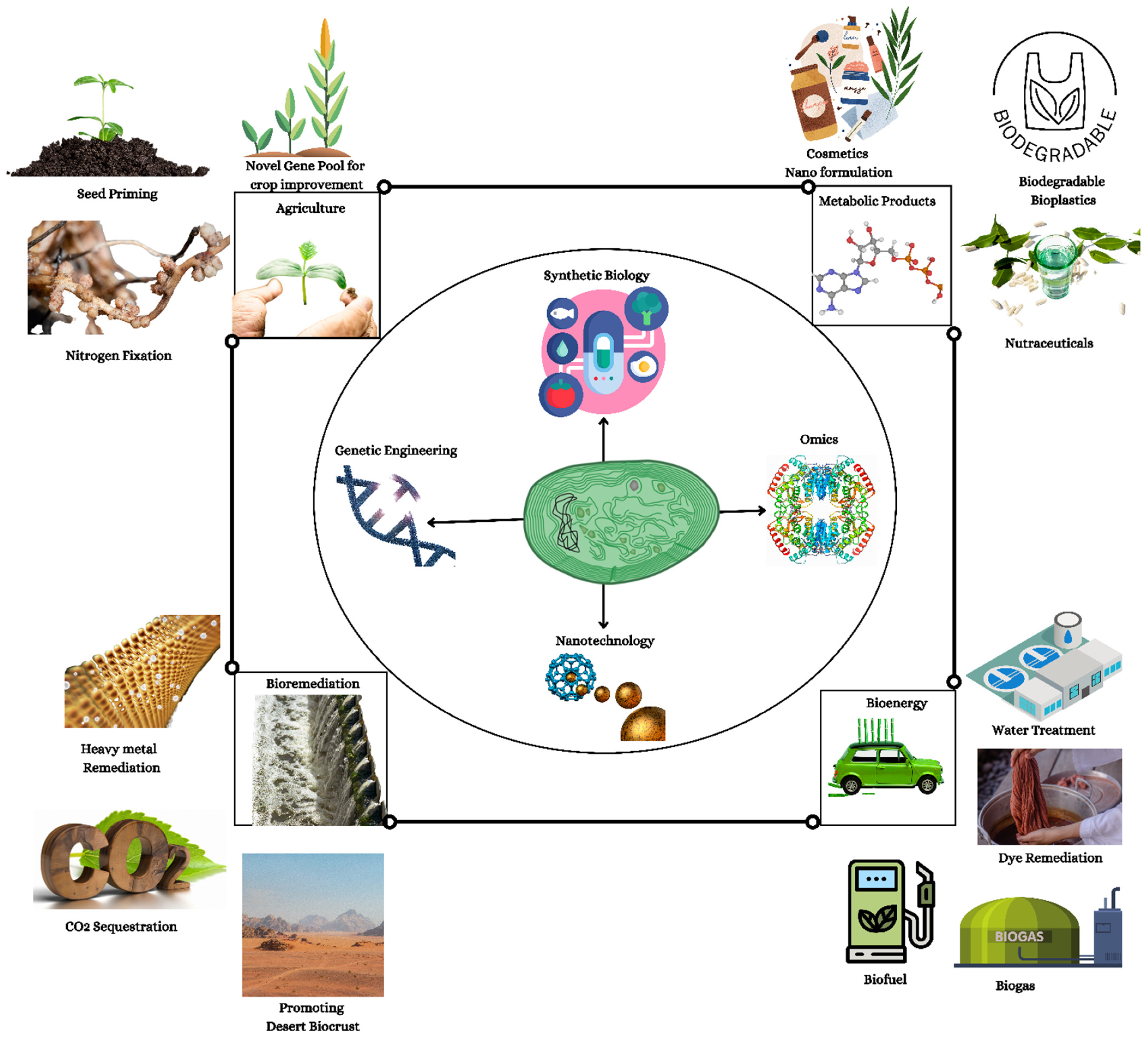
Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications
Abstract
1. Introduction
2. Molecular Genetics of Cyanobacteria
3. Cyanobacteria: Revolutionary Advances in Genome Engineering
4. Enhanced Conversion of Photoenergy by Genetically Engineered Cyanobacteria
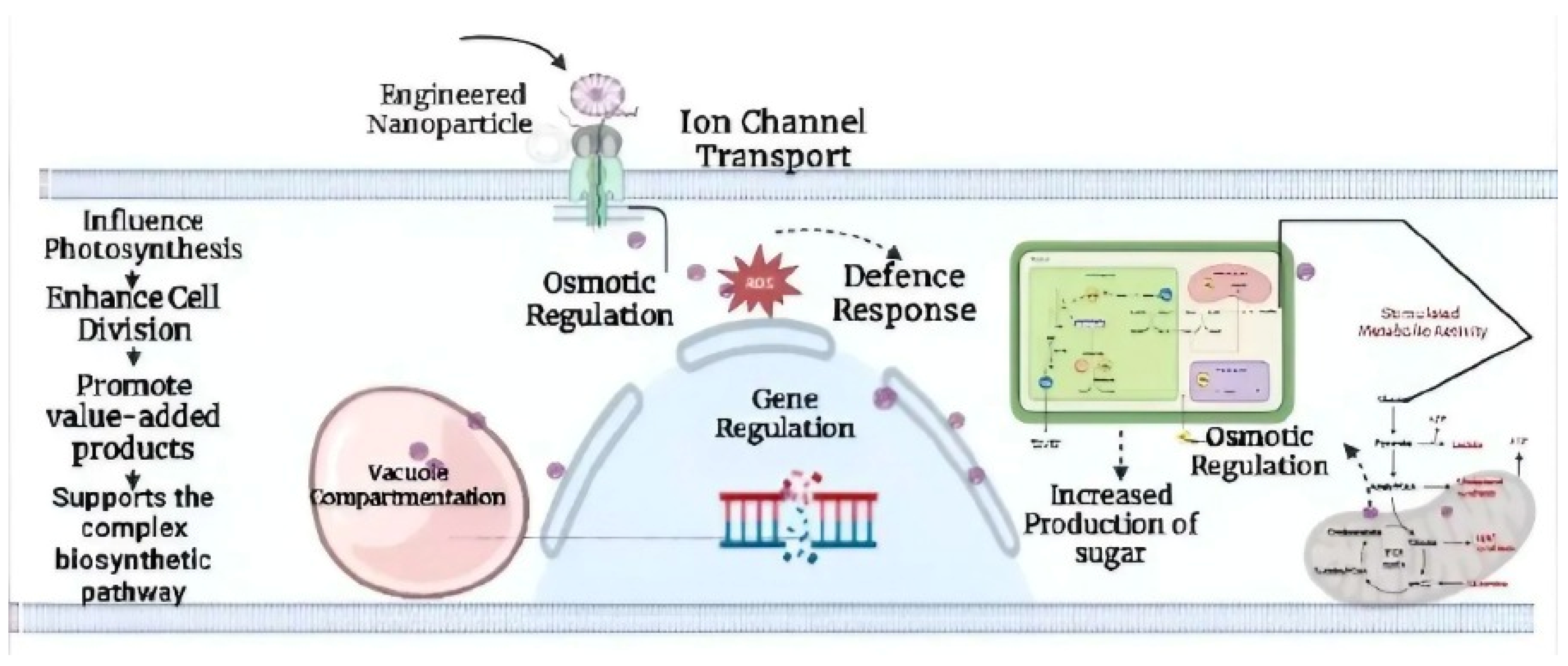
5. The Effects of Nanoparticles on Genetically Engineered Cyanobacteria
6. Genomic and Proteomic Insights on Cyanobacterial Heavy Metal Tolerance
7. Cyanobacterial-Engineered Nanomaterials (NMs): A Heavy Metal Indicator
8. Aquaculture’s Next Frontier: Genetically Engineered Nanosized Cyanobacteria
9. Genetically Engineered Cyanobacteria Nano-Formulation for High-Value Therapeutics
10. Cyanobacterial Cell Factories—A Plastic Scavengers
11. Crop Improvement Using Cyanobacteria-Mediated Nanoparticle Gene Delivery
12. Cyanobacteria and Nano-Sand-Stabilized Biocrust
13. Application of Cyanobacteria in Carbon Capture
14. Genetically Improved Cyanobacteria for Biofuel Production
14.1. Benefits
- Non-toxic to the surrounding environment.
- Extremely efficient with regard to cost.
- Does not result in the production of any undesirable residues.
- Reduces transportation costs and may be performed immediately on-site.
- May be used in conjunction with several other therapeutic methods.
14.2. Challenges
- Some compounds are partially degradable.
- The outcomes that were seen in the laboratory may turn out differently when the experiment is carried out in the field.
- There is a possibility that the hydrological and geochemical parameters of the site may change over time.
- Water and fertilizers are other issues for large-scale cyanobacterial cultivation.
