The Impact of Intermittent Hypoxemia on Left Atrial Remodeling in Patients with Obstructive Sleep Apnea Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Sample Management
2.2. Overnight PSG Study and Definition of SRBD Metrics
2.3. Definition of LA Size by Echocardiography Measurement
2.4. HL-1 Cell Culture, IHR Stimulation, and Measurement of Cell Viability, LDH Cytotoxicity, Intracellular ROS, and Cell Apoptosis
2.4.1. HL-1 Cell Culture
2.4.2. In Vitro IHR Stimulation
2.4.3. Measurement of Cell Viability by a WST-1 Assay
2.4.4. Measurement of Cytotoxicity by LDH Assay
2.4.5. Measurement of Intracellular ROS
2.4.6. Flow Cytometry Analysis for Cell Apoptosis Measurement
2.5. Exosome Isolation, Purification, Quantification, and Incubation in HL-1 Cells
2.6. Real-Time Quantitative Reverse Transcriptase–Polymerase Chain Reaction Analysis (qRT-PCR) of mRNA Expression in HL-1 Cells
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with and without Significant OSAS
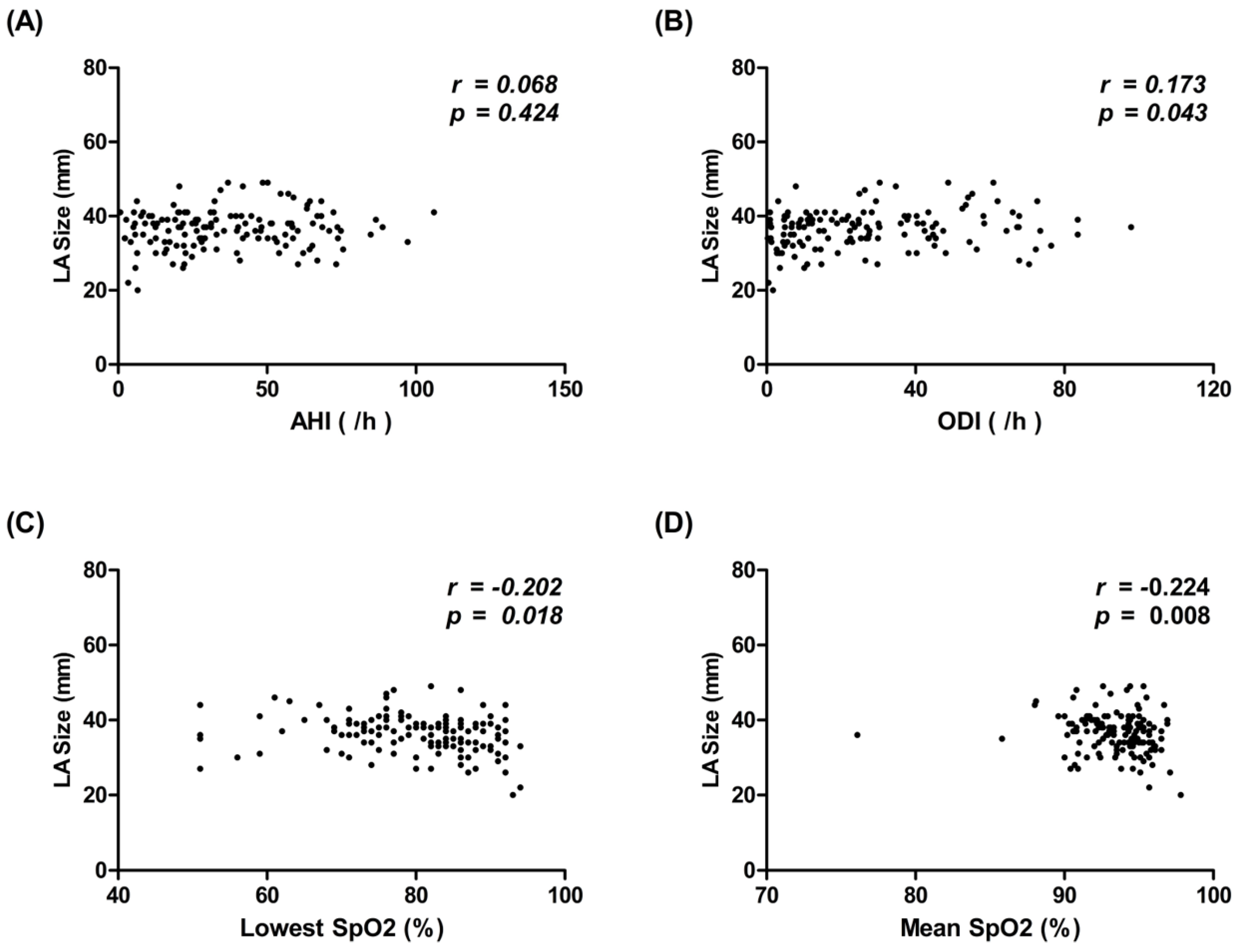
3.2. Correlation between Polysomnography (PSG) Metrics and LA Size in Patients with SRBD
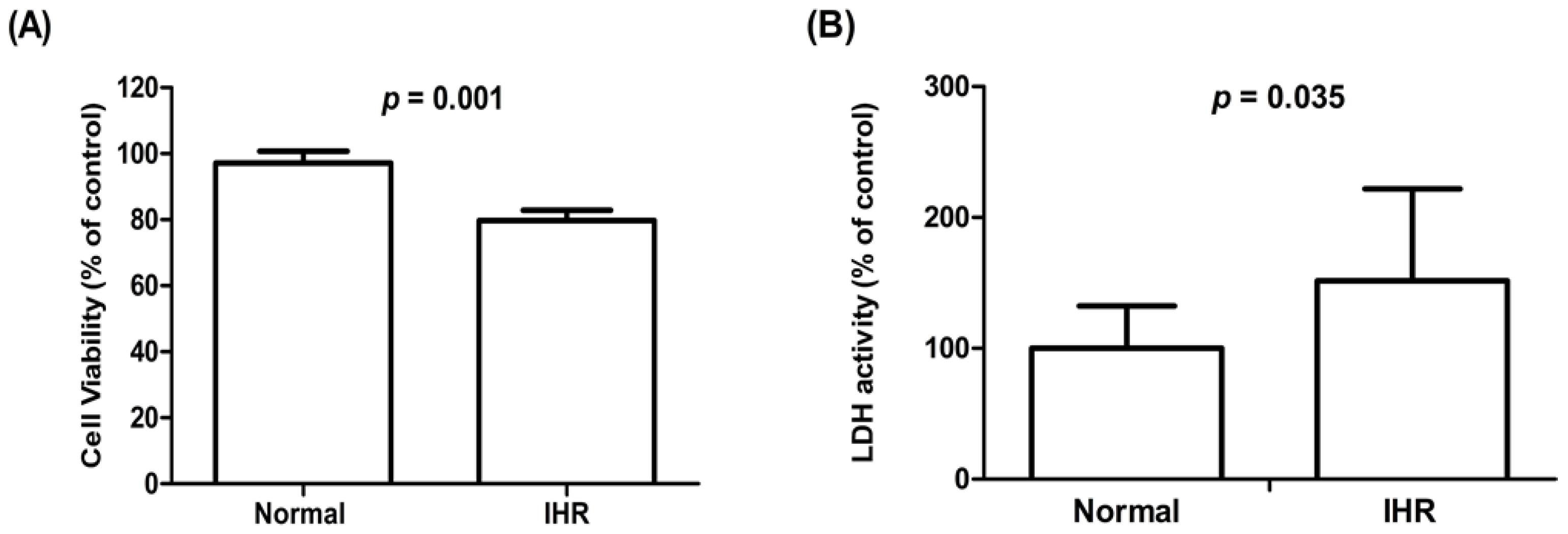
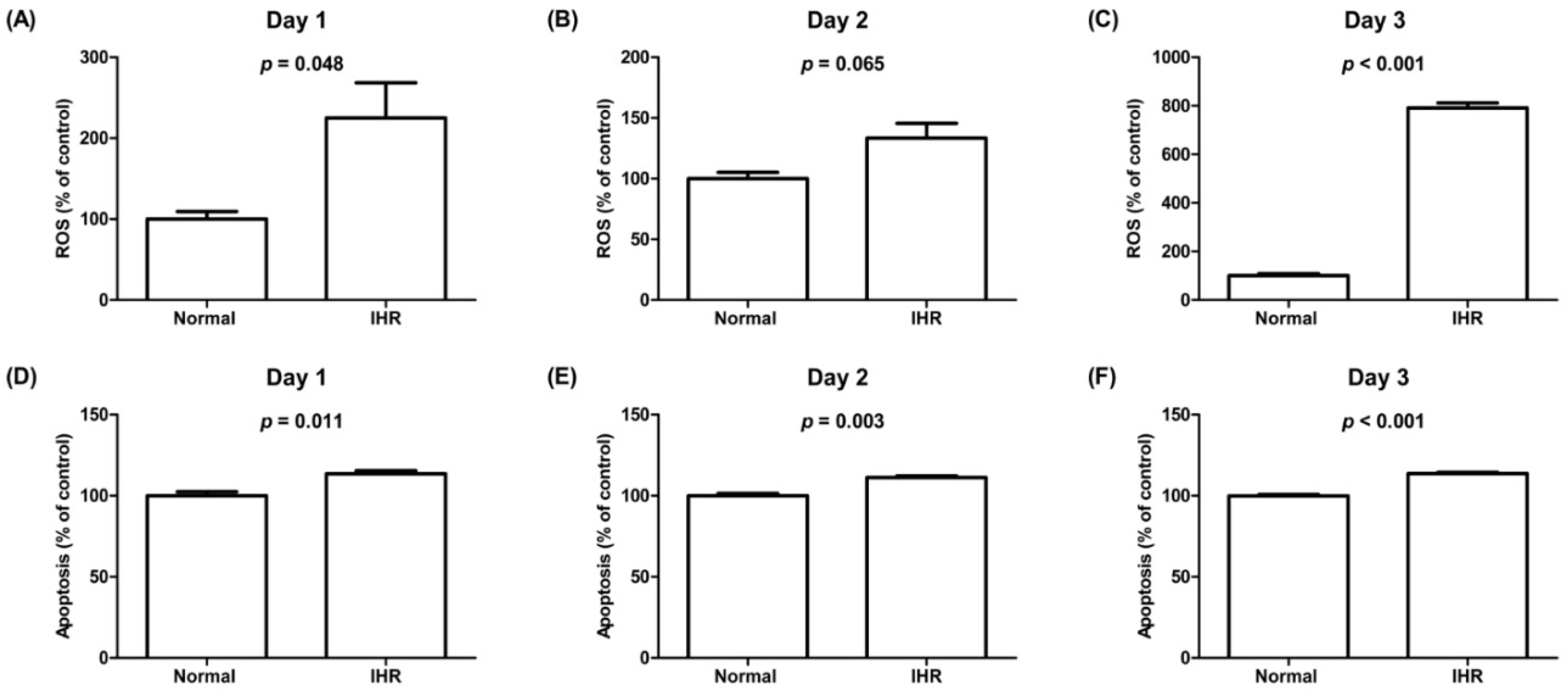
3.3. Cell Viability, Lactate Dehydrogenase (LDH) Activity, ROS Levels, Apoptosis, and the Expression of HIF-1α and Inflammatory Cytokines of HL-1 Cells Exposed to IHR Stimulation or Normoxia
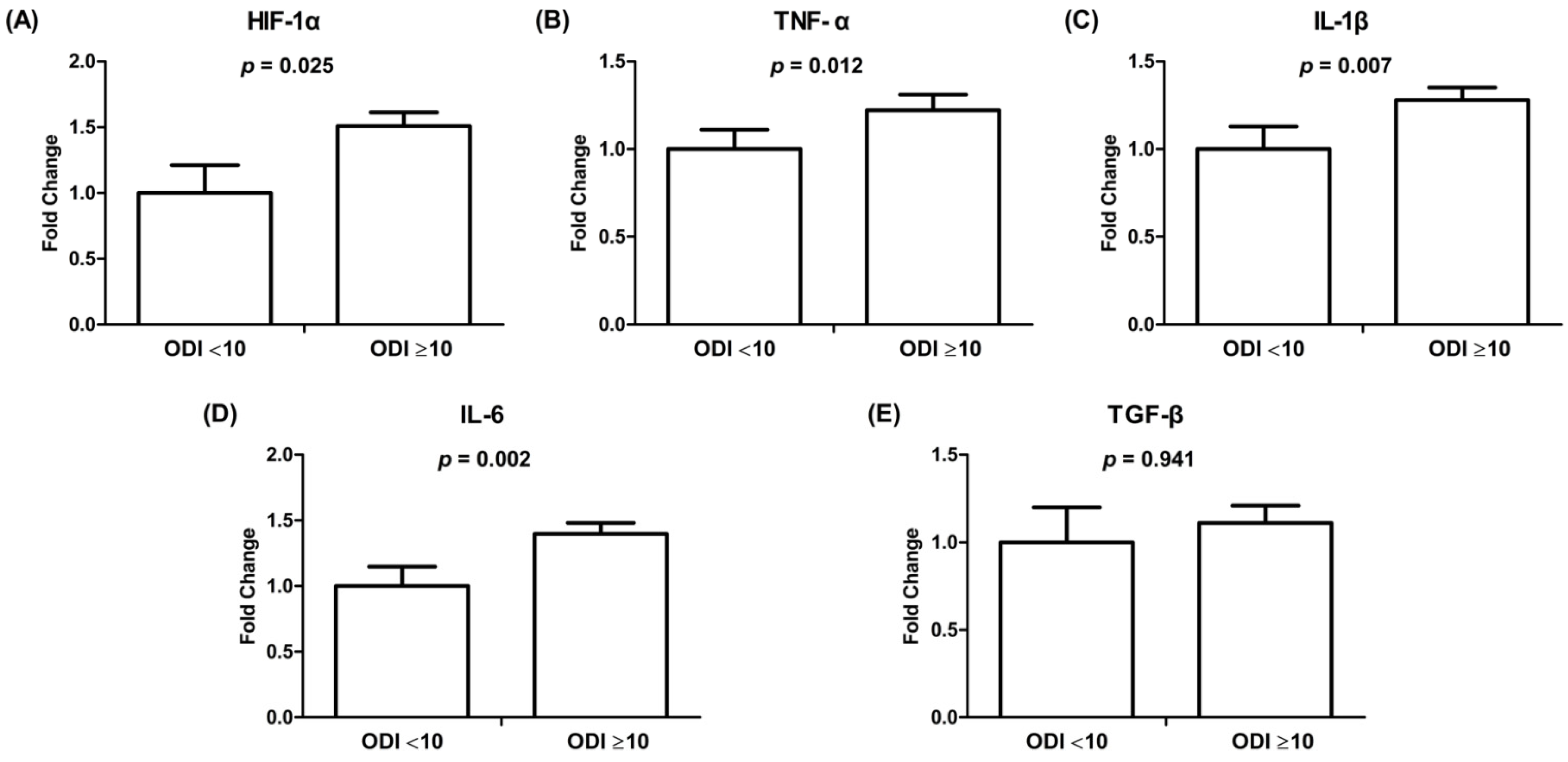
3.4. mRNA Gene Expression of HIF-1α and Inflammatory Cytokines in HL-1 Cells Treated with Exosomes Derived from Patients with and without Significant OSAS
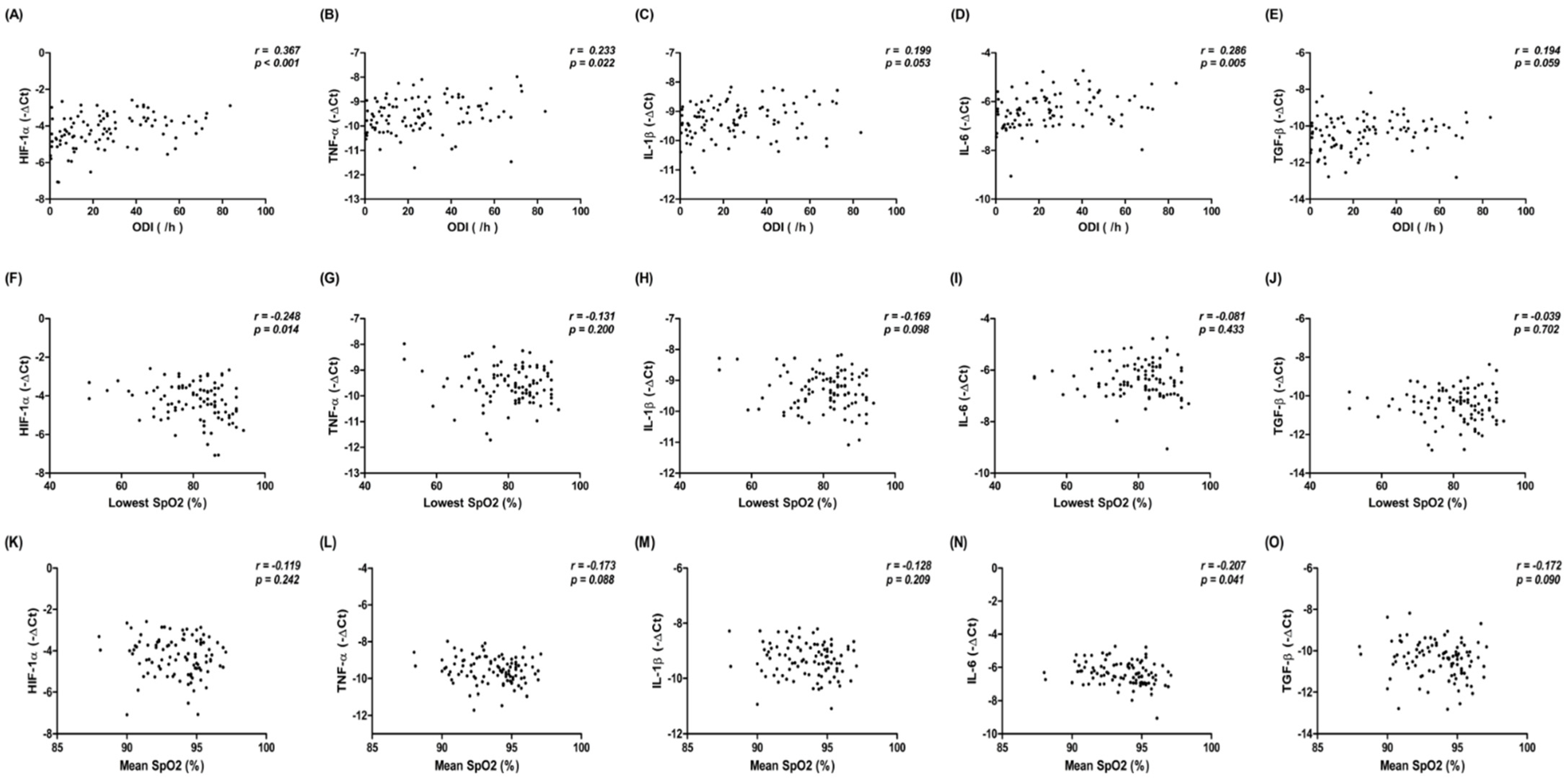
3.5. Correlation between mRNA Gene Expression of HIF-1α and Inflammatory Cytokines in HL-1 Cells Treated with Exosomes from Patients with SRDB, and PSG Metrics in Patients with SRDB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, T.S.; Barnes, M.E.; Gersh, B.J.; Takemoto, Y.; Rosales, A.G.; Bailey, K.R.; Seward, J.B. Prediction of risk for first age-related cardiovascular events in an elderly population: The incremental value of echocardiography. J. Am. Coll. Cardiol. 