Improving Osteosarcoma Treatment: Comparative Oncology in Action
Abstract
1. Introduction
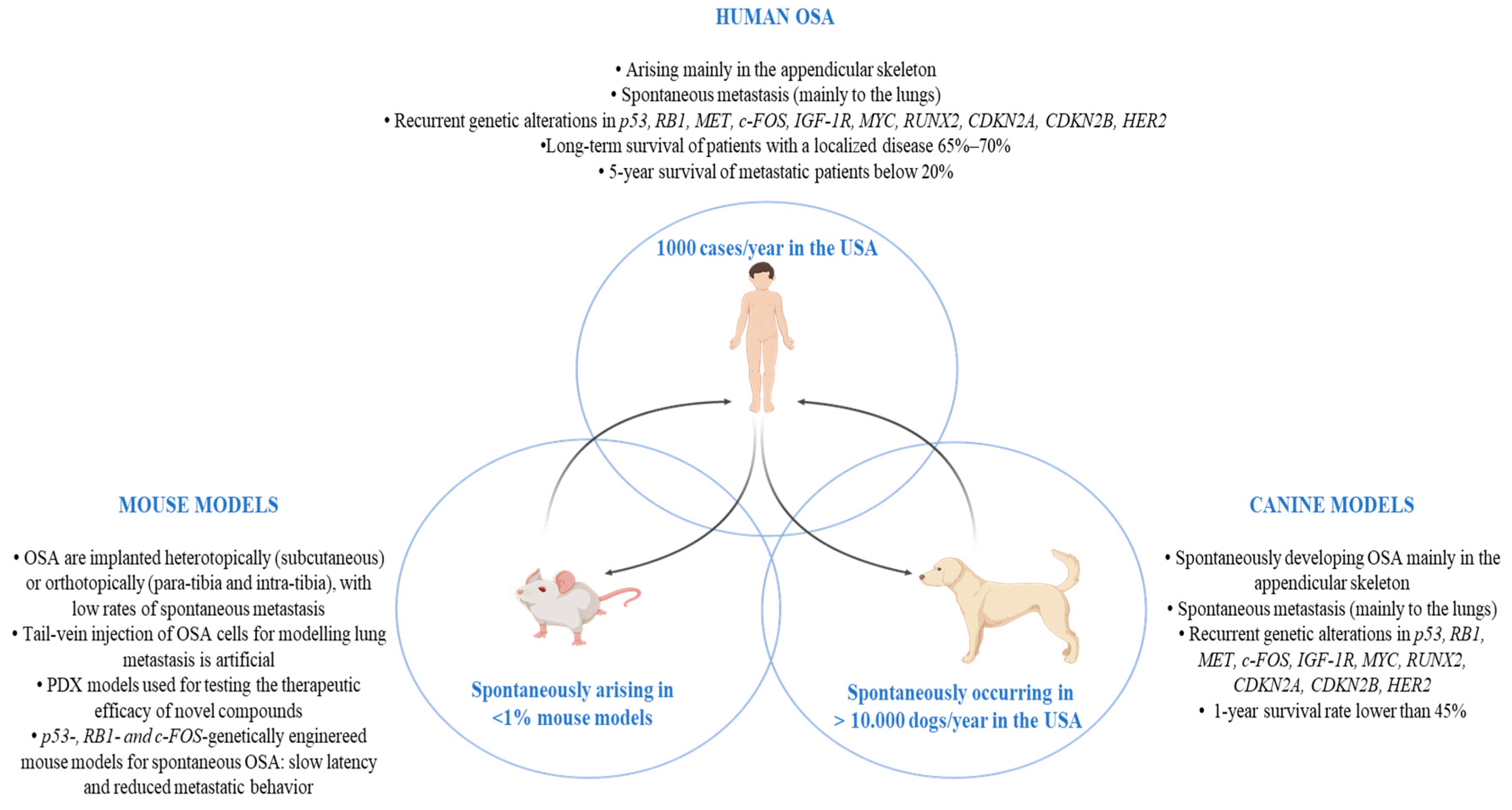
2. Comparative Oncology
3. OSA in Pet Dogs
4. Translational Studies of Combinatorial Chemotherapeutic Approaches
4.1. In Vitro Canine OSA Models
4.2. In Vivo Canine OSA Models
5. Translational Studies of Immune-Based Approaches
5.1. Branded Targets
5.2. Other Immunomodulatory Strategies
6. Chondroitin Sulfate Proteoglycan 4 (CSPG4): A Novel OSA Immunotherapeutic Target
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Zhang, J.; Chen, Y.; Kang, Y.; Liao, Z.; He, Y.; Zhang, C. Novel Immunotherapies for Osteosarcoma. Front. Oncol. 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.; Marcove, R.C.; Caparros, B.; Nirenberg, A.; Kosloff, C.; Andrew, A.N.D. Primary Osteogenic Sarcoma. The Rationale for Preoperative Chemotherapy and Delayed Surgery. Cancer 1979, 43, 2163–2177. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.; Caparros, B.; Huvos, A.G.; Kosloff, C.; Nirenberg, A.; Cacavio, A.; Marcove, R.C.; Lane, J.M.; Mehta, B.; Urban, C. Preoperative Chemotherapy for Osteogenic Sarcoma: Selection of Postoperative Adjuvant Chemotherapy Based on the Response of the Primary Tumor to Preoperative Chemotherapy. Cancer 1982, 49, 1221–1230. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y.; He, Z.; Yang, J.; Sun, B.; Shi, X.; et al. Progress in the Chemotherapeutic Treatment of Osteosarcoma. Oncol. Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029. [Google Scholar] [CrossRef]
- Ferrari, S.; Ruggieri, P.; Cefalo, G.; Tamburini, A.; Capanna, R.; Fagioli, F.; Comandone, A.; Bertulli, R.; Bisogno, G.; Palmerini, E.; et al. Neoadjuvant Chemotherapy with Methotrexate, Cisplatin, and Doxorubicin with or without Ifosfamide in Nonmetastatic Osteosarcoma of the Extremity: An Italian Sarcoma Group Trial ISG/OS-1. J. Clin. Oncol. 2012, 30, 2112–2118. [Google Scholar] [CrossRef]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in Patients with a Poor Response to Preoperative Chemotherapy for Newly Diagnosed High-Grade Osteosarcoma (EURAMOS-1): An Open-Label, International, Randomised Controlled Trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A Review of Current and Future Therapeutic Approaches. Biomed. Eng. Online 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Meyers, P.A.; Heller, G.; Healey, J.H.; Huvos, A.; Applewhite, A.; Sun, M.; LaQuaglia, M. Osteogenic Sarcoma with Clinically Detectable Metastasis at Initial Presentation. J. Clin. Oncol. 1993, 11, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Ahmad, R.; Sultan, I.; Yaser, S.; Shehadeh, A. Osteosarcoma with Metastasis at Initial Diagnosis: Current Outcomes and Prognostic Factors in the Context of a Comprehensive Cancer Center. Mol. Clin. Oncol. 2014, 2, 811. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Tortora, C.; Pota, E.; Di Paola, A.; Di Martino, M.; Di Leva, C.; Di Pinto, D.; Rossi, F. Osteosarcoma in Children: Not Only Chemotherapy. Pharmaceuticals 2021, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and Prognosis with Osteosarcoma: Outcomes in More than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur. J. Cancer 2019, 109, 36. [Google Scholar] [CrossRef]
- Ghosh, J.; Bajpai, J. Chemotherapy for Osteosarcoma: Adverse Effects and Remedial Measures. Pediatr. Hematol. Oncol. J. 2017, 2, 41–47. [Google Scholar] [CrossRef]
- Tian, D.; Feng, K.; Wu, X.; Gao, C.; Hu, L. Analysis of the Efficacy of Multidrug Combination Chemotherapy Regimens for Osteosarcoma and the Management of Chemotherapeutic Reactions. Evid. Based. Complement. Alternat. Med. 2022, 2022, 6510429. [Google Scholar] [CrossRef] [PubMed]
- Moukengue, B.; Lallier, M.; Marchandet, L.; Baud’huin, M.; Verrecchia, F.; Ory, B.; Lamoureux, F. Origin and Therapies of Osteosarcoma. Cancers 2022, 14, 3503. [Google Scholar] [CrossRef]
- Morton, C.L.; Houghton, P.J. Establishment of Human Tumor Xenografts in Immunodeficient Mice. Nat. Protoc. 2007, 2, 247–250. [Google Scholar] [CrossRef]
- Kavirayani, A.M.; Sundberg, J.P.; Foreman, O. Primary Neoplasms of Bones in Mice: Retrospective Study and Review of Literature. Vet. Pathol. 2012, 49, 182–205. [Google Scholar] [CrossRef]
- Beck, J.; Ren, L.; Huang, S.; Berger, E.; Bardales, K.; Mannheimer, J.; Mazcko, C.; LeBlanc, A. Canine and Murine Models of Osteosarcoma. Vet. Pathol. 2022, 59, 399–414. [Google Scholar] [CrossRef]