- Rapidly changing climatic conditions and pollution threaten the economic feasibility of outdoor cyanobacteria farming.
15. Genetically Modified Cyanobacteria and the Threats They Entail
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waterbury, J.B. The cyanobacteria—Isolation, purification and identification. Prokaryotes 2006, 4, 1053–1073. [Google Scholar]
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef]
- Micallef, M.L.; D’Agostino, P.M.; Al-Sinawi, B.; Neilan, B.A.; Moffitt, M.C. Exploring cyanobacterial genomes for natural product biosynthesis pathways. Mar. Genom. 2015, 21, 1–12. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Shen, C.R.; Huang, C.H.; Sung, L.Y.; Wu, M.Y.; Hu, Y.C. CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab. Eng. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Savakis, P.; Hellingwerf, K.J. Engineering cyanobacteria for direct biofuel production from CO2. Curr. Opin. Biotechnol. 2015, 33, 8–14. [Google Scholar] [CrossRef]
- Cai, F.; Axen, S.D.; Kerfeld, C.A. Evidence for the widespread distribution of CRISPR-Cas system in the Phylum Cyanobacteria. RNA Biol. 2013, 10, 687–693. [Google Scholar] [CrossRef]
- Hou, S.; Brenes-Álvarez, M.; Reimann, V.; Alkhnbashi, O.S.; Backofen, R.; Muro-Pastor, A.M.; Hess, W.R. CRISPR-Cas systems in multicellular cyanobacteria. RNA Biol. 2019, 16, 518–529. [Google Scholar] [CrossRef]
- Bhaya, D.; Davison, M.; Barrangou, R. CRISPR-Cas systems in bacteria and archaea: Versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 2011, 45, 273–297. [Google Scholar] [CrossRef]
- Scholz, I.; Lange, S.J.; Hein, S.; Hess, W.R.; Backofen, R. CRISPR-Cas systems in the cyanobacterium Synechocystis sp. PCC6803 exhibit distinct processing pathways involving at least two Cas6 and a Cmr2 protein. PLoS ONE 2013, 8, e56470. [Google Scholar] [CrossRef]
- Sekar, N.; Umasankar, Y.; Ramasamy, R.P. Photocurrent generation by immobilized cyanobacteria via direct electron transport in photo-bioelectrochemical cells. Phys. Chem. Chem. Phys. 2014, 16, 7862–7871. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N.; Ramasamy, R.P. Recent advances in photosynthetic energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 19–33. [Google Scholar] [CrossRef]
- Pisciotta, J.M.; Zou, Y.; Baskakov, I.V. Light-dependent electrogenic activity of cyanobacteria. PLoS ONE 2010, 5, e10821. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N.; Jain, R.; Yan, Y.; Ramasamy, R.P. Enhanced photo-bioelectrochemical energy conversion by genetically engineered cyanobacteria. Biotechnol. BioEng. 2016, 113, 675–679. [Google Scholar] [CrossRef]
- Liu, L.; Choi, S. Enhanced biophotoelectricity generation in cyanobacterial biophotovoltaics with intracellularly biosynthesized gold nanoparticles. J. Power Sources 2021, 506, 230251. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S. A micro-sized bio-solar cell for self-sustaining power generation. Lab Chip 2015, 15, 391–398. [Google Scholar] [CrossRef]
- Zhang, C.C.; Zhou, C.Z.; Burnap, R.L.; Peng, L. Carbon/nitrogen metabolic balance: Lessons from Cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef]
- Sarma, M.K.; Quadir, M.G.A.; Bhaduri, R.; Kaushik, S.; Goswami, P. Composite polymer coated magnetic nanoparticles-based anode enhances dye degradation and power production in microbial fuel cells. Biosens. Bioelectron. 2018, 119, 94–102. [Google Scholar] [CrossRef]
- Kaushik, S.; Sarma, M.K.; Goswami, P. FRET-guided surging of cyanobacterial photosystems improves and stabilizes current in photosynthetic microbial fuel cell. J. Mater Chem. A Mater 2017, 5, 7885–7895. [Google Scholar] [CrossRef]
- McCormick, A.J.; Bombelli, P.; Scott, A.M.; Philips, A.J.; Smith, A.G.; Fisher, A.C.; Howe, C.J. Photosynthetic biofilms in pure culture harness solar energy in a mediatorless bio-photovoltaic cell (BPV) system. Energy Environ. Sci. 2011, 4, 4699–4709. [Google Scholar] [CrossRef]
