The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
3. PSO and AD: Common and Uncommon Denominators
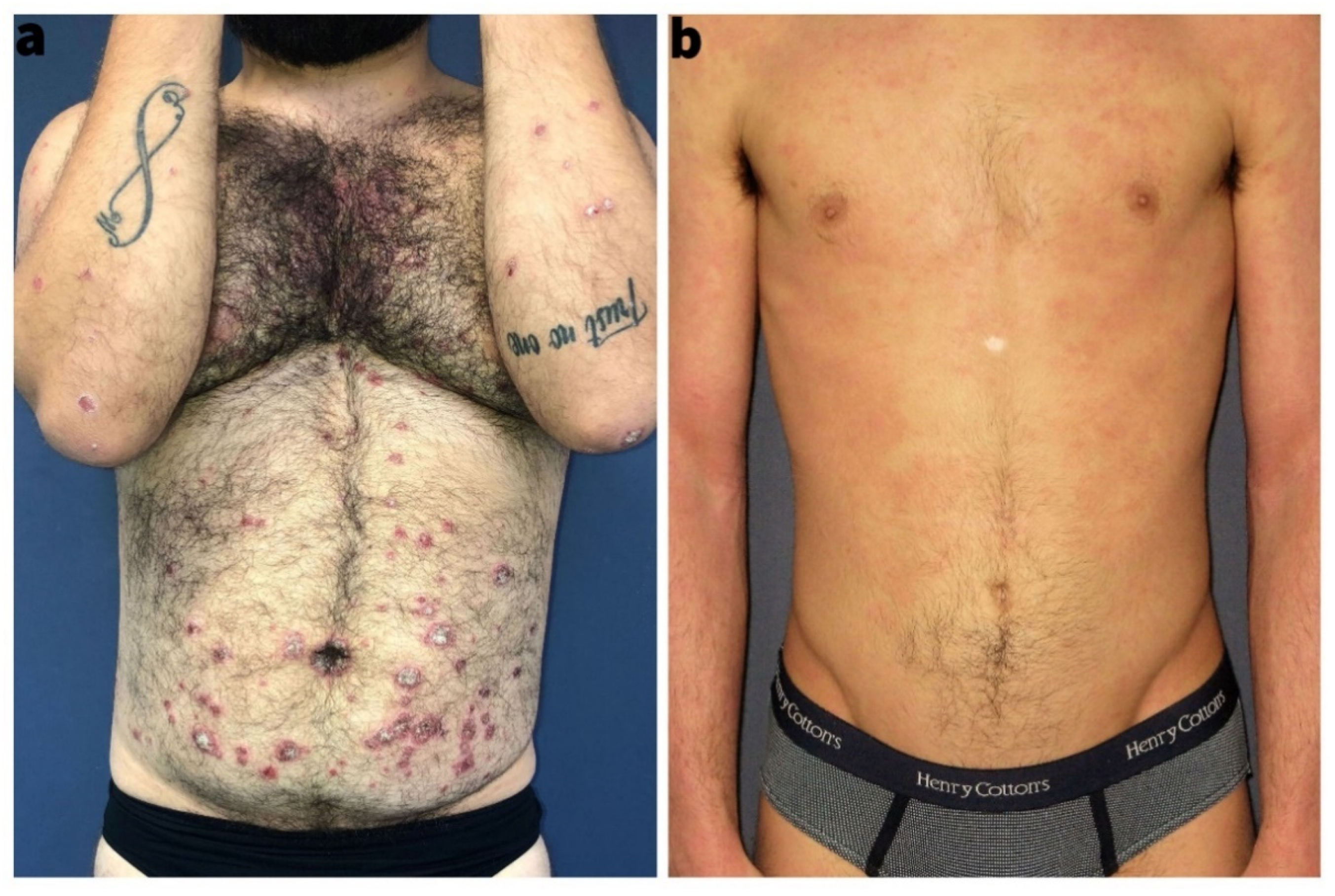
3.1. Epidemiology and Clinical Characteristics
3.2. Genetics
4. Health-Related QoL and Psychological Aspects in PSO and AD Patients
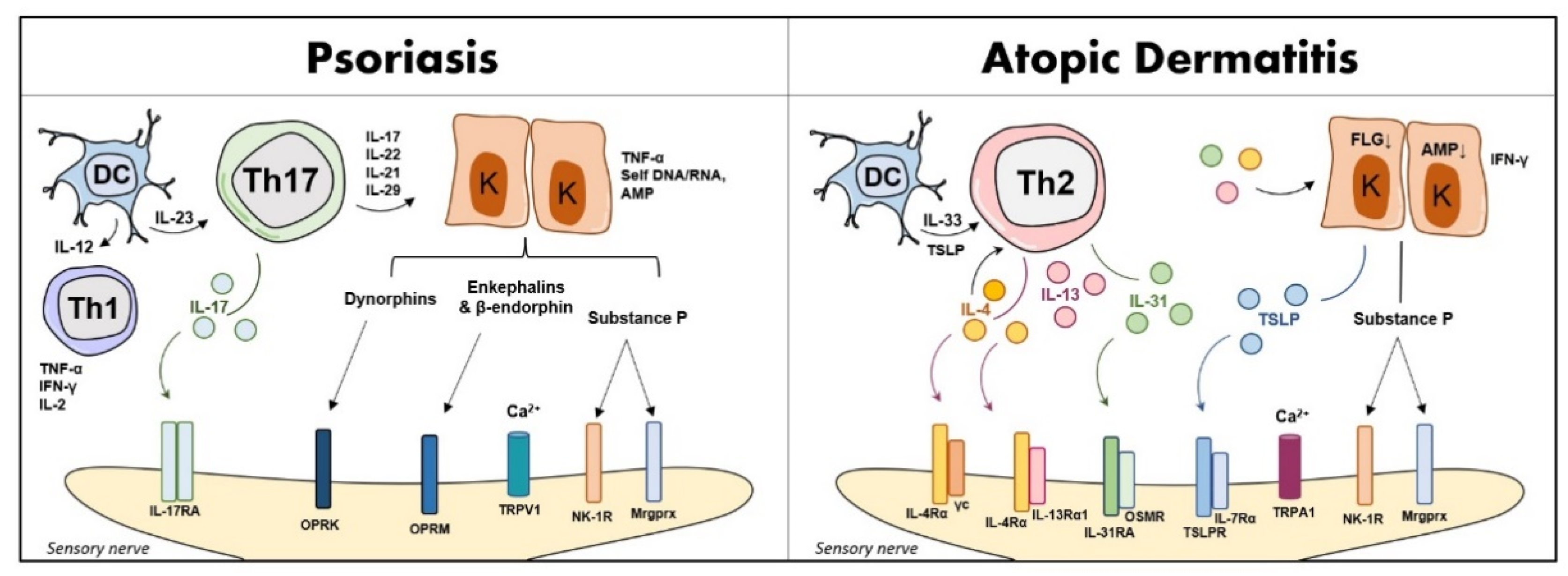
Neuro-Immune Response in PSO and AD
5. The Need for Biomarkers to Predict QoL Impairment in PSO and AD
5.1. Candidate Biomarkers of Disease Activity, and Treatment Response in PSO and AD
5.2. Sociodemographic, Clinical and Therapeutic Factors Associated with QoL Impairment
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziehfreund, S.; Tizek, L.; Hangel, N.; Fritzsche, M.; Weidinger, S.; Smith, C.; Bryce, P.; Greco, D.; Bogaard, E.; Flohr, C.; et al. Requirements and expectations of high-quality biomarkers for atopic dermatitis and psoriasis in 2021-a two-round Delphi survey among international experts. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1467–1476. [Google Scholar] [CrossRef]
- Eyerich, K.; Eyerich, S. Immune response patterns in non-communicable inflammatory skin diseases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 692–703. [Google Scholar] [CrossRef]
- Schäbitz, A.; Eyerich, K.; Garzorz-Stark, N. So close, and yet so far away: The dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J. Intern. Med. 2021, 290, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.; Guttman-Yassky, E. Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Bowcock, A.M.; Cookson, W.O. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Hum. Mol. Genet. 2004, 13, R43–R55. [Google Scholar] [CrossRef]
- Baurecht, H.; Hotze, M.; Brand, S.; Büning, C.; Cormican, P.; Corvin, A.; Ellinghaus, D.; Ellinghaus, E.; Esparza-Gordillo, J.; Fölster-Holst, R.; et al. Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am. J. Hum. Genet. 2015, 96, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, J.C. Human Leukocyte Antigen-Class I Alleles and the Autoreactive T Cell Response in Psoriasis Pathogenesis. Front. Immunol. 2018, 9, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, A.D.; McLean, W.H.; Leung, D.Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tsai, T.F. Overlapping Features of Psoriasis and Atopic Dermatitis: From Genetics to Immunopathogenesis to Phenotypes. Int. J. Mol. Sci. 2022, 23, 5518. [Google Scholar] [CrossRef]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.-H.; Homey, B.; Cao, W.; Wang, Y.-H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, A.; Siewert, K.; Stöhr, J.; Besgen, P.; Kim, S.-M.; Rühl, G.; Nickel, J.; Vollmer, S.; Thomas, P.; Krebs, S.; et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 2015, 212, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Ray-Jones, H.; Eyre, S.; Barton, A.; Warren, R.B. One SNP at a Time: Moving beyond GWAS in Psoriasis. J. Investig. Dermatol. 2016, 136, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Ellinghaus, D.; Baurecht, H.; Esparza-Gordillo, J.; Rodríguez, E.; Matanovic, A.; Marenholz, I.; Hübner, N.; Schaarschmidt, H.; Novak, N.; Michel, S.; et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat. Genet. 2013, 45, 808–812. [Google Scholar] [CrossRef] [Green Version]
- Løset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology 2019, 235, 355–364. [Google Scholar] [CrossRef]