- Sampson, V.B.; Gorlick, R.; Kamara, D.; Kolb, E.A. A Review of Targeted Therapies Evaluated by the Pediatric Preclinical Testing Program for Osteosarcoma. Front. Oncol. 2013, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Landuzzi, L.; Manara, M.C.; Lollini, P.L.; Scotlandi, K. Patient Derived Xenografts for Genome-Driven Therapy of Osteosarcoma. Cells. 2021, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Crenn, V.; Biteau, K.; Amiaud, J.; Dumars, C.; Guiho, R.; Vidal, L.; Le Nail, L.R.; Heymann, D.; Moreau, A.; Gouin, F.; et al. Bone Microenvironment Has an Influence on the Histological Response of Osteosarcoma to Chemotherapy: Retrospective Analysis and Preclinical Modeling. Am. J. Cancer Res. 2017, 7, 2333. [Google Scholar] [PubMed]
- Jacques, C.; Renema, N.; Lezot, F.; Ory, B.; Walkley, C.R.; Grigoriadis, A.E.; Heymann, D. Small Animal Models for the Study of Bone Sarcoma Pathogenesis:Characteristics, Therapeutic Interests and Limitations. J. Bone Oncol. 2018, 12, 7. [Google Scholar] [CrossRef]
- Uluçkan, Ö.; Segaliny, A.; Botter, S.; Santiago, J.M.; Mutsaers, A.J. Preclinical Mouse Models of Osteosarcoma. Bonekey Rep. 2015, 4, 670. [Google Scholar] [CrossRef]
- Luu, H.H.; Kang, Q.; Jong, K.P.; Si, W.; Luo, Q.; Jiang, W.; Yin, H.; Montag, A.G.; Simon, M.A.; Peabody, T.D.; et al. An Orthotopic Model of Human Osteosarcoma Growth and Spontaneous Pulmonary Metastasis. Clin. Exp. Metastasis 2005, 22, 319–329. [Google Scholar] [CrossRef]
- Lin, P.P.; Pandey, M.K.; Jin, F.; Raymond, A.K.; Akiyama, H.; Lozano, G. Targeted Mutation of P53 and Rb in Mesenchymal Cells of the Limb Bud Produces Sarcomas in Mice. Carcinogenesis 2009, 30, 1789–1795. [Google Scholar] [CrossRef]
- Mutsaers, A.J.; Ng, A.J.M.; Baker, E.K.; Russell, M.R.; Chalk, A.M.; Wall, M.; Liddicoat, B.J.J.; Ho, P.W.M.; Slavin, J.L.; Goradia, A.; et al. Modeling Distinct Osteosarcoma Subtypes in Vivo Using Cre:Lox and Lineage-Restricted Transgenic ShRNA. Bone 2013, 55, 166–178. [Google Scholar] [CrossRef]
- Walkley, C.R.; Qudsi, R.; Sankaran, V.G.; Perry, J.A.; Gostissa, M.; Roth, S.I.; Rodda, S.J.; Snay, E.; Dunning, P.; Fahey, F.H.; et al. Conditional Mouse Osteosarcoma, Dependent on P53 Loss and Potentiated by Loss of Rb, Mimics the Human Disease. Genes Dev. 2008, 22, 1662. [Google Scholar] [CrossRef]
- Berman, S.D.; Calo, E.; Landman, A.S.; Danielian, P.S.; Miller, E.S.; West, J.C.; Fonhoue, B.D.; Caron, A.; Bronson, R.; Bouxsein, M.L.; et al. Metastatic Osteosarcoma Induced by Inactivation of Rb and P53 in the Osteoblast Lineage. Proc. Natl. Acad. Sci. USA 2008, 105, 11851. [Google Scholar] [CrossRef]
- Grigoriadis, A.E.; Schellander, K.; Wang, Z.Q.; Wagner, E.F. Osteoblasts Are Target Cells for Transformation in C-Fos Transgenic Mice. J. Cell Biol. 1993, 122, 685. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.J.; Mutsaers, A.J.; Baker, E.K.; Walkley, C.R. Genetically Engineered Mouse Models and Human Osteosarcoma. Clin. Sarcoma Res. 2012, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Donehower, L.A.; Harvey, M.; Slagle, B.L.; McArthur, M.J.; Montgomery, C.A.; Butel, J.S.; Bradley, A. Mice Deficient for P53 Are Developmentally Normal but Susceptible to Spontaneous Tumours. Nature 1992, 356, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Aurisicchio, L.; Impellizeri, J.A.; Cavallo, F. The Importance of Comparative Oncology in Translational Medicine. Cancer Immunol. Immunother. 2015, 64, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Sapino, S.; Chindamo, G.; Peira, E.; Vercelli, C.; Riganti, C.; Manzoli, M.; Gambino, G.; Re, G.; Gallarate, M. Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics 2022, 14, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ram Kumar, R.M.; Arlt, M.J.E.; Kuzmanov, A.; Born, W.; Fuchs, B. Sunitinib Malate (SU-11248) Reduces Tumour Burden and Lung Metastasis in an Intratibial Human Xenograft Osteosarcoma Mouse Model. Am. J. Cancer Res. 2015, 5, 2156. [Google Scholar]
- Rodriguez, C.O.; Crabbs, T.A.; Wilson, D.W.; Cannan, V.A.; Skorupski, K.A.; Gordon, N.; Koshkina, N.; Kleinerman, E.; Anderson, P.M. Aerosol Gemcitabine: Preclinical Safety and in Vivo Antitumor Activity in Osteosarcoma-Bearing Dogs. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 197–206. [Google Scholar] [CrossRef]
- Gordon, N.B.; Kleinerman, E.; Sheshadri, A.; Blanco, D.; Yedururi, S.; Morani, A.; Gill, J.B.; Harrison, D.J.; Herzog, C.E.; Livingston, J.A.; et al. A Phase I Trial of Aerosol Gemcitabine for the Treatment of Patients with Solid Tumors and Lung Metastases. J. Clin. Oncol. 2020, 38, TPS3645. [Google Scholar] [CrossRef]
- Study of Aerosol Gemcitabine in Patients with Solid Tumors and Pulmonary Metastases—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03093909 (accessed on 13 December 2022).
