CrossRef] [PubMed]
- Amir Ali, A.; Vender, R.; Vender, R. The Role of IL-17 in Papulopustular Rosacea and Future Directions. J. Cutan. Med. Surg. 2019, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Eshaghi, H.; Linder, M.T.; Scheynius, A.; Jakobson, E. Positive Atopy Patch Test Reaction to Malassezia furfur in Atopic Dermatitis Correlates with a T Helper 2-like Peripheral Blood Mononuclear Cells Response. J. Investig. Dermatol. 2002, 118, 1044–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
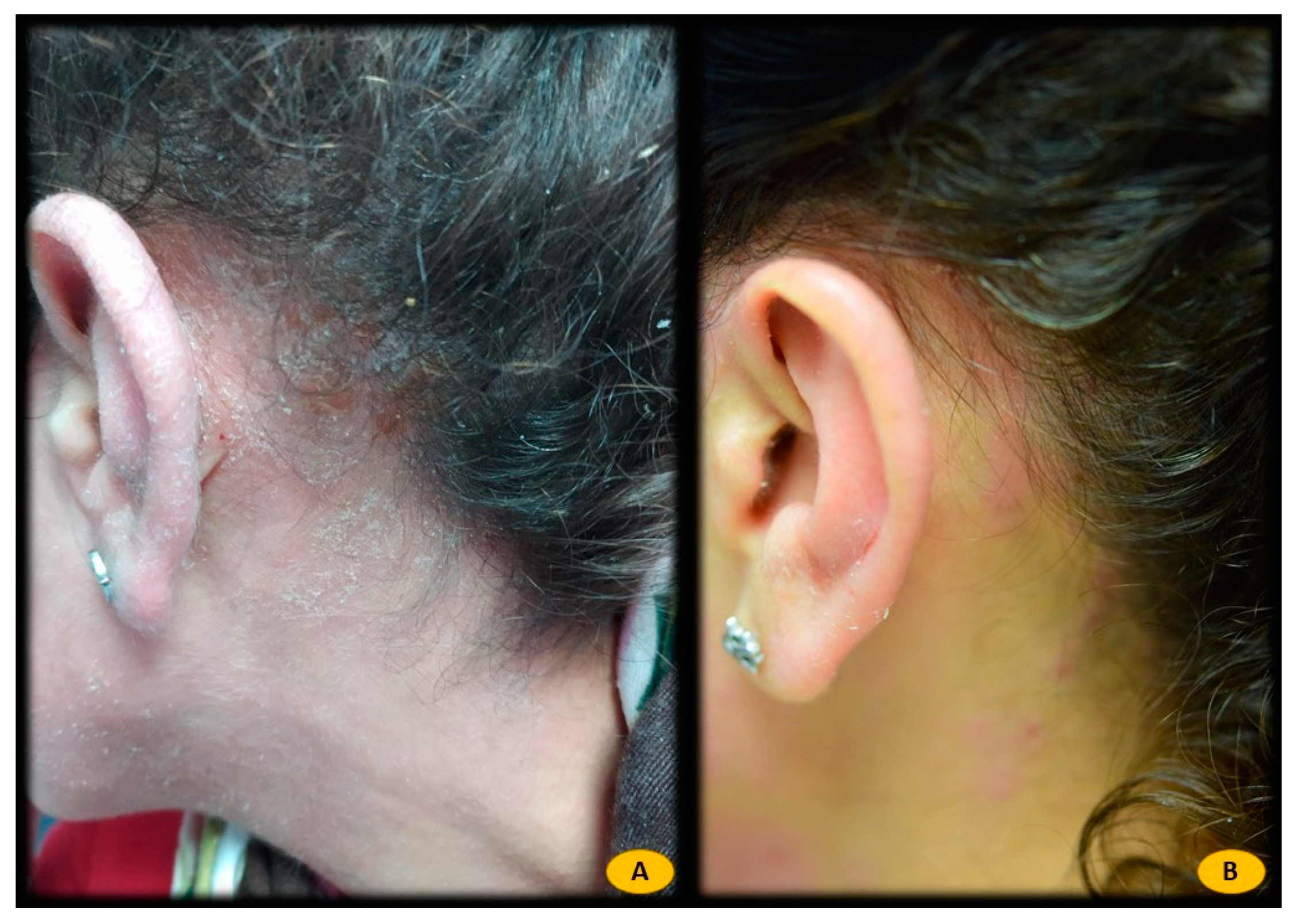
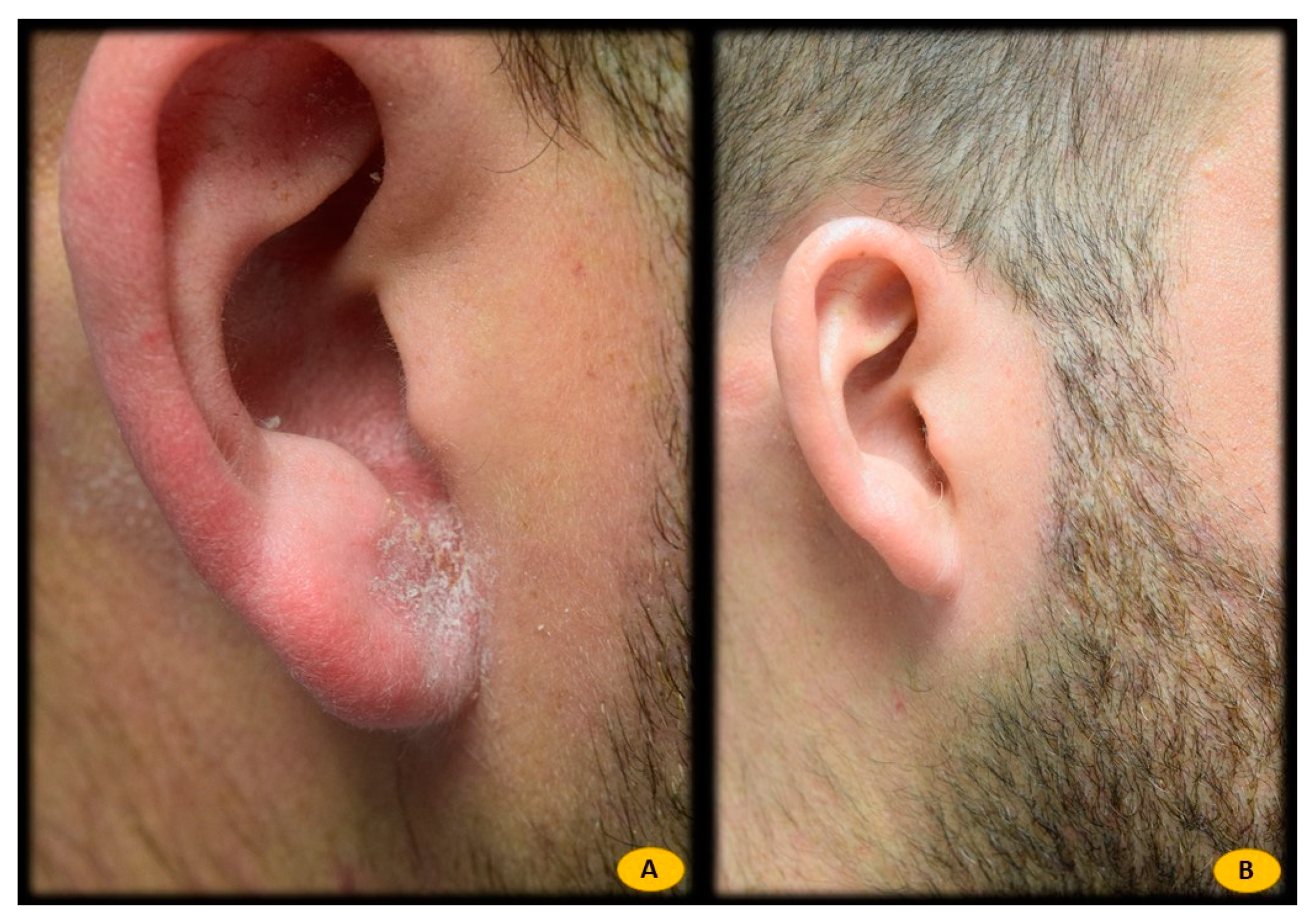
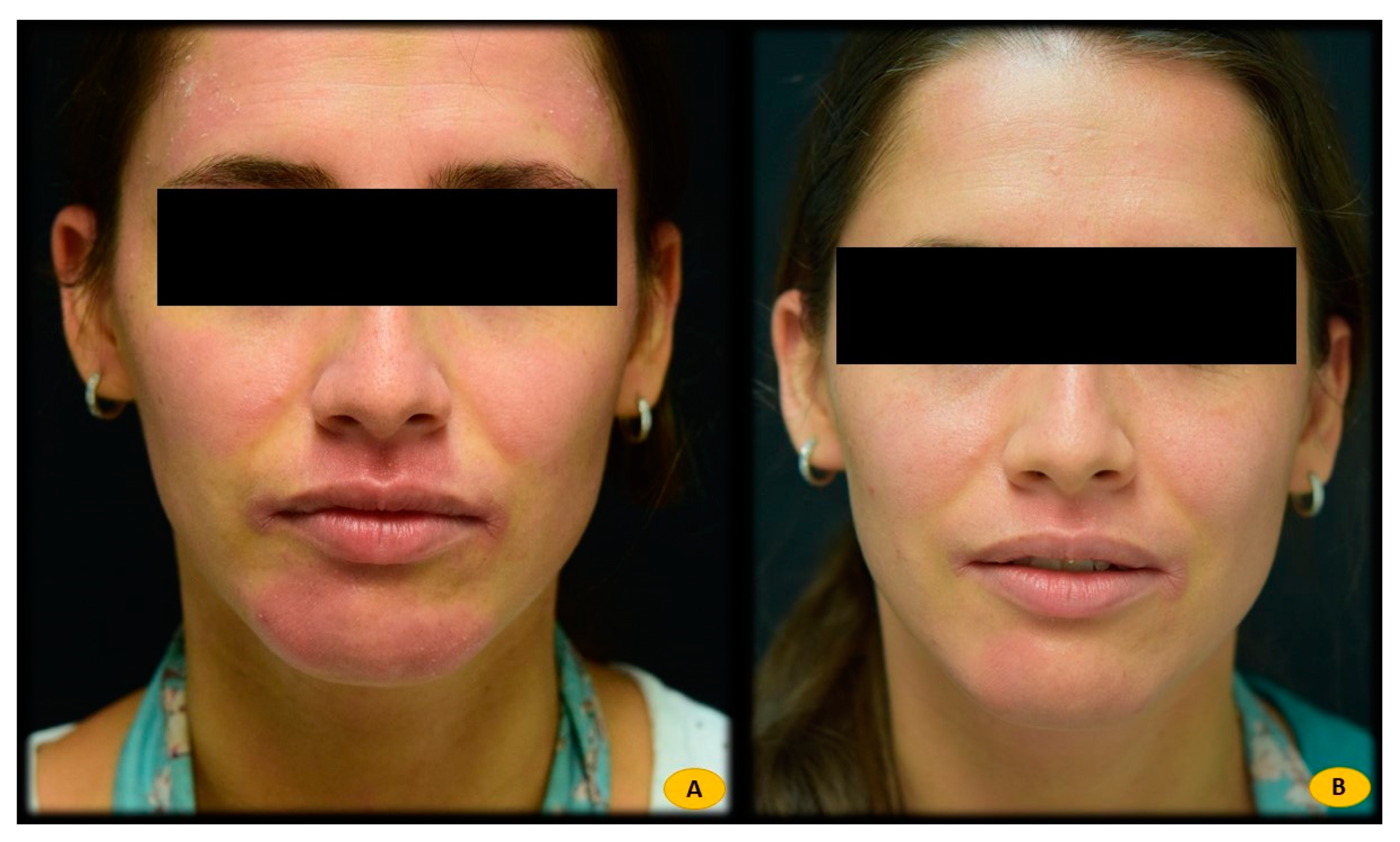
| Atopic Dermatitis | Seborrheic Dermatitis | Healthy Controls | |
|---|---|---|---|
| n | 25 | 14 | 19 |
| Age, years (mean ± SD) | 31.92 ± 10.26 | 38.64 ± 17.08 | 36.58 ± 13.32 |
| Sex ratio (M/F) * | 0.48 | 0.79 | 0.63 |
| Weight (mean ± SD) | 73.24 ± 16.22 | 82.86 ± 4.88 | 72.11 ± 9.78 |
| H&N involvement * | 68% | 100% | 0% |
| Asthma history * | 52% | 0% | 5% |
| Pneumoallergens history * | 88% | 15% | 0% |
| Total IgE, IU/mL (mean ± SD) * | 2210.96 ± 3260.30 | 241.37 ± 481.33 | 36.3 ± 54.56 |
| M. furfur IgE > 0.35 (Ku.arb./L) * | 80% | 0% | 0% |
| Total LDH level (U/L) | 211.44 ± 53.62 | 205.33 ± 35.20 | 189.83 ± 21.71 |
| Total CRP level (mg/L) | 1.44 ± 0.82 | 3.08 ± 2.40 | 1.96 ± 0.99 |
| Dupilumab | No Dupilumab | |
|---|---|---|
| n | 15 | 10 |