- Mucha, S.; Baurecht, H.; Novak, N.; Rodríguez, E.; Bej, S.; Mayr, G.; Emmert, H.; Stölzl, D.; Gerdes, S.; Jung, E.S.; et al. Protein-coding variants contribute to the risk of atopic dermatitis and skin-specific gene expression. J. Allergy Clin. Immunol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef]
- Lundberg, L.; Johannesson, M.; Silverdahl, M.; Hermansson, C.; Lindberg, M. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm. Venereol. 2000, 80, 430–434. [Google Scholar] [PubMed]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Nandi, D.P.V. Biomarkers in Psoriasis: A Neurocutaneous Disease. J. Clin. Dermatol. Ther. 2019, 5, 035. [Google Scholar]
- Ferreira, B.I.; Abreu, J.L.; Reis, J.P.; Figueiredo, A.M. Psoriasis and Associated Psychiatric Disorders: A Systematic Review on Etiopathogenesis and Clinical Correlation. J. Clin. Aesthetic Dermatol. 2016, 9, 36–43. [Google Scholar]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440.e2. [Google Scholar] [CrossRef]
- Rønnstad, A.; Halling-Overgaard, A.S.; Hamann, C.R.; Skov, L.; Egeberg, A.; Thyssen, J.P. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 79, 448–456.e30. [Google Scholar] [CrossRef] [Green Version]
- Pompili, M.; Bonanni, L.; Gualtieri, F.; Trovini, G.; Persechino, S.; Baldessarini, R.J. Suicidal risks with psoriasis and atopic dermatitis: Systematic review and meta-analysis. J. Psychosom. Res. 2021, 141, 110347. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Onken, A.T.; Weidinger, S.; Franke, A.; Nasorri, F.; Pennino, D.; Grosber, M.; Pfab, F.; Schmidt-Weber, C.B.; Mempel, M.; et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N. Engl. J. Med. 2011, 365, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 5, 1254–1264. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Slavich, G.M.; Lucia, A.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Halioua, B.; Chelli, C.; Misery, L.; Taieb, J.; Taieb, C. Sleep Disorders and Psoriasis: An Update. Acta Derm. Venereol. 2022, 102, adv00699. [Google Scholar] [CrossRef]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Björkqvist, M. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GGimeno, D.; Kivimaki, M.; Brunner, E.; Elovainio, M.; De Vogli, R.; Steptoe, A.; Kumari, M.; Lowe, G.D.; Rumley, A.; Marmot, M.; et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009, 39, 413–423. [Google Scholar] [CrossRef]
- Davami, M.H.; Baharlou, R.; Ahmadi Vasmehjani, A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M.R. Elevated IL-17 and TGF-β Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin. Neurosci. 2016, 7, 137–142. [Google Scholar] [CrossRef]
- Hung, Y.Y.; Kang, H.Y.; Huang, K.W.; Huang, T.L. Association between toll-like receptors expression and major depressive disorder. Psychiatry Res. 2014, 220, 283–286. [Google Scholar] [CrossRef]
- Koo, J.; Marangell, L.B.; Nakamura, M.; Armstrong, A.; Jeon, C.; Bhutani, T.; Wu, J.J. Depression and suicidality in psoriasis: Review of the literature including the cytokine theory of depression. J. Eur. Acad. Dermatol. Venereol. 2017, 12, 1999–2009. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Gui, S.; Tian, L.; Xu, S.; Song, X.; Zhong, X.; Chen, Y.; Chen, X.; Yu, Y.; et al. Vascular endothelial growth factor in major depressive disorder, schizophrenia, and bipolar disorder: A network meta-analysis. Psychiatry Res. 2020, 292, 113319. [Google Scholar] [CrossRef]
