Ocular Surface Features in Patients with Parkinson Disease on and off Treatment: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Ocular Surface Parameters in PD
3.1.1. Simple Tests for Tear Film Characterization
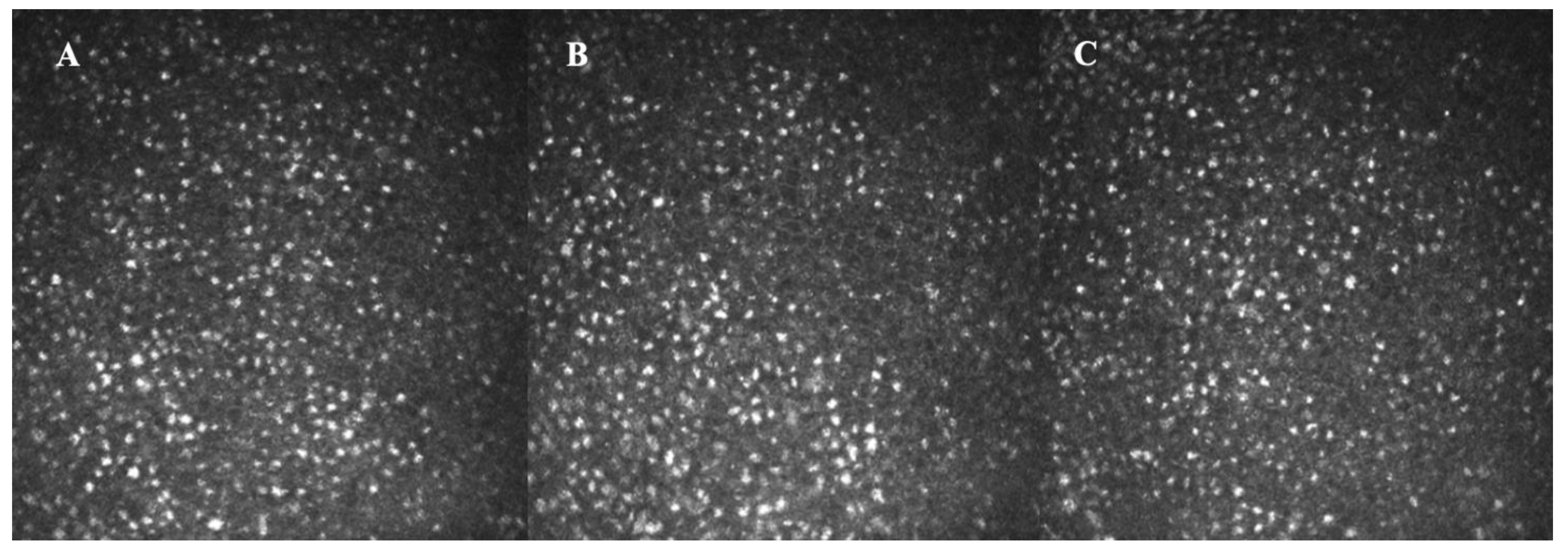
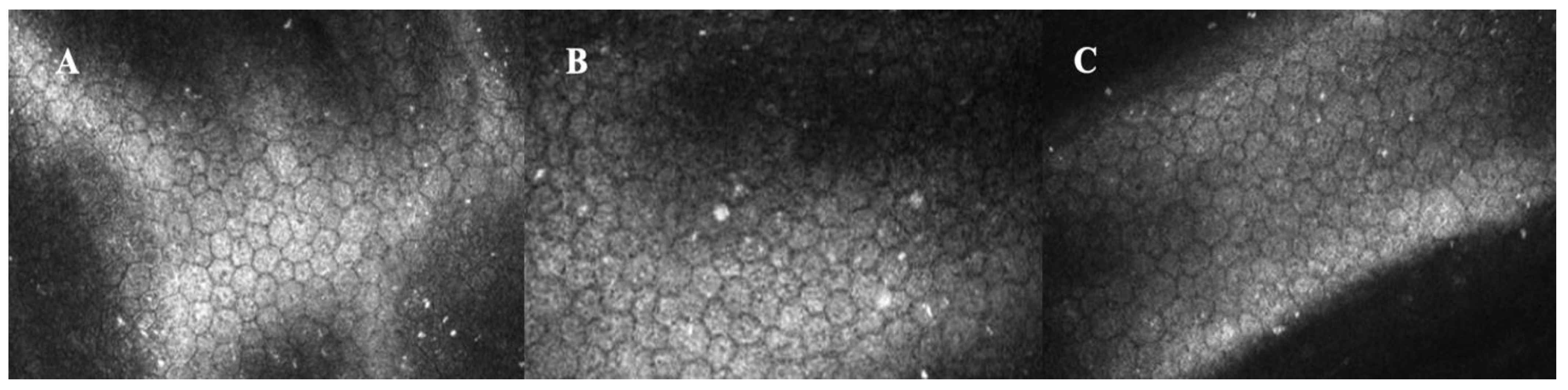
3.1.2. High-Tech Imaging Devices
3.1.3. Biochemical Investigation of Tear Film
3.2. Influence of PD Therapies on Cornea
3.2.1. Epitheliopathy
3.2.2. Corneal Edema
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowacka, B.; Lubiński, W.; Honczarenko, K.; Potemkowski, A.; Safranow, K. Ophthalmological Features of Parkinson Disease. Med. Sci. Monit. 2014, 20, 2243–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcano, P.; Chen, J.J.; Bureau, B.L.; Savica, R. Early Ophthalmologic Features of Parkinson’s Disease: A Review of Preceding Clinical and Diagnostic Markers. J. Neurol. 2019, 266, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Skibell, B.C.; Watts, R.L.; Loupe, D.N.; Drews-Botsch, C.; Newman, N.J. Ophthalmologic Features of Parkinson’s Disease. Neurology 2004, 62, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Hadjikoutis, S.; Morgan, J.E.; Wild, J.M.; Smith, P.E.M. Ocular Complications of Neurological Therapy. Eur. J. Neurol. 2005, 12, 499–507. [Google Scholar] [CrossRef]
- Örnek, N.; Dağ, E.; Örnek, K. Corneal Sensitivity and Tear Function in Neurodegenerative Diseases. Curr. Eye Res. 2015, 40, 423–428. [Google Scholar] [CrossRef]
- Roda, M.; Ciavarella, C.; Giannaccare, G.; Versura, P. Biomarkers in Tears and Ocular Surface: A Window for Neurodegenerative Diseases. Eye Contact Lens Sci. Clin. Pr. 2020, 46, S129–S134. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Nagino, K.; Sung, J.; Oyama, G.; Hayano, M.; Hattori, N.; Okumura, Y.; Fujio, K.; Akasaki, Y.; Huang, T.; Midorikawa-Inomata, A.; et al. Prevalence and Characteristics of Dry Eye Disease in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 18348. [Google Scholar] [CrossRef]
- Demirci, S.; Gunes, A.; Koyuncuoglu, H.R.; Tok, L.; Tok, O. Evaluation of Corneal Parameters in Patients with Parkinson’s Disease. Neurol. Sci. 2016, 37, 1247–1252. [Google Scholar] [CrossRef]
- Tamer, C.; Melek, I.M.; Duman, T.; Öksüz, H. Tear Film Tests in Parkinson’s Disease Patients. Ophthalmology 2005, 112, 1795.e1–1795.e8. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Berlan, M.; Senard, J.M.; Rascol, O.; Montastruc, J.L. Lacrimation in Parkinson’s Disease. Clin. Neuropharmacol. 