- Yang, Y.T.; Yuzbasiyan-Gurkan, V. Sorafenib and Doxorubicin Show Synergistic Effects in Human and Canine Osteosarcoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9345. [Google Scholar] [CrossRef]
- Inkol, J.M.; Poon, A.C.; Mutsaers, A.J. Inhibition of Copper Chaperones Sensitizes Human and Canine Osteosarcoma Cells to Carboplatin Chemotherapy. Vet. Comp. Oncol. 2020, 18, 559–569. [Google Scholar] [CrossRef]
- Sánchez-Céspedes, R.; Accornero, P.; Miretti, S.; Martignani, E.; Gattino, F.; Maniscalco, L.; Gola, C.; Iussich, S.; Martano, M.; Morello, E.; et al. In Vitro and in Vivo Effects of Toceranib Phosphate on Canine Osteosarcoma Cell Lines and Xenograft Orthotopic Models. Vet. Comp. Oncol. 2020, 18, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wouda, R.M.; Hocker, S.E.; Higginbotham, M.L. Safety Evaluation of Combination Carboplatin and Toceranib Phosphate (Palladia) in Tumour-Bearing Dogs: A Phase I Dose Finding Study. Vet. Comp. Oncol. 2018, 16, E52–E60. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.D.; Pankajavally, S.; Pillai, S.; Eason, B.; Iii, H.; Lanigan, L.G.; Martin, C.K.; Werbeck, J.L.; Rosol, T.J. Effect of Zoledronic Acid and Amputation on Bone Invasion and Lung Metastasis of Canine Osteosarcoma in Nude Mice. Clin. Exp. Metastasis. 2011, 28, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Ahn, J.H.; Choi, A.; Cho, W.H.; Lee, J.A.; Kim, D.H.; Seo, J.-H.; Lim, J.S. Efficacy of Pamidronate in Pediatric Osteosarcoma Patients with Low Bone Mineral Density. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Endo-Munoz, L.; Bennett, T.C.; Topkas, E.; Wu, S.Y.; Thamm, D.H.; Brockley, L.; Cooper, M.; Sommerville, S.; Thomson, M.; O’Connell, K.; et al. Auranofin Improves Overall Survival When Combined with Standard of Care in a Pilot Study Involving Dogs with Osteosarcoma. Vet. Comp. Oncol. 2020, 18, 206–213. [Google Scholar] [CrossRef]
- Rainusso, N.; Brawley, V.S.; Ghazi, A.; Hicks, M.J.; Gottschalk, S.; Rosen, J.M.; Ahmed, N. Immunotherapy Targeting HER2 with Genetically Modified T Cells Eliminates Tumor-Initiating Cells in Osteosarcoma. Cancer Gene Ther. 2012, 19, 212–217. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Mata, M.; Vera, J.; Gerken, C.; Rooney, C.M.; Miller, T.; Pfent, C.; Wang, L.L.; Wilson-Robles, H.M.; Gottschalk, S. Towards Immunotherapy with Redirected T Cells in a Large Animal Model: Ex Vivo Activation, Expansion, and Genetic Modification of Canine T Cells. J. Immunother. 2014, 37, 407. [Google Scholar] [CrossRef]
- Mason, N.J.; Gnanandarajah, J.S.; Engiles, J.B.; Gray, F.; Laughlin, D.; Gaurnier-Hausser, A.; Wallecha, A.; Huebner, M.; Paterson, Y. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin. Cancer Res. 2016, 22, 4380–4390. [Google Scholar] [CrossRef]
- Doyle, H.A.; Gee, R.J.; Masters, T.D.; Gee, C.R.; Booth, C.J.; Peterson-Roth, E.; Koski, R.A.; Helfand, S.C.; Price, L.; Bascombe, D.; et al. Vaccine-Induced ErbB (EGFR/HER2)-Specific Immunity in Spontaneous Canine Cancer. Transl. Oncol. 2021, 14, 101205. [Google Scholar] [CrossRef]
- Khanna, C.; Prehn, J.; Hayden, D.; Cassaday, R.D.; Caylor, J.; Jacob, S.; Bose, S.M.; Hong, S.-H.; Hewitt, S.M.; Helman, L.J.; et al. A Randomized Controlled Trial of Octreotide Pamoate Long-Acting Release and Carboplatin versus Carboplatin Alone in Dogs with Naturally Occurring Osteosarcoma: Evaluation of Insulin-like Growth Factor Suppression and Chemotherapy. Clin. Cancer Res. 2002, 8, 2406–2412. [Google Scholar] [PubMed]
- Anderson, P.M.; Bielack, S.S.; Gorlick, R.G.; Skubitz, K.; Daw, N.C.; Herzog, C.E.; Monge, O.R.; Lassaletta, A.; Boldrini, E.; Pápai, Z.; et al. A Phase II Study of Clinical Activity of SCH 717454 (Robatumumab) in Patients with Relapsed Osteosarcoma and Ewing Sarcoma. Pediatr. Blood Cancer 2016, 63, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Melacarne, A.; Aralla, M.; Sabattini, S.; Tiraboschi, L.; Ferrari, V.; Zeira, O.; Balboni, A.; Faroni, E.; Guerra, D.; et al. A Target Animal Effectiveness Study on Adjuvant Peptide-Based Vaccination in Dogs with Non-Metastatic Appendicular Osteosarcoma Undergoing Amputation and Chemotherapy. Cancers. 2022, 14, 1347. [Google Scholar] [CrossRef]
- Melacarne, A.; Ferrari, V.; Tiraboschi, L.; Mishto, M.; Liepe, J.; Aralla, M.; Marconato, L.; Lizier, M.; Pozzi, C.; Zeira, O.; et al. Identification of a Class of Non-Conventional ER-Stress-Response-Derived Immunogenic Peptides. Cell Rep. 2021, 36, 109312. [Google Scholar] [CrossRef] [PubMed]
- Macewen, E.G.; Kurzman, I.D.; Rosenthal, R.C.; Smith, B.W.; Manley, P.A.; Roush, J.K.; Howard, P.E. Therapy for Osteosarcoma in Dogs with Intravenous Injection of Liposome-Encapsulated Muramyl Tripeptide. J. Natl. Cancer Inst. 1989, 81, 935–938. [Google Scholar] [CrossRef]
- Kurzman, I.D.; Macewen, E.G.; Rosenthal, R.C.; Fox, L.E.; Keller, E.T.; Helfand, S.C.; Vail, D.M.; Dubielzig, R.R.; Madewell, B.R.; Rodriguez, C.O.; et al. Adjuvant Therapy for Osteosarcoma in Dogs: Results of Randomized Clinical Trials Using Combined Liposome-Encapsulated Muramyl Tripeptide and Cisplatin. Clin. Cancer Res. 1995, 1, 1595–1601. [Google Scholar]
- Kleinerman, E.S.; Gano, J.B.; Johnston, D.A.; Benjamin, R.S.; Jaffe, N. Efficacy of Liposomal Muramyl Tripeptide (CGP 19835A) in the Treatment of Relapsed Osteosarcoma. Am. J. Clin. Oncol. 1995, 18, 93–99. [Google Scholar] [CrossRef]
- Shi, F.; MacEwen, E.G.; Kurzman, I.D. In Vitro and in Vivo Effect of Doxorubicin Combined with Liposome-Encapsulated Muramyl Tripeptide on Canine Monocyte Activation. Cancer Res. 1993, 53, 3986–3991. [Google Scholar]
- Use of L-MTP-PE for the Treatment of Osteosarcoma-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04571229 (accessed on 13 December 2022).