- Simpson, E.L.; Reynolds, N.J.; Flohr, C.; Paller, A.S.; Silverberg, J.I.; Cork, M.J.; Guttman-Yassky, E.; Irvine, A.D. Response to “Comment on: ‘When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council’”. J. Am. Acad. Dermatol. 2018, 79, e25–e26. [Google Scholar] [CrossRef] [Green Version]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 2, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Li, Y.; Zhou, Y.; Follansbee, T.; Hwang, S.T. Immune mediators and therapies for pruritus in atopic dermatitis and psoriasis. J. Cutan. Immunol. Allergy 2019, 2, 4–14. [Google Scholar] [CrossRef]
- Schwarz, W.; Birau, N.; Hornstein, O.P.; Heubeck, B.; Schönberger, A.; Meyer, C.; Gottschalk, J. Alterations of melatonin secretion in atopic eczema. Acta Derm. Venereol. 1988, 68, 224–229. [Google Scholar]
- Kartha, L.B.; Chandrashekar, L.; Rajappa, M.; Menon, V.; Thappa, D.M.; Ananthanarayanan, P.H. Serum melatonin levels in psoriasis and associated depressive symptoms. Clin. Chem. Lab. Med. 2014, 52, e123–e125. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Lin, M.H.; Lee, J.H.; Lee, P.L.; Dai, Y.S.; Chu, K.H.; Sun, C.; Lin, Y.T.; Wan, K.-S.; Chiang, B.; et al. Melatonin Supplementation for Children with Atopic Dermatitis and Sleep Disturbance: A Randomized Clinical Trial. JAMA Pediatr. 2016, 170, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scuderi, S.A.; Cucinotta, L.; Filippone, A.; Lanza, M.; Campolo, M.; Paterniti, I.; Esposito, E. Effect of Melatonin on Psoriatic Phenotype in Human Reconstructed Skin Model. Biomedicines 2022, 10, 752. [Google Scholar] [CrossRef]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Influence of Itch and Pain on Sleep Quality in Atopic Dermatitis and Psoriasis. Acta Derm. Venereol. 2019, 99, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Steinke, S.; Zeidler, C.; Riepe, C.; Bruland, P.; Soto-Rey, I.; Storck, M.; Augustin, M.; Bobko, S.; Garcovich, S.; Legat, F.J.; et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: Results of the European Academy of Dermatology and Venereology Network on Assessment of Severity and Burden of Pruritus (PruNet) cross-sectional trial. J. Am. Acad. Dermatol. 2018, 79, 457–463.e5. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol. 2016, 51, 263–292. [Google Scholar] [CrossRef]
- Garcovich, S.; Maurelli, M.; Gisondi, P.; Peris, K.; Yosipovitch, G.; Girolomoni, G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines 2021, 9, 303. [Google Scholar] [CrossRef]
- Fowler, E.; Yosipovitch, G. A New Generation of Treatments for Itch. Acta Derm. Venereol. 2020, 100, adv00027. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.H.; Zuo, Y.G. Thymic Stromal Lymphopoietin in Cutaneous Immune-Mediated Diseases. Front. Immunol. 2021, 12, 698522. [Google Scholar] [CrossRef]
- Food and Drug Administration; US Department of Health and Human Services Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Biomarker Qualification: Evidentiary Framework. Guidance for Industry and FDA Staff. Draft Guidance. 2018. Available online: https://www.fda.gov/media/119271/download (accessed on 6 January 2022).
- European Medicines Agency (EMA). Qualification of Novel Methodologies for Medicine Development. Human Regulatory. Research and Development. Opinions and Letters of Support on the Qualification of Novel Methodologies for Medicine Development. Available online: https://www.ema.europa.eu/en/glossary/biomarker (accessed on 6 January 2022).