1994, 17, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Lan, W.; Ong, L.M.; Tong, L. Non-Hormonal Systemic Medications and Dry Eye. Ocul. Surf. 2011, 9, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Benjamin, L.; Snibson, G.R. Meibomian Gland Disease. Classification and Grading of Lid Changes. Eye 1991, 5, 395–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.Y.; Kim, S.H.; Kim, J.H.; Kim, M.H.; Ko, M.K. Schrimer Test in Parkinson’s Disease. J. Korean Med. Sci. 1994, 9, 239–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blinchevsky, S.; Ramasubramanian, A.; Borchman, D.; Sayied, S.; Venkatasubramanian, K. Meibum Lipid Composition and Conformation in Parkinsonism. EC Ophthalmol. 2021, 12, 20. [Google Scholar]
- Ulusoy, E.K.; Ulusoy, D.M. Evaluation of Corneal Sublayers Thickness and Corneal Parameters in Patients with Parkinson’s Disease. Int. J. Neurosci. 2021, 131, 939–945. [Google Scholar] [CrossRef]
- Aksoy, D.; Ortak, H.; Kurt, S.; Cevik, E.; Cevik, B. Central Corneal Thickness and Its Relationship to Parkinson’s Disease Severity. Can. J. Ophthalmol. 2014, 49, 152–156. [Google Scholar] [CrossRef]
- Hamedani, A.G.; Gold, D.R. Eyelid Dysfunction in Neurodegenerative, Neurogenetic, and Neurometabolic Disease. Front. Neurol. 2017, 8, 329. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Hohl, N.; Silburn, P.; O’Gorman, C.; Broadley, S.A. Case-Control Study of Blink Rate in Parkinson’s Disease under Different Conditions. J. Neurol. 2012, 259, 739–744. [Google Scholar] [CrossRef]
- Karson, C.N.; Lewitt, P.A.; Calne, D.B.; Wyatt, R.J. Blink Rates in Parkinsonism. Ann. Neurol. 1982, 12, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Agostino, R.; Bologna, M.; Dinapoli, L.; Gregori, B.; Fabbrini, G.; Accornero, N.; Berardelli, A. Voluntary, Spontaneous, and Reflex Blinking in Parkinson’s Disease. Mov. Disord. 2008, 23, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kass-Iliyya, L.; Javed, S.; Gosal, D.; Kobylecki, C.; Marshall, A.; Petropoulos, I.N.; Ponirakis, G.; Tavakoli, M.; Ferdousi, M.; Chaudhuri, K.R.; et al. Small Fiber Neuropathy in Parkinson’s Disease: A Clinical, Pathological and Corneal Confocal Microscopy Study. Park. Relat Disord. 2015, 21, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ferdousi, M.; Kalteniece, A.; Kass-Iliyya, L.; Petropoulos, I.N.; Malik, R.A.; Kobylecki, C.; Silverdale, M. Corneal Confocal Microscopy Detects Small Fibre Neurodegeneration in Parkinson’s Disease Using Automated Analysis. Sci. Rep. 2020, 10, 20147. [Google Scholar] [CrossRef]
- Che, N.N.; Jiang, Q.H.; Ding, G.X.; Chen, S.Y.; Zhao, Z.X.; Li, X.; Malik, R.A.; Ma, J.J.; Yang, H.Q. Corneal Nerve Fiber Loss Relates to Cognitive Impairment in Patients with Parkinson’s Disease. npj Park. Dis. 2021, 7, 80. [Google Scholar] [CrossRef]