- Regan, D.P.; Chow, L.; Das, S.; Haines, L.; Palmer, E.; Kurihara, J.N.; Coy, J.W.; Mathias, A.; Thamm, D.H.; Gustafson, D.L.; et al. Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined With the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma. Clin. Cancer Res. 2022, 28, 662–676. [Google Scholar] [CrossRef]
- Rebhun, R.B.; York, D.; Cruz, S.M.; Judge, S.J.; Razmara, A.M.; Farley, L.E.; Brady, R.V.; Johnson, E.G.; Burton, J.H.; Willcox, J.; et al. Inhaled Recombinant Human IL-15 in Dogs with Naturally Occurring Pulmonary Metastases from Osteosarcoma or Melanoma: A Phase 1 Study of Clinical Activity and Correlates of Response. J. Immunother. Cancer 2022, 10, e004493. [Google Scholar] [CrossRef]
- Schultz, M. Rudolf Virchow. Emerg. Infect. Dis. 2008, 14, 1480. [Google Scholar] [CrossRef]
- Paoloni, M.C.; Khanna, C. Comparative Oncology Today. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Paoloni, M.; Mazcko, C.; Khanna, C. The Comparative Oncology Trials Consortium: Using Spontaneously Occurring Cancers in Dogs to Inform the Cancer Drug Development Pathway. PLoS Med. 2009, 6, e1000161. [Google Scholar] [CrossRef] [PubMed]
- Barutello, G.; Rolih, V.; Arigoni, M.; Tarone, L.; Conti, L.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. Strengths and Weaknesses of Pre-Clinical Models for Human Melanoma Treatment: Dawn of Dogs’ Revolution for Immunotherapy. Int. J. Mol. Sci. 2018, 19, 799. [Google Scholar] [CrossRef] [PubMed]
- Lequarré, A.S.; Andersson, L.; André, C.; Fredholm, M.; Hitte, C.; Leeb, T.; Lohi, H.; Lindblad-Toh, K.; Georges, M. LUPA: A European Initiative Taking Advantage of the Canine Genome Architecture for Unravelling Complex Disorders in Both Human and Dogs. Vet. J. 2011, 189, 155–159. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Institute of Medicine, Board on Health Care Services; National Cancer Policy Forum. The Role of Clinical Studies for Pets with Naturally Occurring Tumors in Translational Cancer Research: Workshop Summary; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Rao, S.R.; Somarelli, J.A.; Altunel, E.; Selmic, L.E.; Byrum, M.; Sheth, M.U.; Cheng, S.; Ware, K.E.; Kim, S.Y.; Prinz, J.A.; et al. From the Clinic to the Bench and Back Again in One Dog Year: How a Cross-Species Pipeline to Identify New Treatments for Sarcoma Illuminates the Path Forward in Precision Medicine. Front. Oncol. 2020, 10, 117. [Google Scholar] [CrossRef]
- Rodriguez, C.O. Using Canine Osteosarcoma as a Model to Assess Efficacy of Novel Therapies: Can Old Dogs Teach Us New Tricks? Adv. Exp. Med. Biol. 2014, 804, 237–256. [Google Scholar] [CrossRef]
- Fan, T.M.; Khanna, C. Comparative Aspects of Osteosarcoma Pathogenesis in Humans and Dogs. Vet. Sci. 2015, 2, 210–230. [Google Scholar] [CrossRef]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Lazarides, A.L.; Putterman, A.B.; Eward, W.C.; Eward, C. A Dog in the Cancer Fight: Comparative Oncology in Osteosarcoma. In Osteosarcoma—Biology, Behavior and Mechanisms; InTechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Morello, E.; Martano, M.; Buracco, P. Biology, Diagnosis and Treatment of Canine Appendicular Osteosarcoma: Similarities and Differences with Human Osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine Osteosarcoma: A Naturally Occurring Disease to Inform Pediatric Oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Tarone, L.; Barutello, G.; Iussich, S.; Giacobino, D.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. Naturally Occurring Cancers in Pet Dogs as Pre-Clinical Models for Cancer Immunotherapy. Cancer Immunol. Immunother. 2019, 68, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Tarone, L.; Buracco, P.; Cavallo, F.; Riccardo, F. Canine Melanoma and Osteosarcoma Immunotherapy by Means of In Vivo DNA Electroporation. In Electroporation in Veterinary Oncology Practice; Springer: Cham, Switzerland, 2021; pp. 277–304. [Google Scholar] [CrossRef]
- Rowell, J.L.; McCarthy, D.O.; Alvarez, C.E. Dog Models of Naturally Occurring Cancer. Trends Mol. Med. 2011, 17, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.L.; Sivaprakasam, K.; Briones, N.; Zismann, V.; Perdigones, N.; Drenner, K.; Facista, S.; Richholt, R.; Liang, W.; Aldrich, J.; et al. Canine Osteosarcoma Genome Sequencing Identifies Recurrent Mutations in DMD and the Histone Methyltransferase Gene SETD2. Commun. Biol. 2019, 2, 266. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Mazcko, C.N. Improving Human Cancer Therapy through the Evaluation of Pet Dogs. Nat. Rev. Cancer 2020, 20, 727–742. [Google Scholar] [CrossRef]
- Megquier, K.; Turner-Maier, J.; Morrill, K.; Li, X.; Johnson, J.; Karlsson, E.K.; London, C.A.; Gardner, H.L. The Genomic Landscape of Canine Osteosarcoma Cell Lines Reveals Conserved Structural Complexity and Pathway Alterations. PLoS ONE 2022, 17, e0274383. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative Review of Human and Canine Osteosarcoma: Morphology, Epidemiology, Prognosis, Treatment and Genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef]
- Dernell, W.S. Tumours of the Skeletal System. In BSAVA Manual of Canine and Feline Oncology; British Small Animal Veterinary Association: Gloucester, UK, 2011; Chapter 13; pp. 159–177. [Google Scholar] [CrossRef]
- Grignani, G.; Palmerini, E.; Ferraresi, V.; D’Ambrosio, L.; Bertulli, R.; Asaftei, S.D.; Tamburini, A.; Pignochino, Y.; Sangiolo, D.; Marchesi, E.; et al. Sorafenib and Everolimus for Patients with Unresectable High-Grade Osteosarcoma Progressing after Standard Treatment: A Non-Randomised Phase 2 Clinical Trial. Lancet. Oncol. 2015, 16, 98–107. [Google Scholar] [CrossRef]
- Pignochino, Y.; Grignani, G.; Cavalloni, G.; Motta, M.; Tapparo, M.; Bruno, S.; Bottos, A.; Gammaitoni, L.; Migliardi, G.; Camussi, G.; et al. Sorafenib Blocks Tumour Growth, Angiogenesis and Metastatic Potential in Preclinical Models of Osteosarcoma through a Mechanism Potentially Involving the Inhibition of ERK1/2, MCL-1 and Ezrin Pathways. Mol. Cancer 2009, 8, 118. [Google Scholar] [CrossRef]
- Grignani, G.; Palmerini, E.; Dileo, P.; Asaftei, S.D.; D’ambrosio, L.; Pignochino, Y.; Mercuri, M.; Picci, P.; Fagioli, F.; Casali, P.G.; et al. A Phase II Trial of Sorafenib in Relapsed and Unresectable High-Grade Osteosarcoma after Failure of Standard Multimodal Therapy: An Italian Sarcoma Group Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 508–516. [Google Scholar] [CrossRef]