- Suomela, S.; Kainu, K.; Onkamo, P.; Tiala, I.; Himberg, J.; Koskinen, L.; Snellman, E.; Karvonen, S.; Karvonen, J.; Uurasmaa, T.; et al. Clinical associations of the risk alleles of HLA-Cw6 and CCHCR1*WWCC in psoriasis. Acta Derm. Venereol. 2007, 87, 127–134. [Google Scholar] [CrossRef]
- Ramessur, R.; Corbett, M.; Marshall, D.; Acencio, M.L.; Barbosa, I.A.; Dand, N.; Di Meglio, P.; Haddad, S.; Jensen, A.H.; Koopmann, W.; et al. Biomarkers of disease progression in people with psoriasis: A scoping review. Br. J. Dermatol. 2022, 187, 481–493. [Google Scholar] [CrossRef]
- Abji, F.; Pollock, R.A.; Liang, K.; Chandran, V.; Gladman, D.D. Brief Report: CXCL10 Is a Possible Biomarker for the Development of Psoriatic Arthritis Among Patients with Psoriasis. Arthritis Rheumatol. 2016, 68, 2911–2916. [Google Scholar] [CrossRef] [Green Version]
- Brazzelli, V.; Maffioli, P.; Bolcato, V.; Ciolfi, C.; D’Angelo, A.; Tinelli, C.; Derosa, G. Psoriasis and Diabetes, a Dangerous Association: Evaluation of Insulin Resistance, Lipid Abnormalities, and Cardiovascular Risk Biomarkers. Front. Med. 2021, 8, 605691. [Google Scholar] [CrossRef]
- Baran, A.; Stepaniuk, A.; Kiluk, P.; Kaminski, T.W.; Maciaszek, M.; Flisiak, I. Potential Predictive Value of Serum Pentraxin 3 and Paraoxonase 1 for Cardiometabolic Disorders Development in Patients with Psoriasis-Preliminary Data. Metabolites 2022, 12, 580. [Google Scholar] [CrossRef]
- Burlando, M.; Oddenino, G.; Carmisciano, L.; Cozzani, E.; Capurro, N.; Herzum, A.; Parodi, A. Increased serum level of N-terminal Pro-B-type natriuretic peptide in psoriatic patients: A single-center study. Ital. J. Dermatol. Venereol. 2022. advance online publication. [Google Scholar] [CrossRef]
- Elnabawi, Y.A.; Garshick, M.S.; Tawil, M.; Barrett, T.J.; Fisher, E.A.; Sicco, K.L.; Neimann, A.L.; Scher, J.U.; Krueger, J.; Berger, J.S. CCL20 in psoriasis: A potential biomarker of disease severity, inflammation, and impaired vascular health. J. Am. Acad. Dermatol. 2021, 84, 913–920. [Google Scholar] [CrossRef]
- Corbett, M.; Ramessur, R.; Marshall, D.; Acencio, M.L.; Ostaszewski, M.; Barbosa, I.A.; Dand, N.; Di Meglio, P.; Smith, C.H.; Ndlovu, M.; et al. Biomarkers of systemic treatment response in people with psoriasis: A scoping review. Br. J. Dermatol. 2022, 187, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2016, 38, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, K.; Gupta, S.; Gadkari, A.; Hiragun, T.; Kono, T.; Katayama, I.; Demiya, S.; Eckert, L. Burden of atopic dermatitis in Japanese adults: Analysis of data from the 2013 National Health and Wellness Survey. J. Dermatol. 2018, 45, 390–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, T.; Izuhara, K.; Onozuka, D.; Nunomura, S.; Tamagawa-Mineoka, R.; Masuda, K.; Ichiyama, S.; Saeki, H.; Furue, M.; Katoh, N.; et al. Exploration of biomarkers to predict clinical improvement of atopic dermatitis in patients treated with dupilumab: A study protocol. Medicine 2020, 99, e22043. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.N.; Mortz, C.G.; Jensen, T.K.; Barington, T.; Lambertsen, K.L.; Halken, S. Biomarkers in asthma in the context of atopic dermatitis in young children. Pediatr. Allergy Immunol. 2022, 33, e13823. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, J.D.; Suárez-Fariñas, M.; Dhingra, N.; Cardinale, I.; Li, X.; Kostic, A.; Ming, J.E.; Radin, A.R.; Krueger, J.G.; Graham, N.; et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, S.; Kubo, R.; Nishida, E.; Morita, A. Serum interleukin-6 levels in response to biologic treatment in patients with psoriasis. Mod. Rheumatol. 2017, 27, 137–141. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Bianchini, E.; Bartolomeo, R.; Fabiano, A.; Manfredini, M.; Ferrari, F.; Albertini, G.; Pellacani, G.; Cossarizza, A.; et al. Anti-TNF-α Drugs Differently Affect the TNFα-sTNFR System and Monocyte Subsets in Patients with Psoriasis. PLoS ONE 2016, 11, e0167757. [Google Scholar] [CrossRef]
- Medvedeva, I.V.; Stokes, M.E.; Eisinger, D.; LaBrie, S.T.; Ai, J.; Trotter, M.W.B.; Schafer, P.; Yang, R. Large-scale Analyses of Disease Biomarkers and Apremilast Pharmacodynamic Effects. Sci. Rep. 2020, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Tabra, S.A.; Abd Elghany, S.E.; Amer, R.A.; Fouda, M.H.; Abu-Zaid, M.H. Serum interleukin-23 levels: Relation to depression, anxiety, and disease activity in psoriatic arthritis patients. Clin. Rheumatol. 2022, 41, 3391–3399. [Google Scholar] [CrossRef]
- Elsawy, N.A.; Helal, A.; El Shafei, M.; Mikhael, N.L. Serum interleukin 23 in psoriatic arthritis patients: Relation to disease activity, physical function and health related quality of life. Aktuelle Rheumatol. 2020, 45, 460–466. [Google Scholar] [CrossRef]
- von Stebut, E.; Boehncke, W.H.; Ghoreschi, K.; Gori, T.; Kaya, Z.; Thaci, D.; Schäffler, A. IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front. Immunol. 2020, 10, 3096. [Google Scholar] [CrossRef] [Green Version]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007, 120, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, e98006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabenhorst, A.; Hartmann, K. Interleukin-31: A novel diagnostic marker of allergic diseases. Curr. Allergy Asthma Rep. 2014, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Na, C.H.; Chung, J.; Simpson, E.L. Quality of Life and Disease Impact of Atopic Dermatitis and Psoriasis on Children and 460 Their Families. Children 2019, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Finlay, A.Y.; Kelly, S.E. Psoriasis--an index of disability. Clin. Exp. Dermatol. 1987, 12, 8–11. [Google Scholar] [CrossRef]