| Age, years (mean ± SD) | 34.33 ± 10.97 | 28.30 ± 8.33 |
| Sex ratio (M/F) | 0.60 | 0.30 |
| Weight (mean ± SD) * | 79.53 ± 16.42 | 63.80 ± 10.84 |
| H&N involvement * | 100% | 20% |
| Asthma history | 47% | 60% |
| Pneumoallergens history | 87% | 90% |
| Total IgE, IU/mL (mean ± SD) | 1772.48 ± 3053.84 | 2868.67 ± 3610.73 |
| M. furfur IgE > 0.35 (Ku.arb./L) | 93% | 60% |
| Total LDH level (U/L) | 201.93 ± 22.76 | 225.7 ± 80.53 |
| Total CRP level (mg/L) | 1.47 ± 0.92 | 1.40 ± 0.71 |
| Dermatological Diseases | Clinical Features | Diagnostic Approach |
|---|---|---|
| Dupilumab facial redness | Fixed erythema located on the face or neck (may also occur extrafacially) Usually unilateral Resistant to corticosteroids and/or topical calcineurin inhibitors Patient on dupilumab treatment | Clinical Temporal correlation between dupilumab initiation and onset of erythema red facial erythema |
| Seborrhoeic dermatitis | Pityriasiform lesions (whitish “dry” scale) over orange erythema on nasolabial folds, ciliary area, beard or sideburn area, scalp or external auditory canal. | Clinical |
| Rosacea | Persistent malar erythema or with flushing exacerbations Telangiectasias of different calibre Papules and/or pustules | Clinical Dermoscopy Microscopic examination of Demodex folliculorum with tape test, scraping, etc. |