- Wang, J.; Hu, F.; Yu, P.; Wang, J.; Liu, Z.; Bao, Q.; Zhang, W.; Wen, J. Sorafenib Inhibits Doxorubicin-Induced PD-L1 Upregulation to Improve Immunosuppressive Microenvironment in Osteosarcoma. J. Cancer Res. Clin. Oncol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Son, J.K.; Kim, D.H.; Lee, J.; Kim, S.B.; Park, B.Y.; Kim, M.; Lee, S.; Hur, T.Y.; Kim, E.T. Retrospective Evaluation of Toceranib Phosphate (Palladia) for Treatment of Different Tumor Types in 31 Dogs. Korean J. Vet. Res. 2021, 61, 10.1–10.11. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Cortazar, P.; Zhang, J.J.; Tang, S.; Sridhara, R.; Murgo, A.; Justice, R.; Pazdur, R. FDA Approval Summary: Sunitinib for the Treatment of Progressive Well-Differentiated Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors. Oncologist 2012, 17, 1108. [Google Scholar] [CrossRef] [PubMed]
- Zekria, J.; Mansour, M.; Karim, S.M. The Anti-Tumour Effects of Zoledronic Acid. J. Bone Oncol. 2014, 3, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Cates, J.M.M.; Cole, H.A.; Slosky, D.A.; Haro, H.; Ichikawa, J.; Ando, T.; Schwartz, H.S.; Schoenecker, J.G. Pleiotropic Effects of Bisphosphonates on Osteosarcoma. Bone 2014, 63, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Hoddinott, K.; Oblak, M.L.; Wood, G.A.; Boston, S.; Mutsaers, A.J. Effect of Timing of Bisphosphonate Administration on Canine Osteosarcoma Cells Undergoing Radiation Therapy. Can. J. Vet. Res. 2020, 84, 225. [Google Scholar]
- Suva, L.J.; Cooper, A.; Watts, A.E.; Ebetino, F.H.; Price, J.; Gaddy, D. Bisphosphonates in Veterinary Medicine: The New Horizon for Use. Bone 2021, 142, 115711. [Google Scholar] [CrossRef]
- Gesto, S.D.; Cerqueira, M.F.S.A.N.; Fernandes, A.P.; Ramos, J.M. Gemcitabine: A Critical Nucleoside for Cancer Therapy. Curr. Med. Chem. 2012, 19, 1076–1087. [Google Scholar] [CrossRef]
- Nabhan, C.; Krett, N.; Gandhi, V.; Rosen, S. Gemcitabine in Hematologic Malignancies. Curr. Opin. Oncol. 2001, 13, 514–521. [Google Scholar] [CrossRef]
- Palmerini, E.; Jones, R.L.; Marchesi, E.; Paioli, A.; Cesari, M.; Longhi, A.; Meazza, C.; Coccoli, L.; Fagioli, F.; Asaftei, S.; et al. Gemcitabine and Docetaxel in Relapsed and Unresectable High-Grade Osteosarcoma and Spindle Cell Sarcoma of Bone. BMC Cancer 2016, 16, 280. [Google Scholar] [CrossRef]
- McMahon, M.B.; Bear, M.D.; Kulp, S.K.; Pennell, M.L.; London, C.A. Biological Activity of Gemcitabine against Canine Osteosarcoma Cell Lines in Vitro. Am. J. Vet. Res. 2010, 71, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Zhuang, Y.F.; Wang, W.M. Gemcitabine for the Treatment of Patients with Osteosarcoma. Asian Pac. J. Cancer Prev. 2014, 15, 7159–7162. [Google Scholar] [CrossRef] [PubMed]
- Selting, K.A.; Bechtel, S.M.; Espinosa, J.; Henry, C.J.; Tate, D.; Bryan, J.N.; Rajewski, L.; Flesner, B.K.; Decedue, C.; Baltezor, M. Evaluation of Intravenous and Subcutaneous Administration of a Novel, Excipient-Free, Nanoparticulate Formulation of Paclitaxel in Dogs with Spontaneously Occurring Neoplasia. Vet. Comp. Oncol. 2018, 16, 650–657. [Google Scholar] [CrossRef]
- Silva, D.M.; Franciosi, A.I.; Pezzini, P.C.F.; Guérios, S.D. Subcutaneous Administration of Paclitaxel in Dogs with Cancer: A Preliminary Study. Can. Vet. J. 2015, 56, 823. [Google Scholar] [PubMed]
- Khanna, C.; Rosenberg, M.; Vail, D.M. A Review of Paclitaxel and Novel Formulations Including Those Suitable for Use in Dogs. J. Vet. Intern. Med. 2015, 29, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Coccè, V.; Masia, C.; Milani, M.; Salè, E.O.; Alessandri, G.; Parati, E.; Sisto, F.; Pentimalli, F.; Brini, A.T.; et al. Paclitaxel-Releasing Mesenchymal Stromal Cells Inhibit in Vitro Proliferation of Human Mesothelioma Cells. Biomed. Pharmacother. 2017, 87, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Zeira, O.; Ghezzi, E.; Pettinari, L.; Re, V.; Lupi, D.M.; Benali, S.L.; Borgonovo, S.; Alessandri, G.; Petrella, F.; Paroni, R.; et al. Case Report: Microfragmented Adipose Tissue Drug Delivery in Canine Mesothelioma: A Case Report on Safety, Feasibility, and Clinical Findings. Front. Vet. Sci. 2021, 7, 585427. [Google Scholar] [CrossRef]
- Capelli, C.; Frigerio, S.; Lisini, D.; Nava, S.; Gaipa, G.; Belotti, D.; Cabiati, B.; Budelli, S.; Lazzari, L.; Bagnarino, J.; et al. A Comprehensive Report of Long-Term Stability Data for a Range ATMPs: A Need to Develop Guidelines for Safe and Harmonized Stability Studies. Cytotherapy 2022, 24, 544–556. [Google Scholar] [CrossRef]
- Ganapathy, D.; Sekar, D.; Preethi, A.; Shanmugam, R. Clinical Impact of Medicinal Herbs in the Treatment of Osteosarcoma. Ann. Rom. Soc. Cell Biol. 2021, 25, 2503–2508. [Google Scholar]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules. 2022, 27, 3209. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Zahedipour, F.; Bolourinezhad, M.; Teng, Y.; Sahebkar, A. The Multifaceted Therapeutic Mechanisms of Curcumin in Osteosarcoma: State-of-the-Art. J. Oncol. 2021, 2021, 3006853. [Google Scholar] [CrossRef]
- Xu, C.; Wang, M.; Guo, W.; Sun, W.; Liu, Y. Curcumin in Osteosarcoma Therapy: Combining With Immunotherapy, Chemotherapeutics, Bone Tissue Engineering Materials and Potential Synergism With Photodynamic Therapy. Front. Oncol. 2021, 11, 672490. [Google Scholar] [CrossRef] [PubMed]
- BİLDİK, A.; BAYAR, İ.; AŞICI, G.S.E.; KIRAL, F.; ULUTAŞ, P.A. Cytotoxic and Apoptotic Eff Ects of Curcumin on D-17 Canine Osteosarcoma Cell Line. Kafkas Üniversitesi Vet. Fakültesi Derg. 2021, 27, 465–473. [Google Scholar] [CrossRef]
- Stanić, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.S.; York, D.; Johnson, E.; Al-Nadaf, S.; Skorupski, K.A.; Rodriguez, C.O.; Burton, J.H.; Guerrero, T.; Sein, K.; Wittenburg, L.; et al. In Vitro and In Vivo Activity of Liposome Encapsulated Curcumin for Naturally Occurring Canine Cancers. Vet. Comp. Oncol. 2018, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Soltaninejad, H.; Ghorani-Azam, A.; Nafisi-Moghadam, R.; Haddadzadegan, N.; Ansari, M.; Saeed-Banadaki, S.H.; Sobhan, M.R.; Mozafari, S.; Zahedi, M. Slow Release Curcumin-Containing Soy Protein Nanoparticles as Anticancer Agents for Osteosarcoma: Synthesis and Characterization. Prog. Biomater. 2022, 11, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.N.M.; Rahim, N.F.C.; Hussin, Y.; Yeap, S.K.; Masarudin, M.J.; Mohamad, N.E.; Akhtar, M.N.; Osman, M.A.; Cheah, Y.K.; Alitheen, N.B. Anti-Metastatic and Anti-Angiogenic Effects of Curcumin Analog DK1 on Human Osteosarcoma Cells In Vitro. Pharm. 2021, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Constanze, B.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Evidence That TNF-β Suppresses Osteoblast Differentiation of Mesenchymal Stem Cells and Resveratrol Reverses It through Modulation of NF-ΚB, Sirt1 and Runx2. Cell Tissue Res. 2020, 381, 83–98. [Google Scholar] [CrossRef]