- Andréasson, M.; Lagali, N.; Badian, R.A.; Utheim, T.P.; Scarpa, F.; Colonna, A.; Allgeier, S.; Bartschat, A.; Köhler, B.; Mikut, R.; et al. Parkinson’s Disease with Restless Legs Syndrome—An in Vivo Corneal Confocal Microscopy Study. npj Park. Dis. 2021, 7, 4. [Google Scholar] [CrossRef]
- Misra, S.L.; Kersten, H.M.; Roxburgh, R.H.; Danesh-Meyer, H.V.; McGhee, C.N.J. Corneal Nerve Microstructure in Parkinson’s Disease. J. Clin. Neurosci. 2017, 39, 53–58. [Google Scholar] [CrossRef]
- Reddy, V.C.; Patel, S.V.; Hodge, D.O.; Leavitt, J.A. Corneal Sensitivity, Blink Rate, and Corneal Nerve Density in Progressive Supranuclear Palsy and Parkinson Disease. Cornea 2013, 32, 631–635. [Google Scholar] [CrossRef]
- Arrigo, A.; Rania, L.; Calamuneri, A.; Postorino, E.I.; Mormina, E.; Gaeta, M.; Marino, S.; di Lorenzo, G.; Quartarone, A.; Anastasi, G.; et al. Early Corneal Innervation and Trigeminal Alterations in Parkinson Disease: A Pilot Study. Cornea 2018, 37, 448–454. [Google Scholar] [CrossRef]
- Podgorny, P.J.; Suchowersky, O.; Romanchuk, K.G.; Feasby, T.E. Evidence for Small Fiber Neuropathy in Early Parkinson’s Disease. Park. Relat. Disord. 2016, 28, 94–99. [Google Scholar] [CrossRef]
- Lim, S.H.; Ferdousi, M.; Kalteniece, A.; Mahfoud, Z.R.; Petropoulos, I.N.; Malik, R.A.; Kobylecki, C.; Silverdale, M. Corneal Confocal Microscopy Identifies Parkinson’s Disease with More Rapid Motor Progression. Mov. Disord. 2021, 36, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Che, N.; Ding, G.; Chen, S.; Li, D.; Li, X.; Ma, J.; Yang, H. Measurement of Corneal Nerve Fiber Parameters in Patients with Parkinson’s Disease. Natl. Med. J. China 2021, 101, 498–503. [Google Scholar] [CrossRef]
- Daggumilli, S.; Vanathi, M.; Ganger, A.; Goyal, V.; Tandon, R. Corneal Evaluation in Patients With Parkinsonism on Long-Term Amantadine Therapy. Cornea 2019, 38, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Boerger, M.; Funke, S.; Leha, A.; Roser, A.E.; Wuestemann, A.K.; Maass, F.; Bähr, M.; Grus, F.; Lingor, P. Proteomic Analysis of Tear Fluid Reveals Disease-Specific Patterns in Patients with Parkinson’s Disease—A Pilot Study. Park. Relat. Disord. 2019, 63, 3–9. [Google Scholar] [CrossRef]
- Çomoǧlu, S.S.; Güven, H.; Acar, M.; Öztürk, G.; Koçer, B. Tear Levels of Tumor Necrosis Factor-Alpha in Patients with Parkinson’s Disease. Neurosci. Lett. 2013, 553, 63–67. [Google Scholar] [CrossRef]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Feigenbaum, D.; Freire, D.; Mack, W.J.; Okamoto, C.T.; Lew, M.F. Levels of Oligomeric α-Synuclein in Reflex Tears Distinguish Parkinson’s Disease Patients from Healthy Controls. Biomark. Med. 2019, 13, 1447–1457. [Google Scholar] [CrossRef]