- Tschörtner, J.; Lai, B.; Krömer, J.O. Biophotovoltaics: Green power generation from sunlight and water. Front. Microbiol. 2019, 10, 866. [Google Scholar] [CrossRef]
- Hondo, S.; Takahashi, M.; Osanai, T.; Matsuda, M.; Hasunuma, T.; Tazuke, A.; Nakahira, Y.; Chohnan, S.; Hasegawa, M.; Asayama, M. Genetic engineering and metabolite profiling for overproduction of polyhydroxybutyrate in cyanobacteria. J. BioSci. BioEng. 2015, 120, 510–517. [Google Scholar] [CrossRef]
- Wang, B.; Pugh, S.; Nielsen, D.R.; Zhang, W.; Meldrum, D.R. Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab. Eng. 2013, 16, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Martínez, M.; Perez-Zabaleta, M.; Gustavsson, M.; Quillaguamán, J.; Larsson, G.; van Maris, A.J.A. The role of the acyl-CoA thioesterase “YciA” in the production of (R)-3-hydroxybutyrate by recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 3693–3704. [Google Scholar] [CrossRef]
- Akiyama, H.; Okuhata, H.; Onizuka, T.; Kanai, S.; Hirano, M.; Tanaka, S.; Sasaki, K.; Miyasaka, H. Antibiotics-free stable polyhydroxyalkanoate (PHA) production from carbon dioxide by recombinant cyanobacteria. Bioresour. Technol. 2011, 102, 11039–11042. [Google Scholar] [CrossRef]
- Feng, L.-J.; Li, J.-W.; Xu, E.G.; Sun, X.-D.; Zhu, F.-P.; Ding, Z.; Tian, H.; Dong, S.-S.; Xia, P.-F.; Yuan, X.-Z. Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria. Environ. Sci. Nano 2019, 6, 3072–3079. [Google Scholar] [CrossRef]
- Cui, J.; Xie, Y.; Sun, T.; Chen, L.; Zhang, W. Deciphering and engineering photosynthetic cyanobacteria for heavy metal bioremediation. Sci. Total Environ. 2021, 761, 144111. [Google Scholar] [CrossRef]
- Chakdar, H.; Thapa, S.; Srivastava, A.; Shukla, P. Genomic and proteomic insights into the heavy metal bioremediation by cyanobacteria. J. Hazard. Mater. 2022, 424, 127609. [Google Scholar] [CrossRef]
- Ferrari, S.G.; Silva, P.G.; González, D.M.; Navoni, J.A.; Silva, H.J. Arsenic tolerance of cyanobacterial strains with potential use in biotechnology. Rev. Argent. Microbiol. 2013, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Newby, R.; Lee, L.H.; Perez, J.L.; Tao, X.; Chu, T. Characterization of zinc stress response in Cyanobacterium Synechococcus sp. IU 625. Aquat. Toxicol. 2017, 186, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Badarau, A.; Dennison, C. Thermodynamics of copper and zinc distribution in the cyanobacterium Synechocystis PCC 6803. Proc. Natl. Acad. Sci. USA 2011, 108, 13007–13012. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, T.; Li, S.; Chen, L.; Zhang, W. Adaptive laboratory evolution of cadmium tolerance in Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2018, 11, 205. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Blanc-Garin, V.; Chauvat, F. Genetic, genomics, and responses to stresses in cyanobacteria: Biotechnological implications. Genes 2021, 12, 500. [Google Scholar] [CrossRef]
- Voth, W.; Jakob, U. Stress-activated chaperones: A first line of defense. Trends BioChem. Sci. 2017, 42, 899–913. [Google Scholar] [CrossRef]
- Alidoust, L.; Soltani, N.; Modiri, S.; Haghighi, O.; Azarivand, A.; Khajeh, K.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Cadmium uptake capacity of an indigenous cyanobacterial strain, Nostoc entophytum ISC32: New insight into metal uptake in microgravity-simulating conditions. Microbiology 2016, 162, 246–255. [Google Scholar] [CrossRef]
- Alidoust, L.; Zahiri, H.S.; Maleki, H.; Soltani, N.; Vali, H.; Noghabi, K.A. Nostoc entophytum cell response to cadmium exposure: A possible role of chaperon proteins GroEl and HtpG in cadmium-induced stress. Ecotoxicol. Environ. Saf. 2019, 169, 40–49. [Google Scholar] [CrossRef]
- de Souza, P.O.; Sinhor, V.; Crizel, M.G.; Pires, N.; Filho, P.J.S.; Picoloto, R.S.; Duarte, F.A.; Pereira, C.M.P.; Mesko, M.F. Bioremediation of chromium and lead in wastewater from chemistry laboratories promotes by cyanobacteria. Bioresour. Technol. Rep. 2022, 19, 101161. [Google Scholar] [CrossRef]