- De Luca, A.; Bellavia, D.; Raimondi, L.; Carina, V.; Costa, V.; Fini, M.; Giavaresi, G. Multiple Effects of Resveratrol on Osteosarcoma Cell Lines. Pharmaceuticals. 2022, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Xie, Y.; Zhang, J.; Wang, Q.; Xu, X. Resveratrol Induces Apoptosis in Human Osteosarcoma MG63 Cells. Chin J Clin Oncol 2008, 5, 361–366. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, D. Resveratrol Eliminates Cancer Stem Cells of Osteosarcoma by STAT3 Pathway Inhibition. PLoS ONE 2018, 13, e0205918. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol Suppresses Cross-Talk between Colorectal Cancer Cells and Stromal Cells in Multicellular Tumor Microenvironment: A Bridge between In Vitro and In Vivo Tumor Microenvironment Study. Molecules. 2020, 25, 4292. [Google Scholar] [CrossRef]
- Carlson, A.; Alderete, K.S.; Grant, M.K.O.; Seelig, D.M.; Sharkey, L.C.; Zordoky, B.N.M. Anticancer Effects of Resveratrol in Canine Hemangiosarcoma Cell Lines. Vet. Comp. Oncol. 2018, 16, 253. [Google Scholar] [CrossRef]
- Ege, B.; Yumrutas, O.; Ege, M.; Pehlivan, M.; Bozgeyik, I. Pharmacological Properties and Therapeutic Potential of Saffron (Crocus Sativus L.) in Osteosarcoma. J. Pharm. Pharmacol. 2020, 72, 56–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.Z.; Wen, X.D.; Shoyama, Y.; Yuan, C.S. Role of Saffron and Its Constituents on Cancer Chemoprevention. Pharm. Biol. 2013, 51, 920. [Google Scholar] [CrossRef]
- Levine, C.B.; Bayle, J.; Biourge, V.; Wakshlag, J.J. Cellular Effects of a Turmeric Root and Rosemary Leaf Extract on Canine Neoplastic Cell Lines. BMC Vet. Res. 2017, 13, 388. [Google Scholar] [CrossRef]
- Li, X.; Huang, T.; Jiang, G.; Gong, W.; Qian, H.; Zou, C. Synergistic Apoptotic Effect of Crocin and Cisplatin on Osteosarcoma Cells via Caspase Induced Apoptosis. Toxicol. Lett. 2013, 221, 197–204. [Google Scholar] [CrossRef]
- Liptak, J.M.; Dernell, W.S.; Ehrhart, N.; Lafferty, M.H.; Monteith, G.J.; Withrow, S.J. Cortical Allograft and Endoprosthesis for Limb-Sparing Surgery in Dogs with Distal Radial Osteosarcoma: A Prospective Clinical Comparison of Two Different Limb-Sparing Techniques. Vet. Surg. 2006, 35, 518–533. [Google Scholar] [CrossRef]
- Jeys, L.M.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A. Post Operative Infection and Increased Survival in Osteosarcoma Patients: Are They Associated? Ann. Surg. Oncol. 2007, 14, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; Olea-Popelka, F.; Sefton, J.; Aldridge, C.F.; Withrow, S.J.; Lafferty, M.H.; Rebhun, R.B.; Kent, M.S.; Ehrhart, N. Evaluation of Outcome and Prognostic Factors for Dogs Living Greater than One Year after Diagnosis of Osteosarcoma: 90 Cases (1997–2008). J. Am. Vet. Med. Assoc. 2014, 245, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma (SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed Cell Death 1 (PD-1) Targeting in Patients with Advanced Osteosarcomas: Results from the PEMBROSARC Study. Eur. J. Cancer 2019, 119, 151–157. [Google Scholar] [CrossRef]
- Meftahpour, V.; Aghebati-Maleki, A.; Fotouhi, A.; Safarzadeh, E.; Aghebati-Maleki, L. Prognostic Significance and Therapeutic Potentials of Immune Checkpoints in Osteosarcoma. EXCLI J. 2022, 21, 250. [Google Scholar] [CrossRef]
- Miao, Y.R.; Thakkar, K.N.; Qian, J.; Kariolis, M.S.; Huang, W.; Nandagopal, S.; Tat, T.; Yang, C.; Diep, A.N.; Cherf, G.M.; et al. Neutralization of PD-L2 Is Essential for Overcoming Immune Checkpoint Blockade Resistance in Ovarian Cancer. Clin. Cancer Res. 2021, 27, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zheng, B.; Huang, Y.; Wang, S.; Bao, X.; Liu, K.; Guo, W. Osteosarcoma Cell Intrinsic PD-L2 Signals Promote Invasion and Metastasis via the RhoA-ROCK-LIMK2 and Autophagy Pathways. Cell Death Dis. 2019, 10, 261. [Google Scholar] [CrossRef]
- Solinas, C.; Aiello, M.; Rozali, E.; Lambertini, M.; Willard-Gallo, K.; Migliori, E. Programmed Cell Death-Ligand 2: A Neglected But Important Target in the Immune Response to Cancer? Transl. Oncol. 2020, 13, 100811. [Google Scholar] [CrossRef]
- Stevenson, V.B.; Perry, S.N.; Todd, M.; Huckle, W.R.; LeRoith, T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet. Pathol. 2021, 58, 692–698. [Google Scholar] [CrossRef]
- Flint, A.F.; U’Ren, L.; Legare, M.E.; Withrow, S.J.; Dernell, W.; Hanneman, W.H. Overexpression of the ErbB-2 Proto-Oncogene in Canine Osteosarcoma Cell Lines and Tumors. Vet. Pathol. 2004, 41, 291–296. [Google Scholar] [CrossRef]
- Mardanpour, K.; Rahbar, M.; Mardanpour, S. Coexistence of HER2, Ki67, and P53 in Osteosarcoma: A Strong Prognostic Factor. N. Am. J. Med. Sci. 2016, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Salsman, V.S.; Yvon, E.; Louis, C.U.; Perlaky, L.; Wels, W.S.; Dishop, M.K.; Kleinerman, E.E.; Pule, M.; Rooney, C.M.; et al. Immunotherapy for Osteosarcoma: Genetic Modification of T Cells Overcomes Low Levels of Tumor Antigen Expression. Mol. Ther. 2009, 17, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2)-Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, J.C.; Rodeck, U.; Snook, A.E. Listeria Monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines. 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, M.; Paterson, Y.; Wood, L.M. Clinical Experience and Recent Advances in the Development of Listeria-Based Tumor Immunotherapies. Front. Immunol. 2021, 12, 642316. [Google Scholar] [CrossRef]
- Musser, M.L.; Berger, E.P.; Parsons, C.; Kathariou, S.; Johannes, C.M. Vaccine Strain Listeria Monocytogenes Abscess in a Dog: A Case Report. BMC Vet. Res. 2019, 15, 467. [Google Scholar] [CrossRef]
- Musser, M.L.; Berger, E.P.; Tripp, C.D.; Clifford, C.A.; Bergman, P.J.; Johannes, C.M. Safety Evaluation of the Canine Osteosarcoma Vaccine, Live Listeria Vector. Vet. Comp. Oncol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Maniscalco, L.; Iussich, S.; Morello, E.; Martano, M.; Gattino, F.; Miretti, S.; Biolatti, B.; Accornero, P.; Martignani, E.; Sánchez-Céspedes, R.; et al. Increased Expression of Insulin-like Growth Factor-1 Receptor Is Correlated with Worse Survival in Canine Appendicular Osteosarcoma. Vet. J. 2015, 205, 272–280. [Google Scholar] [CrossRef]