- Hamm-Alvarez, S.F.; Okamoto, C.T.; Janga, S.R.; Feigenbaum, D.; Edman, M.C.; Freire, D.; Shah, M.; Ghanshani, R.; Mack, W.J.; Lew, M.F. Oligomeric α-Synuclein Is Increased in Basal Tears of Parkinson’s Patients. Biomark. Med. 2018, 13, 941–952. [Google Scholar] [CrossRef]
- Acera, A.; Gómez-Esteban, J.C.; Murueta-Goyena, A.; Galdos, M.; Azkargorta, M.; Elortza, F.; Ruzafa, N.; Ibarrondo, O.; Pereiro, X.; Vecino, E. Potential Tear Biomarkers for the Diagnosis of Parkinson’s Disease—A Pilot Study. Proteomes 2022, 10, 4. [Google Scholar] [CrossRef]
- Bogdanov, V.; Kim, A.; Nodel, M.; Pavlenko, T.; Pavlova, E.; Blokhin, V.; Chesnokova, N.; Ugrumov, M. A Pilot Study of Changes in the Level of Catecholamines and the Activity of α-2-Macroglobulin in the Tear Fluid of Patients with Parkinson’s Disease and Parkinsonian Mice. Int. J. Mol. Sci. 2021, 22, 4736. [Google Scholar] [CrossRef]
- Kim, A.R.; Nodel, M.R.; Pavlenko, T.A.; Chesnokova, N.B.; Yakhno, N.N.; Ugrumov, M.V. Tear Fluid Catecholamines as Biomarkers of the Parkinson’s Disease: A Clinical and Experimental Study. Acta Nat. 2019, 11, 99–103. [Google Scholar] [CrossRef]
- Kim, A.; Nigmatullina, R.; Zalyalova, Z.; Soshnikova, N.; Krasnov, A.; Vorobyeva, N.; Georgieva, S.; Kudrin, V.; Narkevich, V.; Ugrumov, M. Upgraded Methodology for the Development of Early Diagnosis of Parkinson’s Disease Based on Searching Blood Markers in Patients and Experimental Models. Mol. Neurobiol. 2019, 56, 3437–3450. [Google Scholar] [CrossRef] [PubMed]
- Fraunfelder, F.T.; Meyer, S.M. Amantadine and Corneal Deposits. Am. J. Ophthalmol. 1990, 110, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, J.T. Vision Loss Associated with Amantadine Hydrochloride Use. JAMA J. Am. Med. Assoc. 1977, 237, 1200. [Google Scholar] [CrossRef]
- Blanchard, D.L. Amantadine Caused Corneal Edema. Cornea 1990, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Nogaki, H.; Morimatsu, M. Superficial Punctate Keratitis and Corneal Abrasion Due to Amantadine Hydrochloride. J. Neurol. 1993, 240, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaka, A.; Chikama, T.; Kiuchi, Y. Amantadine Can Induce Intra-Epithelial Deposition in the Cornea. Am. J. Ophthalmol. Case Rep. 2020, 19, 100852. [Google Scholar] [CrossRef]
- Ghaffariyeh, A.; Honarpisheh, N. Amantadine-Associated Corneal Edema. Park. Relat. Disord. 2010, 16, 427. [Google Scholar] [CrossRef]
- Hessen, M.M.; Vahedi, S.; Khoo, C.T.; Vakili, G.; Eghrari, A.O. Clinical and Genetic Investigation of Amantadine-Associated Corneal Edema. Clin. Ophthalmol. 2018, 12, 1367–1371. [Google Scholar] [CrossRef] [Green Version]
- Hotehama, A.; Mimura, T.; Usui, T.; Kawashima, H.; Amano, S. Sudden Onset of Amantadine-Induced Reversible Bilateral Corneal Edema in an Elderly Patient: Case Report and Literature Review. Jpn J. Ophthalmol. 2011, 55, 71–74. [Google Scholar] [CrossRef]