2003, 42, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Pritchett, A.M.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J.; Karon, B.L.; Redfield, M.M. Diastolic dysfunction and left atrial volume: A population-based study. J. Am. Coll. Cardiol. 2005, 45, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Hoit, B.D. Left Atrial Remodeling: More Than Just Left Atrial Enlargement. Circ. Cardiovasc. Imaging 2017, 10, e006036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111. [Google Scholar] [CrossRef]
- Molina, C.E.; Abu-Taha, I.H.; Wang, Q.; Rosello-Diez, E.; Kamler, M.; Nattel, S.; Ravens, U.; Wehrens, X.H.T.; Hove-Madsen, L.; Heijman, J.; et al. Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients With and Without Atrial Fibrillation. Front. Physiol. 2018, 9, 1383. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Xu, B.; Xiang, Y.; Wu, L.; Zhang, Y.; Ma, X.; Tong, S.; Shu, M.; Song, Z.; Li, Y.; et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int. J. Cardiol. 2013, 169, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Alegret, J.M.; Aragones, G.; Elosua, R.; Beltran-Debon, R.; Hernandez-Aguilera, A.; Romero-Menor, C.; Camps, J.; Joven, J. The relevance of the association between inflammation and atrial fibrillation. Eur. J. Clin. Investig. 2013, 43, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Liuba, I.; Ahlmroth, H.; Jonasson, L.; Englund, A.; Jonsson, A.; Safstrom, K.; Walfridsson, H. Source of inflammatory markers in patients with atrial fibrillation. Europace 2008, 10, 848–853. [Google Scholar] [CrossRef]
- Guo, Y.; Apostalakis, S.; Blann, A.D.; Lip, G.Y. Plasma CX3CL1 levels and long term outcomes of patients with atrial fibrillation: The West Birmingham Atrial Fibrillation Project. Cerebrovasc. Dis. 2014, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Zhang, W.; Chen, Y.; Ma, L.; Zhang, H.; Wang, F. Significance of hypoxia-inducible factor-1alpha expression with atrial fibrosis in rats induced with isoproterenol. Exp. Ther. Med. 2014, 8, 1677–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepperell, J.C.; Ramdassingh-Dow, S.; Crosthwaite, N.; Mullins, R.; Jenkinson, C.; Stradling, J.R.; Davies, R.J. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised parallel trial. Lancet 2002, 359, 204–210. [Google Scholar] [CrossRef]