- Whalley, D.; McKenna, S.; Dewar, A.; Erdman, R.; Kohlmann, T.; Niero, M.; Cook, S.; Crickx, B.; Herdman, M.; Frech, F.; et al. A new instrument for assessing quality of life in atopic dermatitis: International development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br. J. Dermatol. 2004, 150, 274–283. [Google Scholar] [CrossRef]
- Lewis-Jones, M.S.; Finlay, A.Y. The Children’s Dermatology Life Quality Index (CDLQI): Initial validation and practical use. Br. J. Dermatol. 1995, 132, 942–949. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- WHO. Preamble to the Constitution of the World Health Organisation as Adopted by the International Health Conference; World Health Organization: New Yourk, NY, USA, 1946. [Google Scholar]
- Schuster, B.; Ziehfreund, S.; Schielein, M.C.; Tizek, L.; Biedermann, T.; Peifer, C.; Zink, A. Adding happiness to complement the Dermatology Quality of Life Index in psoriasis and atopic dermatitis healthcare: A cross-sectional study. Adding happiness to complement the Dermatology Quality of Life Index in psoriasis and atopic dermatitis healthcare: A cross-sectional study. Eur. J. Dermatol. 2022, 32, 220–226. [Google Scholar]
- Paudyal, P.; Apfelbacher, C.; Jones, C.; Siddiqui, S.; El-Turki, A.; De Giovanni, C.; Smith, H. “DLQI Seems to be ‘Action’, and Skindex-29 Seems to be ‘Emotion’”: Qualitative Study of the Perceptions of Patients with Psoriasis or Eczema on Two Common Dermatology-specific Quality of Life Measures. Acta Derm. Venereol. 2020, 100, adv00105. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Megna, M.; Amerio, P.; Argenziano, G.; Babino, G.; Bardazzi, F.; Bianchi, L.; Caldarola, G.; Chiricozzi, A.; Conti, A.; et al. Patients’ demographic and socioeconomic characteristics influence the therapeutic decision-making process in psoriasis. PLoS ONE 2020, 15, e0237267. [Google Scholar] [CrossRef] [PubMed]
- Lesner, K.; Reich, A.; Szepietowski, J.C.; Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Kupfer, J.; Salek, S.; et al. Determinants of Psychosocial Health in Psoriatic Patients: A Multi-national Study. Acta Derm. Venereol. 2017, 97, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Kaczmarczyk, R.; Zink, A.; Balato, A.; PSES study group. Sociodemographic, clinical and therapeutic factors as predictors of life quality impairment in psoriasis: A cross-sectional study in Italy. Dermatol. Ther. 2022, 35, e15622. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, B.; Cybulski, M.; Jankowiak, B.; Krajewska-Kułak, E. Acceptance of Illness, Satisfaction with Life, Sense of Stigmatization, and Quality of Life among People with Psoriasis: A Cross-Sectional Study. Dermatol. Ther. 2020, 10, 413–430. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, J.M.; Feldman, S.R.; Stern, R.S.; Thomas, J.; Rolstad, T.; Margolis, D.J. Determinants of quality of life in patients with psoriasis: A study from the US population. J. Am. Acad. Dermatol. 2004, 51, 704–708. [Google Scholar] [CrossRef]
- Kumsa, S.M.; Tadesse, T.A.; Woldu, M.A. Management practice, quality of life and associated factors in psoriasis patients attending a dermatological center in Ethiopia. PLoS ONE 2021, 16, e0260243. [Google Scholar] [CrossRef]
- Nayak, P.B.; Girisha, B.S.; Noronha, T.M. Correlation between Disease Severity, Family Income, and Quality of Life in Psoriasis: A Study from South India. Indian Dermatol. Online J. 2018, 9, 165–169. [Google Scholar]
- Kowalewska, B.; Jankowiak, B.; Niedżwiecka, B.; Krajewska-Kułak, E.; Niczyporuk, W.; Khvorik, D.F. Relationships between the acceptance of illness, quality of life and satisfaction with life in psoriasis. Postepy Dermatol. Alergol. 2020, 37, 948–955. [Google Scholar] [CrossRef]
- Napolitano, M.; Mastroeni, S.; Fania, L.; Pallotta, S.; Fusari, R.; Uras, C.; Panebianco, A.; Cavani, A.; Abeni, D.; Cavani, A.; et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin. Exp. Dermatol. 2020, 45, 705–711. [Google Scholar] [CrossRef]
- Guillet, C.; Seeli, C.; Nina, M.; Maul, L.V.; Maul, J.T. The impact of gender and sex in psoriasis: What to be aware of when treating women with psoriasis. Int. J. Womens Dermatol. 2022, 8, e010. [Google Scholar] [CrossRef] [PubMed]
- Grozdev, I.; Kast, D.; Cao, L.; Carlson, D.; Pujari, P.; Schmotzer, B.; Babineau, D.; Kern, E.; McCormick, T.; Cooper, K.D.; et al. Physical and mental impact of psoriasis severity as measured by the compact Short Form-12 Health Survey (SF-12) quality of life tool. J. Investig. Dermatol. 2012, 132, 1111–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, N.; Augustin, M.; Langenbruch, A.; Mrowietz, U.; Reich, K.; Thaci, D.; Boehncke, W.H.; Kirsten, N.; Danckworth, A.; Sommer, R. Disease burden and treatment needs of patients with psoriasis in sexually-sensitive and visible body areas: Results from a large-scale survey in routine care. Eur. J. Dermatol. 2020, 30, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Torres, R.M.; Pita-Fernandez, S.; Fonseca, E. Quality of life and related factors in a cohort of plaque-type psoriasis patients in La Coruna, Spain. Int. J. Dermatol. 2014, 53, e507–e511. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.G.; Agner, T.; Clausen, M.L.; Thomsen, S.F. Quality of life and disease severity in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1760–1767. [Google Scholar] [CrossRef]