| Demodicosis | Facial itching and/or burning sensation (especially on the cheeks) Erythematous dilatation of facial follicular openings seen on dermoscopy | Clinical Dermoscopy Microscopic examination of Demodex folliculorum with tape test, scraping, etc. |
| Dermatitis perioralis | Monomorphous papular rash localised in the perioral region Asymptomatic | Clinical |
| Allergic contact dermatitis | Erythematous, scaly, very pruritic rash. Special patterns: palpebral, hairline, lateral facial and cervical sides, usually symmetrical | Patch testing with standard and specific series |
| Airborne dermatitis | Facial and cervical erythematous-squamous rash, with involvement of the eyelids, retroauricular, and submandibular areas. Respect of the nasal triangle | Allergological study by means of: - Specific IgE in serum for pneumoallergens. - Allergen specific prick test |
| Malassezia head/neck dermatitis | Facial and cervical rash mainly localised in seborrheic-like areas Patients on dupilumab treatment Age from adolescence onwards (infrequent in childhood) Refractoriness to topical corticosteroids and/or topical calcineurin inhibitors May appear de novo or be persistent after starting dupilumab treatment | Malassezia furfur-specific IgE serum (>0.35 IU/mL) Clinical response to treatment with topical and/or oral antifungals |
| Topical steroid withdrawal | More frequent in women Prolonged use of topical corticosteroids Erythematous hypersensitive skin appearance Local sensation of itching, heat, pain, burning, etc. | Anamnesis (confirmation of chronic use of topical corticosteroids on the face) Clinical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Triviño, F.J.; Ayén-Rodríguez, Á. Study of Hypersensitivity to Malassezia furfur in Patients with Atopic Dermatitis with Head and Neck Pattern: Is It Useful as a Biomarker and Therapeutic Indicator in These Patients? Life 2022, 12, 299. https://doi.org/10.3390/life12020299
Navarro-Triviño FJ, Ayén-Rodríguez Á. Study of Hypersensitivity to Malassezia furfur in Patients with Atopic Dermatitis with Head and Neck Pattern: Is It Useful as a Biomarker and Therapeutic Indicator in These Patients? Life. 2022; 12(2):299. https://doi.org/10.3390/life12020299
Chicago/Turabian StyleNavarro-Triviño, Francisco José, and Ángela Ayén-Rodríguez. 2022. "Study of Hypersensitivity to Malassezia furfur in Patients with Atopic Dermatitis with Head and Neck Pattern: Is It Useful as a Biomarker and Therapeutic Indicator in These Patients?" Life 12, no. 2: 299. https://doi.org/10.3390/life12020299
APA StyleNavarro-Triviño, F. J., & Ayén-Rodríguez, Á. (2022). Study of Hypersensitivity to Malassezia furfur in Patients with Atopic Dermatitis with Head and Neck Pattern: Is It Useful as a Biomarker and Therapeutic Indicator in These Patients? Life, 12(2), 299. https://doi.org/10.3390/life12020299