- Gami, A.S.; Pressman, G.; Caples, S.M.; Kanagala, R.; Gard, J.J.; Davison, D.E.; Malouf, J.F.; Ammash, N.M.; Friedman, P.A.; Somers, V.K. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004, 110, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glantz, H.; Thunstrom, E.; Johansson, M.C.; Wallentin Guron, C.; Uzel, H.; Ejdeback, J.; Nasic, S.; Peker, Y. Obstructive sleep apnea is independently associated with worse diastolic function in coronary artery disease. Sleep Med. 2015, 16, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Schla, H.F.I. Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry. JACC Heart Fail. 2016, 4, 116–125. [Google Scholar] [CrossRef]
- Lyons, O.D.; Chan, C.T.; Elias, R.M.; Bradley, T.D. Relationship of left atrial size to obstructive sleep apnea severity in end-stage renal disease. Sleep Med. 2014, 15, 1314–1318. [Google Scholar] [CrossRef]
- Holtstrand Hjalm, H.; Fu, M.; Hansson, P.O.; Zhong, Y.; Caidahl, K.; Mandalenakis, Z.; Morales, D.; Ergatoudes, C.; Rosengren, A.; Grote, L.; et al. Association between left atrial enlargement and obstructive sleep apnea in a general population of 71-year-old men. J. Sleep Res. 2018, 27, 252–258. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Konecny, T.; Kara, T.; Somers, V.K. Obstructive sleep apnea and hypertension: An update. Hypertension 2014, 63, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkas, A.; Tiihonen, P.; Julkunen, P.; Mervaala, E.; Toyras, J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med. Biol. Eng. Comput. 2013, 51, 697–708. [Google Scholar] [CrossRef]