- Beikert, F.C.; Langenbruch, A.K.; Radtke, M.A.; Kornek, T.; Purwins, S.; Augustin, M. Willingness to pay and quality of life in patients with atopic dermatitis. Arch. Dermatol. Res. 2014, 306, 279–286. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, T.F.; Wong, K.Y.; Chow, C.M.; Chuh, A.; Ng, P.C. Does age or gender influence quality of life in children with atopic dermatitis? Clin. Exp. Dermatol. 2008, 33, 705–709. [Google Scholar] [CrossRef]
- Kiebert, G.; Sorensen, S.V.; Revicki, D.; Fagan, S.C.; Doyle, J.J.; Cohen, J.; Fivenson, D. Atopic dermatitis is associated with a decrement in health-related quality of life. Int. J. Dermatol. 2002, 41, 151–158. [Google Scholar] [CrossRef]
- Kim, D.H.; Li, K.; Seo, S.J.; Jo, S.J.; Yim, H.W.; Kim, C.M.; Kim, K.H.; Kim, D.W.; Kim, M.B.; Kim, J.W.; et al. Quality of life and disease severity are correlated in patients with atopic dermatitis. J. Korean Med. Sci. 2012, 27, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Janković, S.; Ražnatović-Đurović, M.; Ćirković, A.; Janković, J. Does gender influence quality of life in children with atopic dermatitis? Scr. Med. 2019, 50, 19–24. [Google Scholar] [CrossRef]
- Holm, E.A.; Esmann, S.; Jemec, G.B.E. Does visible atopic dermatitis affect quality of life more in women than in men? Gend. Med. 2004, 1, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Z.; Bong, Y.B.; Tan, L.L.; Lim, S.X.; Yong, A.S.W.; Ch’ng, C.C.; Tan, M.P.; Ismail, R. Determinants of quality of life and psychological status in adults with psoriasis. Arch. Dermatol. Res. 2018, 310, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Peifer, C.; Ziehfreund, S.; Tizek, L.; Biedermann, T.; Zink, A.; Schielein, M.C. Happiness and depression in psoriasis: A cross-sectional study in Germany. Qual. Life Res. 2022, 31, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Miniszewska, J.; Chodkiewicz, J.; Ograczyk-Piotrowska, A.; Zalewska-Janowska, A. Life satisfaction and health related quality of life—The same or a different construct? A survey in psoriasis patients. Health Psychol. Rep. 2020, 8, 219–227. [Google Scholar] [CrossRef]
- Xu, X.; van Galen, L.S.; Koh, M.J.A.; Bajpai, R.; Thng, S.; Yew, Y.W.; Ho, V.P.Y.; Alagappan, U.; Jarbrink, K.S.A.; Car, J. Factors influencing quality of life in children with atopic dermatitis and their caregivers: A cross-sectional study. Sci. Rep. 2019, 9, 15990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernyshov, P.; Ho, R.C.; Monti, F.; Jirakova, A.; Velitchko, S.; Hercogova, J.; Neri, E. An International Multi-center Study on Self-assessed and Family Quality of Life in Children with Atopic Dermatitis. Acta Dermatovenerol. Croat. 2015, 23, 247–253. [Google Scholar] [PubMed]
- Raznatovic Durovic, M.; Jankovic, J.; Tomic Spiric, V.; Relic, M.; Sojevic Timotijevic, Z.; Cirkovic, A.; Duric, S.; Jankovic, S. Does age influence the quality of life in children with atopic dermatitis? PLoS ONE 2019, 14, e0224618. [Google Scholar] [CrossRef]
- Andersen, L.; Nyeland, M.E.; Nyberg, F. Higher self-reported severity of atopic dermatitis in adults is associated with poorer self-reported health-related quality of life in France, Germany, the U.K. and the U.S.A. Br. J. Dermatol. 2020, 182, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Chrostowska-Plak, D.; Reich, A.; Szepietowski, J.C. Relationship between itch and psychological status of patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e239–e242. [Google Scholar] [CrossRef]
- Sondermann, W.; Fiege, O.; Korber, A.; Scherbaum, N. Psychological burden of psoriatic patients in a German university hospital dermatology department. J. Dermatol. 2021, 48, 794–806. [Google Scholar] [CrossRef]
- Sondermann, W.; Schreiber, A.; Korber, A.; Fiege, O.; Scherbaum, N.; Benson, S.; Schedlowski, M. Psychosocial burden and body mass index are associated with dermatology-related quality of life in psoriasis patients. Eur. J. Dermatol. 2020, 30, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Houghton, K.; Patil, D.; Gomez, B.; Feldman, S.R. Correlation Between Change in Psoriasis Area and Severity Index and Dermatology Life Quality Index in Patients with Psoriasis: Pooled Analysis from Four Phase 3 Clinical Trials of Secukinumab. Dermatol. Ther. 2021, 11, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Beck, K.M.; Sanchez, I.M.; Koo, J.; Liao, W. The impact of genital psoriasis on quality of life: A systematic review. Psoriasis 2018, 8, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darjani, A.; Heidarzadeh, A.; Golchay, J.; Sadr-Eshkevari, S.; Alizadeh, N.; Arami, M.; Nichhah, N. Quality of life in psoriatic patients: A study using the short form-36. Int. J. Prev. Med. 2014, 5, 1146–1152. [Google Scholar]
- Sampogna, F.; Tabolli, S.; Abeni, D.; The Idi Multipurpose Psoriasis Research on Vital Experiences (Improve) Investigators. The impact of changes in clinical severity on psychiatric morbidity in patients with psoriasis: A follow-up study. Br. J. Dermatol. 2007, 157, 508–513. [Google Scholar] [CrossRef]