- Roy, A.S.; Hazarika, J.; Manikandan, N.A.; Pakshirajan, K.; Syiem, M.B. Heavy metal removal from multicomponent system by the cyanobacterium Nostoc muscorum: Kinetics and interaction study. Appl. BioChem. Biotechnol. 2015, 175, 3863–3874. [Google Scholar] [CrossRef]
- Chevalier, P.; Proulx, D.; Lessard, P.; Vincent, W.F.; de La Noüe, J. Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J. Appl. Phycol. 2000, 12, 105–112. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Pal, R. Response of cyanobacteria to arsenic toxicity. J. Appl. Phycol. 2011, 23, 293–299. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, X.-P.; Sun, H.; Han, S.-J.; Li, W.-F.; Cui, N.; Lin, G.-M.; Zhang, J.-Y.; Cheng, W.; Cao, D.-D.; et al. Coordinating carbon and nitrogen metabolic signaling through the cyanobacterial global repressor NdhR. Proc. Natl. Acad. Sci. USA 2017, 115, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Iconomou, D.; Sotiroudis, T.; Israilides, C.; Muylaert, K. Exploration of using stripped ammonia and ash from poultry litter for the cultivation of the cyanobacterium Arthrospira platensis and the green microalga Chlorella vulgaris. Bioresour. Technol. 2015, 196, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Shing, W.L.; Surif, S.; Heng, L.Y. Toxicity biosensor for the evaluation of cadmium toxicity based on photosynthetic behavior of cyanobacteria Anabaena torulosa. Asian J. Biochem. 2008, 3, 162–168. [Google Scholar] [CrossRef]
- Kaufman, A.J. Cyanobacteria at work. Nat. GeoSci. 2014, 7, 253–254. [Google Scholar] [CrossRef]
- Jabłońska, J.; Tawfik, D.S. The evolution of oxygen-utilizing enzymes suggests early biosphere oxygenation. Nat. Ecol. Evol. 2021, 5, 442–448. [Google Scholar] [CrossRef]
- Yin, X.-X.; Wang, L.H.; Bai, R.; Huang, H.; Sun, G.-X. Accumulation and transformation of arsenic in the blue-green alga Synechocysis sp. PCC6803. Water Air Soil Pollut. 2012, 223, 1183–1190. [Google Scholar] [CrossRef]
- Hurtado-Gallego, J.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Use of cyanobacterial luminescent bioreporters to report on the environmental impact of metallic nanoparticles. Sensors 2019, 19, 3597. [Google Scholar] [CrossRef]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Lombard, J.; Soule, T.; Dunaj, S.; Wu, S.H.; Wojciechowski, M.F. Timing the evolutionary advent of cyanobacteria and the later great oxidation event using gene phylogenies of a sunscreen. mBio 2019, 10, e00561-19. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.A.; Sigman, D.M.; Morel, F.M.M. The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean–Proterozoic boundary? Inorg. Chim. Acta 2003, 356, 308–318. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Zehr, J.P.; Capone, D.G. Changing perspectives in marine nitrogen fixation. Science 2020, 368, eaay9514. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; de Baar, H.J.W.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef]
- Keren, N.; Aurora, R.; Pakrasi, H.B. Critical roles of bacterioferritins in iron storage and proliferation of cyanobacteria. Plant Physiol. 2004, 135, 1666–1673. [Google Scholar] [CrossRef]
- Schoffman, H.; Lis, H.; Shaked, Y.; Keren, N. Iron-nutrient interactions within phytoplankton. Front. Plant Sci. 2016, 7, 1223. [Google Scholar] [CrossRef]
- Cherchi, C.; Lin, Y.; Gu, A.Z. Nano-titanium dioxide exposure impacts nitrogen metabolism pathways in cyanobacteria. Environ. Eng. Sci. 2021, 38, 469–480. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; González-García, C.; Leganés, F.; Fernández-Piñas, F. Effect of pH, EDTA, and anions on heavy metal toxicity toward a bioluminescent cyanobacterial bioreporter. Arch. Environ. Contam. Toxicol. 2009, 57, 477–487. [Google Scholar] [CrossRef]
- Heidorn, T.; Camsund, D.; Huang, H.H.; Lindberg, P.; Oliveira, P.; Stensjö, K.; Lindblad, P. Synthetic biology in cyanobacteria engineering and analyzing novel functions. Methods Enzym. 2011, 497, 539–579. [Google Scholar]