- Broqueza, J.; Prabaharan, C.B.; Andrahennadi, S.; Allen, K.J.H.; Dickinson, R.; Macdonald-Dickinson, V.; Dadachova, E.; Uppalapati, M. Novel Human Antibodies to Insulin Growth Factor 2 Receptor (IGF2R) for Radioimmunoimaging and Therapy of Canine and Human Osteosarcoma. Cancers 2021, 13, 2208. [Google Scholar] [CrossRef]
- Karkare, S.; Allen, K.J.H.; Jiao, R.; Malo, M.E.; Dawicki, W.; Helal, M.; Godson, D.L.; Dickinson, R.; MacDonald-Dickinson, V.; Yang, R.; et al. Detection and Targeting Insulin Growth Factor Receptor Type 2 (IGF2R) in Osteosarcoma PDX in Mouse Models and in Canine Osteosarcoma Tumors. Sci. Rep. 2019, 9, 11476. [Google Scholar] [CrossRef]
- Broqueza, J.; Prabaharan, C.B.; Allen, K.J.H.; Jiao, R.; Fisher, D.R.; Dickinson, R.; Macdonald-Dickinson, V.; Uppalapati, M.; Dadachova, E. Radioimmunotherapy Targeting IGF2R on Canine-Patient-Derived Osteosarcoma Tumors in Mice and Radiation Dosimetry in Canine and Pediatric Models. Pharmaceuticals 2022, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Magee, K.; Marsh, I.R.; Turek, M.M.; Grudzinski, J.; Aluicio-Sarduy, E.; Engle, J.W.; Kurzman, I.D.; Zuleger, C.L.; Oseid, E.A.; Jaskowiak, C.; et al. Safety and Feasibility of an in Situ Vaccination and Immunomodulatory Targeted Radionuclide Combination Immuno-Radiotherapy Approach in a Comparative (Companion Dog) Setting. PLoS ONE 2021, 16, e0255798. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The Addition of Muramyl Tripeptide to Chemotherapy Improves Overall Survival--a Report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Chou, A.J. Muramyl Tripeptide-Phosphatidyl Ethanolamine Encapsulated in Liposomes (L-MTP-PE) in the Treatment of Osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 307–321. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Dernell, W.S.; Correa, M.T.; Lafferty, M.; Devitt, C.M.; Kuntz, C.A.; Straw, R.C.; Withrow, S.J. Improved Survival Associated with Postoperative Wound Infection in Dogs Treated with Limb-Salvage Surgery for Osteosarcoma. Ann. Surg. Oncol. 2005, 12, 1073–1083. [Google Scholar] [CrossRef]
- Ando, K.; Mori, K.; Corradini, N.; Redini, F.; Heymann, D. Mifamurtide for the Treatment of Nonmetastatic Osteosarcoma. Expert Opin. Pharmacother. 2011, 12, 285–292. [Google Scholar] [CrossRef]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma Treatment—Where Do We Stand? A State of the Art Review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Georgoudaki, A.M.; Prokopec, K.E.; Boura, V.F.; Hellqvist, E.; Sohn, S.; Östling, J.; Dahan, R.; Harris, R.A.; Rantalainen, M.; Klevebring, D.; et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep. 2016, 15, 2000–2011. [Google Scholar] [CrossRef]
- Punzo, F.; Bellini, G.; Tortora, C.; Di Pinto, D.; Argenziano, M.; Pota, E.; Di Paola, A.; Di Martino, M.; Rossi, F. Mifamurtide and TAM-like Macrophages: Effect on Proliferation, Migration and Differentiation of Osteosarcoma Cells. Oncotarget 2020, 11, 687–698. [Google Scholar] [CrossRef]
- Regan, D.P.; Coy, J.W.; Chahal, K.K.; Chow, L.; Kurihara, J.N.; Guth, A.M.; Kufareva, I.; Dow, S.W. The Angiotensin Receptor Blocker Losartan Suppresses Growth of Pulmonary Metastases via AT1R-Independent Inhibition of CCR2 Signaling and Monocyte Recruitment. J. Immunol. 2019, 202, 3087–3102. [Google Scholar] [CrossRef]
- Robinson, T.O.; Schluns, K.S. The Potential and Promise of IL-15 in Immuno-Oncogenic Therapies. Immunol. Lett. 2017, 190, 159. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Dubois, S.; Miljkovic, M.D.; Conlon, K.C. IL-15 in the Combination Immunotherapy of Cancer. Front. Immunol. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.; Colvin Wanshura, L.E.; Yang, J.; Carlson, J.; Xiang, B.; Li, G.; Ferrone, S.; Dudek, A.Z.; Turley, E.A.; McCarthy, J.B. CSPG4, a Potential Therapeutic Target, Facilitates Malignant Progression of Melanoma. Pigment Cell Melanoma Res. 2011, 24, 1148–1157. [Google Scholar] [CrossRef]
- Rolih, V.; Barutello, G.; Iussich, S.; De Maria, R.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. CSPG4: A Prototype Oncoantigen for Translational Immunotherapy Studies. J. Transl. Med. 2017, 15, 151. [Google Scholar] [CrossRef]
- Nicolosi, P.A.; Dallatomasina, A.; Perris, R. Theranostic Impact of NG2/CSPG4 Proteoglycan in Cancer. Theranostics. 2015, 5, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Beard, R.E.; Abate-Daga, D.; Rosati, S.F.; Zheng, Z.; Wunderlich, J.R.; Rosenberg, S.A.; Morgan, R.A. Gene Expression Profiling Using Nanostring Digital RNA Counting to Identify Potential Target Antigens for Melanoma Immunotherapy. Clin. Cancer Res. 2013, 19, 4941–4950. [Google Scholar] [CrossRef] [PubMed]
- Borner, P.J.; Thallmair, M.; Gage, F.H. Defining the NG2-Expressing Cell of the Adult CNS. J. Neurocytol. 2002, 31, 469–480. [Google Scholar] [CrossRef]
- Sato, S.; Tang, Y.J.; Wei, Q.; Hirata, M.; Weng, A.; Han, I.; Okawa, A.; Takeda, S.; Whetstone, H.; Nadesan, P.; et al. Mesenchymal Tumors Can Derive from Ng2/Cspg4-Expressing Pericytes with β-Catenin Modulating the Neoplastic Phenotype. Cell Rep. 2016, 16, 917–927. [Google Scholar] [CrossRef]
- Riccardo, F.; Tarone, L.; Iussich, S.; Giacobino, D.; Arigoni, M.; Sammartano, F.; Morello, E.; Martano, M.; Gattino, F.; De Maria, R.; et al. Identification of CSPG4 as a Promising Target for Translational Combinatorial Approaches in Osteosarcoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919855491. [Google Scholar] [CrossRef]
- Riccardo, F.; Tarone, L.; Camerino, M.; Giacobino, D.; Iussich, S.; Barutello, G.; Arigoni, M.; Conti, L.; Bolli, E.; Quaglino, E.; et al. Antigen Mimicry as an Effective Strategy to Induce CSPG4-Targeted Immunity in Dogs with Oral Melanoma: A Veterinary Trial. J. Immunother. Cancer 2022, 10, e004007. [Google Scholar] [CrossRef]
- Beard, R.E.; Zheng, Z.; Lagisetty, K.H.; Burns, W.R.; Tran, E.; Hewitt, S.M.; Abate-Daga, D.; Rosati, S.F.; Fine, H.A.; Ferrone, S.; et al. Multiple Chimeric Antigen Receptors Successfully Target Chondroitin Sulfate Proteoglycan 4 in Several Different Cancer Histologies and Cancer Stem Cells. J. Immunother. Cancer 2014, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Leuci, V.; Donini, C.; Grignani, G.; Rotolo, R.; Mesiano, G.; Fiorino, E.; Gammaitoni, L.; D’Ambrosio, L.; Merlini, A.; Landoni, E.; et al. CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes. Clin. Cancer Res. 2020, 26, 6321–6334. [Google Scholar] [CrossRef] [PubMed]