- Hughes, B.; Feiz, V.; Flynn, S.B.; Brodsky, M.C. Reversible Amantadine-Induced Corneal Edema in an Adolescent. Cornea 2004, 23, 823–824. [Google Scholar] [CrossRef]
- Jeng, B.H.; Galor, A.; Lee, M.S.; Meisler, D.M.; Hollyfield, J.G.; Schoenfield, L.; McMahon, J.T.; Langston, R.H.S. Amantadine-Associated Corneal Edema. Potentially Irreversible Even after Cessation of the Medication. Ophthalmology 2008, 115, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Chuck, R.S. Sudden Bilateral Corneal Oedema in a Patient with Parkinson’s Disease. Acta Ophthalmol. 2011, 89, 198–199. [Google Scholar] [CrossRef] [PubMed]
- French, D.D.; Margo, C.E. Postmarketing Surveillance of Corneal Edema, Fuchs Dystrophy, and Amantadine Use in the Veterans Health Administration. Cornea 2007, 26, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Jeong, J.H.; Kim, M.K.; Wee, W.R.; Lee, J.H.; Jeon, B.S. The Effect of Amantadine on Corneal Endothelium in Subjects with Parkinson’s Disease. Ophthalmology 2010, 117, 1214–1219. [Google Scholar] [CrossRef]
- Dudley, C.E.; Morell, A.J.; Duffey, M.E.; Patel, S.P. Effects of Amantadine on Corneal Endothelium. Exp. Eye Res. 2019, 181, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Tu, H.P.; Lin, C.P.; Chang, C.H.; Cheng, K.C.; Lin, C.C.; Hsu, S.L. Amantadine Use as a Risk Factor for Corneal Edema: A Nationwide Cohort Study in Taiwan. Am. J. Ophthalmol. 2016, 171, 122–129. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzzi, M.; Giannaccare, G.; Cennamo, M.; Bernabei, F.; Rothschild, P.-R.; Vagge, A.; Scorcia, V.; Mencucci, R. Ocular Surface Features in Patients with Parkinson Disease on and off Treatment: A Narrative Review. Life 2022, 12, 2141. https://doi.org/10.3390/life12122141
Buzzi M, Giannaccare G, Cennamo M, Bernabei F, Rothschild P-R, Vagge A, Scorcia V, Mencucci R. Ocular Surface Features in Patients with Parkinson Disease on and off Treatment: A Narrative Review. Life. 2022; 12(12):2141. https://doi.org/10.3390/life12122141
Chicago/Turabian StyleBuzzi, Matilde, Giuseppe Giannaccare, Michela Cennamo, Federico Bernabei, Pierre-Raphael Rothschild, Aldo Vagge, Vincenzo Scorcia, and Rita Mencucci. 2022. "Ocular Surface Features in Patients with Parkinson Disease on and off Treatment: A Narrative Review" Life 12, no. 12: 2141. https://doi.org/10.3390/life12122141
APA StyleBuzzi, M., Giannaccare, G., Cennamo, M., Bernabei, F., Rothschild, P.-R., Vagge, A., Scorcia, V., & Mencucci, R. (2022). Ocular Surface Features in Patients with Parkinson Disease on and off Treatment: A Narrative Review. Life, 12(12), 2141. https://doi.org/10.3390/life12122141