- Turchin, I.; Bourcier, M. The Role of Interleukins in the Pathogenesis of Dermatological Immune-Mediated Diseases. Adv. Ther. 2022, 39, 4474–4508. [Google Scholar] [CrossRef]
- Jing, D.; Xiao, H.; Shen, M.; Chen, X.; Han, X.; Kuang, Y.; Zhu, W.; Xiao, Y. Association of Psoriasis With Anxiety and Depression: A Case-Control Study in Chinese Patients. Front. Med. 2021, 8, 771645. [Google Scholar] [CrossRef]
- Caldarola, G.; De Simone, C.; Talamonti, M.; Moretta, G.; Fossati, B.; Bianchi, L.; Fargnoli, M.C.; Peris, K. Prevalence of cutaneous comorbidities in psoriatic patients and their impact on quality of life. Eur. J. Dermatol. 2019, 29, 192–196. [Google Scholar]
- Pavlova, N.T.; Kioskli, K.; Smith, C.; Picariello, F.; Rayner, L.; Moss-Morris, R. Psychosocial aspects of obesity in adults with psoriasis: A systematic review. Skin Health Dis. 2021, 1, e33. [Google Scholar] [CrossRef]
- Karpinska-Mirecka, A.; Bartosinska, J.; Krasowska, D. The Impact of Hypertension, Diabetes, Lipid Disorders, Overweight/Obesity and Nicotine Dependence on Health-Related Quality of Life and Psoriasis Severity in Psoriatic Patients Receiving Systemic Conventional and Biological Treatment. Int. J. Environ. Res. Public Health 2021, 18, 13167. [Google Scholar] [CrossRef]
- Augustin, M.; Reich, K.; Blome, C.; Schafer, I.; Laass, A.; Radtke, M.A. Nail psoriasis in Germany: Epidemiology and burden of disease. Br. J. Dermatol. 2010, 163, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Innamorati, M.; Quinto, R.M.; Imperatori, C.; Lora, V.; Graceffa, D.; Fabbricatore, M.; Lester, D.; Contardi, A.; Bonifati, C. Health-related quality of life and its association with alexithymia and difficulties in emotion regulation in patients with psoriasis. Compr. Psychiatry 2016, 70, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, I.Y.K.; Ashcroft, D.M.; Warren, R.B.; Lunt, M.; McElhone, K.; Smith, C.H.; Reynolds, N.J.; Griffiths, C.E.M. Comparative effectiveness of biological therapies on improvements in quality of life in patients with psoriasis. Br. J. Dermatol. 2017, 177, 1410–1421. [Google Scholar] [CrossRef] [Green Version]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
- Tsianakas, A.; Luger, T.A.; Radin, A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: Results from a randomized, placebo-controlled clinical trial. Br. J. Dermatol. 2018, 178, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Boguniewicz, M.; Sher, L.; Gooderham, M.J.; Beck, L.A.; Guttman-Yassky, E.; Pariser, D.; Blauvelt, A.; et al. Efficacy and Safety of Dupilumab in Adolescents with Uncontrolled Moderate to Severe Atopic Dermatitis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 44–56. [Google Scholar] [CrossRef]
- Kimball, A.B.; Bensimon, A.G.; Guerin, A.; Yu, A.P.; Wu, E.Q.; Okun, M.M.; Bao, Y.; Gupta, S.R.; Mulani, P.M. Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: Subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trial. Am. J. Clin. Dermatol. 2011, 12, 51–62. [Google Scholar] [CrossRef]
- Upasham, S.; Bhide, A.; Lin, K.C.; Prasad, S. Point-of-use sweat biosensor to track the endocrine-inflammation relationship for chronic disease monitoring. Future Sci. 2021, 7, FSO628. [Google Scholar] [CrossRef]
- Kiani, C.; Kain, A.; Zink, A. Wearables and smart skin as new tools for clinical practice and research in dermatology. JEADV Clin. Pract. 2022, 1, 66–68. [Google Scholar] [CrossRef]
- Chun, K.S.; Kang, Y.J.; Lee, J.Y.; Nguyen, M.; Lee, B.; Lee, R.; Jo, H.H.; Allen, E.; Chen, H.; Kim, J.; et al. A skin-conformable wireless sensor to objectively quantify symptoms of pruritus. Sci. Adv. 2021, 7, eabf9405. [Google Scholar] [CrossRef]
- Kotru, S.; Klimuntowski, M.; Ridha, H.; Uddin, Z.; Askhar, A.A.; Singh, G.; Howlader, M. Electrochemical sensing: A prognostic tool in the fight against COVID-19. Trends Analyt. Chem. 2021, 136, 116198. [Google Scholar] [CrossRef] [PubMed]
- Marques-Deak, A.; Cizza, G.; Eskandari, F.; Torvik, S.; Christie, I.C.; Sternberg, E.M.; Phillips, T.M. Premenopausal, Osteoporosis Women, Alendronate, Depression Study Group Measurement of cytokines in sweat patches and plasma in healthy women: Validation in a controlled study. J. Immunol. Methods. 2006, 315, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, B.; Lin, K.C.; Pali, M.; Sankhala, D.; Muthukumar, S.; Prasad, S. Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng. Transl. Med. 2021, 6, e10220. [Google Scholar] [CrossRef] [PubMed]
- Cizza, G.; Marques, A.H.; Eskandari, F.; Christie, I.C.; Torvik, S.; Silverman, M.N.; Phillips, T.M.; Sternberg, E.M. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: The POWER study. Biol. Psychiatry 2008, 64, 907–911. [Google Scholar] [CrossRef] [Green Version]
- Jagannath, B.; Lin, K.C.; Pali, M.; Sankhala, D.; Muthukumar, S.; Prasad, S. A Sweat-based Wearable Enabling Technology for Real-time Monitoring of IL-1β and CRP as Potential Markers for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1533–1542. [Google Scholar] [CrossRef]
| Biomarker | Main Points | Relevant Therapeutics |
|---|---|---|
| IL-6 | Useful for assessing disease activity, developing of mental health in patients with PSO and for predicting responsiveness of joint symptoms to biologic treatments [37,68] | Tocilizumab |