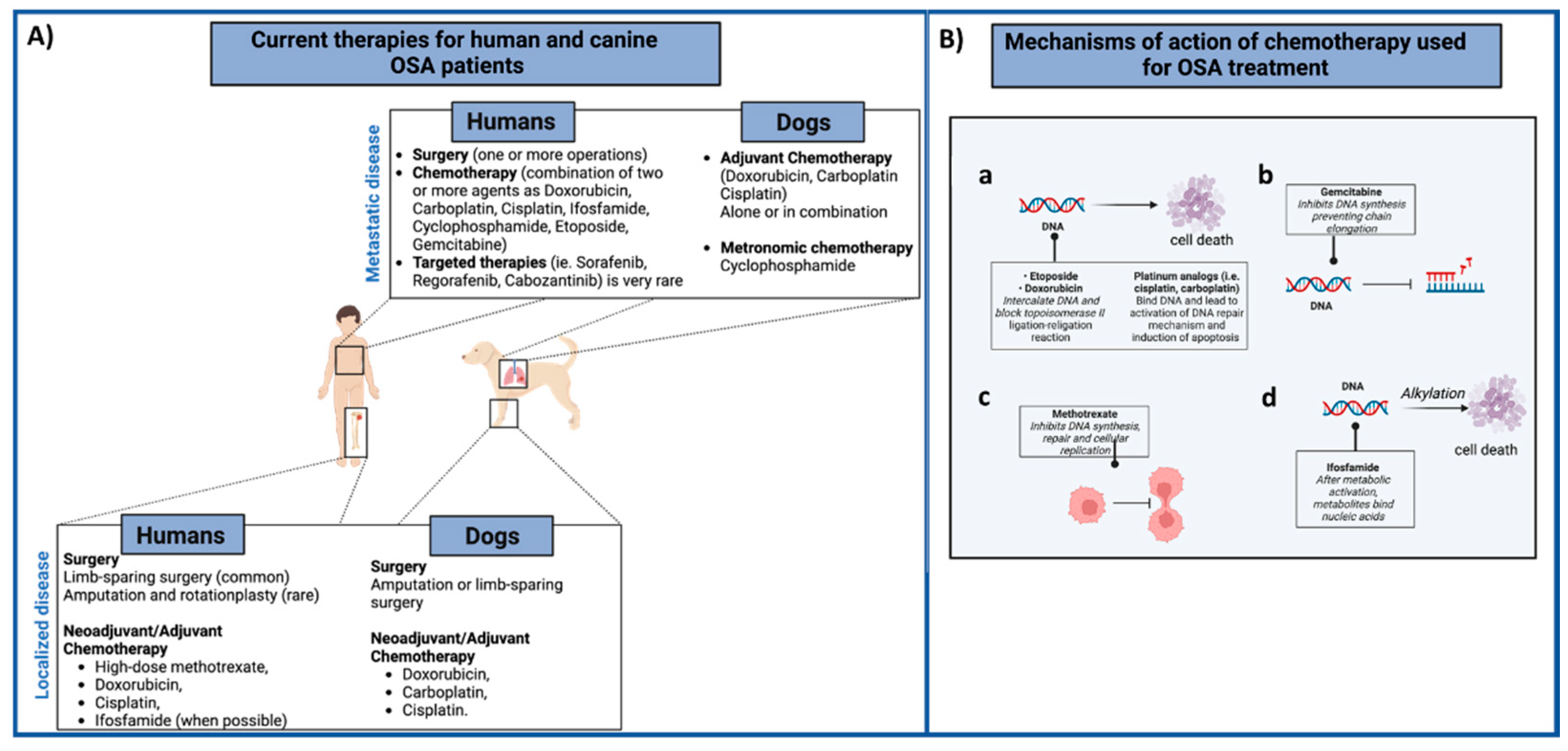
| Treatment | Testing | Results | Authors | |
|---|---|---|---|---|
| Single treatments (chemotherapy or targeted therapies) | Doxorubicin-loaded nanoparticles | Human and dog cell lines, in vitro | Increased drug uptake and cytotoxicity in chemo resistant cell lines | Chirio et al., 2022 [35] |
| Sunitinib (TKI) | Human cell lines in vitro Human PDX models in vivo | Reduced tumour burden, microvessel density and suppression of pulmonary metastasis | Ram et al., 2015 [36] | |
| Gemcitabine | Pivotal study in metastatic OSA-bearing dogs Phase I clinical trial | Aerosol administration of Gemcitabine, control of lung metastasis Treatment of human patients with solid tumors and lung metastasis (including OSA) | Rodriguez et al., 2010 [37]; Gordon et al., 2020 [38]; Ongoing https://clinicaltrials.gov/ct2/show/NCT03093909, (accessed on 13 December 2022) [39] | |
| Combination therapies (chemotherapy + targeted therapies/other compounds) | Doxorubicin + Sorafenib (multi-kinase inhibitor) | Human and dog cell lines, in vitro | Synergistic effects on tumor cell proliferation | Yang et al., 2022 [40] |
| Platinum-based chemotherapy + copper transporter inhibitors | Human and dog cell lines, in vitro | Decreased tumor cell proliferation, migration and clonogenic potential, increased apoptosis | Inkol et al., 2020 [41] | |
| Carboplatin+ Toracenib phosphate (TKRi targeting VEGFR, PDGFR; CSF1R; FLT3) | Canine OSA cell lines, in vitro Canine PDX models, in vivo Prospective phase I veterinary trial | Decreased tumor cells growth, migration and invasion, in vitro Reduced tumor size in vivo 2 OSA-bearing dogs enrolled, experienced progressive disease | Sanchez-Céspedez et al., 2020 [42]; Wouda et al., 2018 [43] | |
| Zoledronic acid and pamidronate alone or combined with chemotherapy | Orthotopic canine PDX model Retrospective study | Inhibition of osteolysis following engraftment and decreased metastasis Decreased bone pain after chemotherapy | Wolfe et al., 2011 [44]; Lim et al., 2016 [45] | |
| Carboplatin+ Auronafin | Pilot veterinary trial | Delayed metastasis, improved overall survival in stage II OSA-bearing dogs | Endo-Munoz et al., 2020 [46] | |
| Branded targets | HER-2 CAR-T cells therapy | OSA tumor initiating cells and human PDX models, in vivo and in vitro Human patients Canine OSA patients | Reduction of tumorigenicity in vitro and reduction of sarcosphere forming efficacy in vivo Tumor and metastasis regression, but severe adverse effects (cytokine storm) Human and canine HER2+ cells killing with no cytokine storm induction | Rainusso et al., 2012 [47]; Morgan et al., 2010 [48]; Mata et al., 2014 [49] |
| Listeria monocytogenes (Lm)-based vaccine expressing a chimeric human HER2 fusion protein | Phase I dose escalation trial in OSA-bearing dogs Phase Ib clinical trial (ADXS31-164 ) | The adjuvant vaccination impaired the development of lung metastasis and in prolonging the overall survival Adult patients with HER2+ tumors, licensed for pediatric OSA patients | Mason et al., 2016 [50]; Results not published yet | |
| EGFR/HER2 peptide-based vaccination | Phase I/II veterinary trial in dogs with OSA and other solid tumors | Induction of antibody response and inhibition of the ErbB/HER2 signaling in both canine and human cells in vitro, | Doyle et al., 2021 [51] | |
| Anti- IGF-1R monoclonal antibody (Romatumumab) | Randomized controlled veterinary trial in OSA-bearing dogs Clinical trial in relapsed human OSA patients | No improvement in the survival as compared to chemotherapy alone Few patients displayed complete response, with most of the others developing progressive disease | Khanna et al., 2002 [52]; Anderson et al., 2016 [53] | |
| Other immunomodulatory strategies | Non-conventional ER-stress-response-derived immunogenic peptides (ERstrePs) released upon Salmonella infection | Veterinary clinical trial including OSA bearing dogs | Development of speific immunity, delayed metastasis and improved survival as compared to historical controls | Marconato et al., 2022 [54]; Melacarne et al., 2021 [55] |
| Liposome-encapsulated lipophilic derivative of muramyl dipeptide (L-MTP-PE ) | Randomized clinical veterinary trial in OSA-bearing dogs Phase I/II clinical trial | Hampered metastatic spread and improved the survival, it was able to enhance both monocyte activation and the cytotoxic activity of macrophages against OSA cells Newly diagnosed or relapsed OSA patients | Macewen et al., 1989 [56]; Kurzman et al., 1995 [57]; Kleinerman et al., 1995 [58]; Shi et al., 1993 [59]; Ongoing https://clinicaltrals.gov/ct2/show/NCT04571229, (accessed on 13 December 2022) [60] | |
| Losartan + toceranib phosphate (Palladia) | Veterinary clinical trial in dogs with metastatic OSA | 50% of canine patients showed improved survival and achieved stsble disease | Regan et al., 2022 [61] | |
| Inhaled rhIL-15 monotherapy | Phase I veterinary trial in advanced canine OSA patients | Prolonged survival following the induction of a NK-mediated cytotoxic response | Rebhun et al., 2022 [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarone, L.; Mareschi, K.; Tirtei, E.; Giacobino, D.; Camerino, M.; Buracco, P.; Morello, E.; Cavallo, F.; Riccardo, F. Improving Osteosarcoma Treatment: Comparative Oncology in Action. Life 2022, 12, 2099. https://doi.org/10.3390/life12122099
Tarone L, Mareschi K, Tirtei E, Giacobino D, Camerino M, Buracco P, Morello E, Cavallo F, Riccardo F. Improving Osteosarcoma Treatment: Comparative Oncology in Action. Life. 2022; 12(12):2099. https://doi.org/10.3390/life12122099
Chicago/Turabian StyleTarone, Lidia, Katia Mareschi, Elisa Tirtei, Davide Giacobino, Mariateresa Camerino, Paolo Buracco, Emanuela Morello, Federica Cavallo, and Federica Riccardo. 2022. "Improving Osteosarcoma Treatment: Comparative Oncology in Action" Life 12, no. 12: 2099. https://doi.org/10.3390/life12122099
APA StyleTarone, L., Mareschi, K., Tirtei, E., Giacobino, D., Camerino, M., Buracco, P., Morello, E., Cavallo, F., & Riccardo, F. (2022). Improving Osteosarcoma Treatment: Comparative Oncology in Action. Life, 12(12), 2099. https://doi.org/10.3390/life12122099