- Mediano, O.; Barcelo, A.; de la Pena, M.; Gozal, D.; Agusti, A.; Barbe, F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur. Respir. J. 2007, 30, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldenburg, O.; Wellmann, B.; Buchholz, A.; Bitter, T.; Fox, H.; Thiem, U.; Horstkotte, D.; Wegscheider, K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur. Heart J. 2016, 37, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Szmyd, B.; Szemraj, J.; Stawski, R.; Sochal, M.; Bialasiewicz, P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1alpha protein. J. Clin. Sleep Med. 2020, 16, 1761–1768. [Google Scholar] [CrossRef]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012, 14, 1036–1045. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Liao, J.; Liu, R.; Yin, L.; Pu, Y. Expression profiling of exosomal miRNAs derived from human esophageal cancer cells by Solexa high-throughput sequencing. Int. J. Mol. Sci. 2014, 15, 15530–15551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Chen, Y.C.; Chang, Y.T.; Wang, H.T.; Liu, W.H.; Chong, S.Z.; Lin, P.T.; Hsu, P.Y.; Su, M.C.; Lin, M.C. GJA1 Expression and Left Atrial Remodeling in the Incidence of Atrial Fibrillation in Patients with Obstructive Sleep Apnea Syndrome. Biomedicines 2021, 9, 1463. [Google Scholar] [CrossRef]
- Chen, Y.L.; Su, M.C.; Liu, W.H.; Wang, C.C.; Lin, M.C.; Chen, M.C. Influence and predicting variables of obstructive sleep apnea on cardiac function and remodeling in patients without congestive heart failure. J. Clin. Sleep Med. 2014, 10, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redline, S.; Budhiraja, R.; Kapur, V.; Marcus, C.L.; Mateika, J.H.; Mehra, R.; Parthasarthy, S.; Somers, V.K.; Strohl, K.P.; Sulit, L.G.; et al. The scoring of respiratory events in sleep: Reliability and validity. J. Clin. Sleep Med. 2007, 3, 169–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wang, H.T.; Chen, H.C.; Chai, H.T.; Lee, Y.W.; Liu, W.H. Localization of right ventricular non-apical lead position: Comparison of three-dimensional echocardiography, computed tomography, and fluoroscopic imaging. J. Int. Med. Res. 2021, 49, 300060521996159. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Guo, B.F.; Chen, Y.L.; Tsai, T.H.; Fu, M.; Chua, S.; Chen, M.C. Right ventricular outflow tract pacing causes intraventricular dyssynchrony in patients with sick sinus syndrome: A real-time three-dimensional echocardiographic study. J. Am. Soc. Echocardiogr. 2010, 23, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Sickinger, S.; Frotschnig, S.; Grimm, M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim. Biophys. Acta 2015, 1853, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Hsu, P.Y.; Su, M.C.; Chin, C.H.; Liou, C.W.; Wang, T.Y.; Lin, Y.Y.; Lee, C.P.; Lin, M.C.; Hsiao, C.C. miR-21-5p Under-Expression in Patients with Obstructive Sleep Apnea Modulates Intermittent Hypoxia with Re-Oxygenation-Induced-Cell Apoptosis and Cytotoxicity by Targeting Pro-Inflammatory TNF-alpha-TLR4 Signaling. Int. J. Mol. Sci. 2020, 21, 999. [Google Scholar] [CrossRef] [Green Version]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Lydic, T.A.; Townsend, S.; Adda, C.G.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.Y.; Hsi, E.; Wang, T.M.; Lin, R.T.; Liao, Y.C.; Juo, S.H. MicroRNA let-7g possesses a therapeutic potential for peripheral artery disease. J. Cell Mol. Med. 2017, 21, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.V.; Claycomb, W.C. Hypoxia regulates the expression of the adrenomedullin and HIF-1 genes in cultured HL-1 cardiomyocytes. Biochem. Biophys. Res. Commun. 1999, 265, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Kainulainen, S.; Toyras, J.; Oksenberg, A.; Korkalainen, H.; Sefa, S.; Kulkas, A.; Leppanen, T. Severity of Desaturations Reflects OSA-Related Daytime Sleepiness Better Than AHI. J. Clin. Sleep Med. 2019, 15, 1135–1142. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Brown, C.D.; Zizi, F.; Ogedegbe, G.; Boutin-Foster, C.; Gorga, J.; McFarlane, S.I. Cardiovascular disease risk reduction with sleep apnea treatment. Expert Rev. Cardiovasc. Ther. 2010, 8, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Tsai, C.M.; Su, M.C.; Lin, M.C.; Lin, H.C.; Lee, W.J.; Hsieh, K.S.; Niu, C.K.; Yu, H.R. Application of desaturation index in post-surgery follow-up in children with obstructive sleep apnea syndrome. Eur. Arch. Otorhino-Laryngol. 