| TNF-α | Correlates with disease activity, systemic treatment response in PSO and major depression [37,61,69] | Adalimumab, etanercept, infliximab |
| IL-17 | Related to disease activity, systemic treatment response in PSO [70] and major depressive disorder [35] | Secukinumab, ixekizumab, brodalumab |
| IL-23 | Correlates with depression, anxiety and disease activity in PsA [71]. It may serve for identifying joint activity or skin severity but not QoL or physical function [72] | Ustekinumab, guselkumab, risankizumab and tildrakizumab |
| HLA-Cw6 | Associated with PSO severity and progression, as well as obesity and metabolic syndrome [54,73] | - |
| CCL20 | Strongly associated with vascular endothelial inflammation, it reflects systemic inflammation and may serve as indicator of impaired vascular health in PSO [60] | - |
| PON1 | Described as potential indicator of the liver disorders in PSO [58] | - |
| PTX3 | Protective role regarding the development of cardiometabolic disorders, especially in overweight and obese patients with PSO [58] | - |
| CXCL10 | Associated with the development of PsA among patients with PSO [56] | - |
| IL-4Rα | Correlates with disease activity and systemic treatment response in AD [66] | Dupilumab |
| IL-13 | Related to disease activity and impairment of skin barrier in AD. It may activate itch signaling and scratching [64,74,75,76] | Tralokinumab |
| IL-31 | Correlates with severity of allergic diseases [77] and good/poor clinical outcome of the anti-IL 4 receptor-α antibody dupilumab during the treatment of patients with moderate-to-severe AD [66] | Nemolizumab |
| Factors | Psoriasis | Atopic Dermatitis |
|---|---|---|
| Sex | Women showed a higher QoL impairment compared to men [89,91,97,98] | NS difference in QoL impairment between genders [102,103,105]. However, female sex was associated with low QoL in a Danish study [99] |
| Age | Young patients showed higher QoL impairment in 2 studies [89,106]. NS differences in QoL impairment between age in 4 studies [91,92,98,108] | NS differences in QoL between young and adult patients by age [103]. Patients aged 16+ years showed a more impaired QoL than patients aged 4–15 years [99] |
| Place of residence | Urban areas showed more impaired QoL than rural areas [89]. Differences in QoL between 13 European countries: higher QoL in Spain, and lower QoL in Italy, especially in Southern Italy [87,88] | - |
| Educational level | A significant association was found between primary educational status and poor QoL [91] | - |
| Net salary | Lower income was associated with impaired QoL [92] | - |
| Disease duration/age at onset | Short disease duration had a higher impact on QoL in patients [98]. NS differences between impaired QoL and age at onset [76] or disease duration [92,108] | NS association between disease duration and QoL in patients with AD [113] |
| Disease Severity | Higher PASI score was associated with impaired QoL [92,98,108,115,116] | Higher SCORAD showed more impaired QoL [100,103,105,113] |
| Disease localization | Isolated involvement of scalp, trunk, intertriginous, palmoplantar and nail PSO was associated with a higher QoL impairment [88,106,125] | Isolated involvement of face, hand, genital and foot eczema was associated with low QoL [99,100]. Involvement of visible regions showed more impaired QoL than no involvement of visible regions in women [105] |
| Comorbidities | Patients with comorbidities such as hypertension, diabetes, lipid disorders, overweight/obesity, PSA, depression and anxiety had poorer QoL [98,106,124] | Adults with AD concomitant other atopic diseases including asthma, allergic rhinitis, allergic conjunctivitis experienced greater QoL impairment than the patients with AD alone [103]. NS differences in QoL impairment between patients with AD only and patients with comorbid atopic diseases [99,113] |
| Therapy | Topical therapy only was mostly associated with QoL impairment compared to topical therapy plus conventional systemic treatment [91]. Phototherapy and non-biological systemic therapy had a moderate effect on patients’ life, whereas biologics targeting TNF-α, IL-12/23 and IL-17 showed to improve patients’ QoL [88,98,131] | Topical corticosteroids only were mostly associated with QoL impairment compared to dupilumab therapy plus topical corticosteroids [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balato, A.; Zink, A.; Babino, G.; Buononato, D.; Kiani, C.; Eyerich, K.; Ziehfreund, S.; Scala, E. The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers. Life 2022, 12, 2026. https://doi.org/10.3390/life12122026
Balato A, Zink A, Babino G, Buononato D, Kiani C, Eyerich K, Ziehfreund S, Scala E. The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers. Life. 2022; 12(12):2026. https://doi.org/10.3390/life12122026
Chicago/Turabian StyleBalato, Anna, Alexander Zink, Graziella Babino, Dario Buononato, Charlotte Kiani, Kilian Eyerich, Stefanie Ziehfreund, and Emanuele Scala. 2022. "The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers" Life 12, no. 12: 2026. https://doi.org/10.3390/life12122026
APA StyleBalato, A., Zink, A., Babino, G., Buononato, D., Kiani, C., Eyerich, K., Ziehfreund, S., & Scala, E. (2022). The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers. Life, 12(12), 2026. https://doi.org/10.3390/life12122026