2017, 274, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhou, N.; Yuan, J.; Lu, L.; Zhang, Q.; Wei, M.; Zou, Y.; Yuan, L. Heat shock transcription factor 1 regulates exercise-induced myocardial angiogenesis after pressure overload via HIF-1alpha/VEGF pathway. J. Cell Mol. Med. 2020, 24, 2178–2188. [Google Scholar] [CrossRef]
- Morand, J.; Arnaud, C.; Pepin, J.L.; Godin-Ribuot, D. Chronic intermittent hypoxia promotes myocardial ischemia-related ventricular arrhythmias and sudden cardiac death. Sci. Rep. 2018, 8, 2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.F.; Pandey, S.; Day, C.H.; Chen, Y.F.; Jiang, A.Z.; Ho, T.J.; Chen, R.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Synergistic effect of HIF-1alpha and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell Physiol. 2018, 233, 3660–3671. [Google Scholar] [CrossRef]
- Lu, D.; Li, N.; Yao, X.; Zhou, L. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn. J. Basic Med. Sci. 2017, 17, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Gabryelska, A.; Stawski, R.; Sochal, M.; Szmyd, B.; Bialasiewicz, P. Influence of one-night CPAP therapy on the changes of HIF-1alpha protein in OSA patients: A pilot study. J. Sleep Res. 2020, 29, e12995. [Google Scholar] [CrossRef]
- Bonello, S.; Zahringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Gorlach, A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.I.; Hsu, Y.C.; Lien, C.F.; Lin, J.H.; Chiu, H.W.; Yang, K.T. Non-lethal levels of oxidative stress in response to short-term intermittent hypoxia enhance ca(2)(+) handling in neonatal rat cardiomyocytes. Cell Physiol. Biochem. 2014, 33, 513–527. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Isaacs, J.S.; Lee, S.; Trepel, J.; Liu, Z.G.; Neckers, L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem. J. 2003, 370, 1011–1017. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Ikejiri, A.; Nagai, S.; Goda, N.; Kurebayashi, Y.; Osada-Oka, M.; Takubo, K.; Suda, T.; Koyasu, S. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int. Immunol. 2012, 24, 137–146. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S.; Charbonneau, M.; Grandmont, S.; Richard, D.E.; Dubois, C.M. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J. Biol. Chem. 2006, 281, 24171–24181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.J.; Chung, H.S.; Lee, B.R.; Kim, S.J.; Yoo, S.J.; Hong, S.H.; Kim, H.M. Expression of proinflammatory cytokines via HIF-1alpha and NF-kappaB activation on desferrioxamine-stimulated HMC-1 cells. Biochem. Biophys. Res. Commun. 2003, 306, 805–811. [Google Scholar] [CrossRef]
- Fang, H.Y.; Hughes, R.; Murdoch, C.; Coffelt, S.B.; Biswas, S.K.; Harris, A.L.; Johnson, R.S.; Imityaz, H.Z.; Simon, M.C.; Fredlund, E.; et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood 2009, 114, 844–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhopalwala, H.; Dewaswala, N.; Liu, S.; Scott, C.G.; Welper, J.M.; Akinnusotu, O.; Bos, J.M.; Ommen, S.R.; Ackerman, M.J.; Pellikka, P.A.; et al. Conversion of left atrial volume to diameter for automated estimation of sudden cardiac death risk in hypertrophic cardiomyopathy. Echocardiography 2021, 38, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chuang, J.H.; Wang, H.T.; Chen, H.C.; Liu, W.H.; Yang, M.Y. Altered Expression of Circadian Clock Genes in Patients with Atrial Fibrillation Is Associated with Atrial High-Rate Episodes and Left Atrial Remodeling. Diagnostics 2021, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Liao, P.; Elsaid, H.; Islam, S.; Shapiro, C.M.; Sun, Y. Oxygen desaturation index from nocturnal oximetry: A sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth. Analg. 2012, 114, 993–1000. [Google Scholar] [CrossRef] [PubMed]

| Variables | ODI ≥ 10 (per hour) (n = 109) | ODI < 10 (per hour) (n = 45) | p-Value |
|---|---|---|---|
| Age | 55.8 ± 11.0 | 53.3 ± 9.8 | 0.188 |
| Sex (male) | 81 (74.3%) | 30 (66.7%) | 0.336 |
| Smoking | 20 (18.3%) | 11 (24.4%) | 0.391 |
| BMI | 26.5 ± 3.3 | 24.2 ± 3.1 | <0.001 |
| Waistline | 85.6 ± 8.3 | 81.7 ± 8.2 | 0.063 |
| DM | 13 (11.9%) | 3 (6.7%) | 0.331 |
| HTN | 42 (38.5%) | 15 (33.3%) | 0.543 |
| HF | 3 (2.8%) | 0 (0%) | 0.261 |
| Stroke | 4 (3.7%) | 2 (4.4%) | 0.821 |
| CAD | 5 (4.6%) | 2 (4.4%) | 0.969 |
| AF | 20 (18.3%) | 6 (13.3) | 0.518 |
| Paroxysmal AF | 9 (8.3%) | 4 (8.9%) | |
| Persistent AF | 11 (10.1%) | 2 (4.4%) | |
| Thyroid disorder | 2 (1.8%) | 0 (0%) | 0.360 |
| Polysomnography | |||
| Epworth sleepiness scale | 9.3 (6.0–12.0) | 8.0 (5.0–12.0) | 0.254 |
| AHI (per hour) | 45.4 (27.7–43.2) | 13.2 (5.7–20.2) | <0.001 |
| ODI (per hour) | 36.5 (19.7–48.4) | 4.8 (2.7–7.1) | <0.001 |
| Lowest SpO2 (%) | 76.7 (72.0–84.0) | 87.9 (86.0–91.0) | <0.001 |
| Mean SpO2 (%) | 92.8 (91.8–94.6) | 94.7 (93.6–96.0) | <0.001 |
| LA size by echocardiography | 37.0 ± 5.0 | 34.6 ± 5.6 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Chen, Y.-C.; Wang, H.-T.; Chang, Y.-T.; Fang, Y.-N.; Hsueh, S.; Liu, W.-H.; Lin, P.-T.; Hsu, P.-Y.; Su, M.-C.; et al. The Impact of Intermittent Hypoxemia on Left Atrial Remodeling in Patients with Obstructive Sleep Apnea Syndrome. Life 2022, 12, 148. https://doi.org/10.3390/life12020148
Chen Y-L, Chen Y-C, Wang H-T, Chang Y-T, Fang Y-N, Hsueh S, Liu W-H, Lin P-T, Hsu P-Y, Su M-C, et al. The Impact of Intermittent Hypoxemia on Left Atrial Remodeling in Patients with Obstructive Sleep Apnea Syndrome. Life. 2022; 12(2):148. https://doi.org/10.3390/life12020148
Chicago/Turabian StyleChen, Yung-Lung, Yung-Che Chen, Hui-Ting Wang, Ya-Ting Chang, Yen-Nan Fang, Shukai Hsueh, Wen-Hao Liu, Pei-Ting Lin, Po-Yuan Hsu, Mao-Chang Su, and et al. 2022. "The Impact of Intermittent Hypoxemia on Left Atrial Remodeling in Patients with Obstructive Sleep Apnea Syndrome" Life 12, no. 2: 148. https://doi.org/10.3390/life12020148
APA StyleChen, Y.-L., Chen, Y.-C., Wang, H.-T., Chang, Y.-T., Fang, Y.-N., Hsueh, S., Liu, W.-H., Lin, P.-T., Hsu, P.-Y., Su, M.-C., Huang, K.-T., & Lin, M.-C. (2022). The Impact of Intermittent Hypoxemia on Left Atrial Remodeling in Patients with Obstructive Sleep Apnea Syndrome. Life, 12(2), 148. https://doi.org/10.3390/life12020148