- Matinha-Cardoso, J.; Coutinho, F.; Lima, S.; Eufrásio, A.; Carvalho, A.P.; Oliva-Teles, A.; Bessa, J.; Tamagnini, P.; Serra, C.R.; Oliveira, P. Novel protein carrier system based on cyanobacterial nano-sized extracellular vesicles for application in fish. Microb. Biotechnol. 2022, 15, 2191–2207. [Google Scholar] [CrossRef] [PubMed]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Beisson, F.; Riekhof, W. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015, 82, 504–522. [Google Scholar] [CrossRef]
- Liang, Y.; Maeda, Y.; Sunaga, Y.; Muto, M.; Matsumoto, M.; Yoshino, T.; Tanaka, T. Biosynthesis of polyunsaturated fatty acids in the oleaginous marine diatom Fistulifera sp. strain JPCC DA0580. Mar. Drugs 2013, 11, 5008–5023. [Google Scholar] [CrossRef]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H.B. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front. Microbiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Atalah, E.; Cruz, C.H.; Izquierdo, M.; Rosenlund, G.; Caballero, M.; Valencia, A.; Robaina, L. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for seabream (Sparus aurata). Aquaculture 2007, 270, 178–185. [Google Scholar] [CrossRef]
- Cohen, Y.; Gurevitz, M. The Cyanobacteria—Ecology, Physiology and Molecular Genetics. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 1074–1098. [Google Scholar]
- Wang, Z.; Chen, Q.; Zhang, J.; Guan, T.; Chen, Y.; Shi, W. Critical roles of cyanobacteria as reservoir and source for antibiotic resistance genes. Environ. Int. 2020, 144, 106034. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Oh, J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol 2016, 82, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.O.; Seo, H.; Oh, J. Marine biopolymer-based nanomaterials as a novel platform for theranostic applications. Polym. Rev. 2017, 57, 631–667. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Lee, J.-B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef]
- Kulshreshtha, A.J.A.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.-M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.-K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef]
- Voráčová, K.; Hájek, J.; Mareš, J.; Urajová, P.; Kuzma, M.; Cheel, J.; Villunger, A.; Kapuscik, A.; Bally, M.; Novák, P.; et al. The cyanobacterial metabolite nocuolin a is a natural oxadiazine that triggers apoptosis in human cancer cells. PLoS ONE 2017, 12, e0172850. [Google Scholar] [CrossRef]
- Bajaj, S.; Asati, V.; Singh, J.; Roy, P.P. 1,3,4-Oxadiazoles: An emerging scaffold to target growth factors, enzymes and kinases as anticancer agents. Eur. J. Med. Chem. 2015, 97, 124–141. [Google Scholar] [CrossRef]
- Semenov, S.G.; Makarova, M.V. 1,2,3-Oxadiazole rings in the aromatic compounds: A quantum-chemical investigation. Russ. J. Gen. Chem. 2011, 81, 1555–1557. [Google Scholar] [CrossRef]
- Todres, Z.V. Chalcogenadiazoles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mohareb, R.M.; Schatz, J. Anti-tumor and anti-leishmanial evaluations of 1,3,4-oxadiazine, pyran derivatives derived from cross-coupling reactions of β-bromo-6H-1,3,4-oxadiazine derivatives. Bioorg. Med. Chem. 2011, 19, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. BioChem. BioPhys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Romay Ch Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Plavšić, M.; Terzic, S.; Ahel, M.; van den Berg, C.M.G. Folic acid in coastal waters of the Adriatic Sea. Mar. Freshw. Res. 2002, 53, 1245. [Google Scholar] [CrossRef]
- Liu, Y.; Cockell, C.S.; Wang, G.; Hu, C.; Chen, L.; de Philippis, R. Control of Lunar and Martian dust—Experimental insights from artificial and natural cyanobacterial and algal crusts in the desert of Inner Mongolia, China. Astrobiology 2008, 8, 75–86. [Google Scholar] [CrossRef]
- Sumiya, E.; Shimogawa, H.; Sasaki, H.; Tsutsumi, M.; Yoshita, K.; Ojika, M.; Suenaga, K.; Uesugi, M. Cell-morphology profiling of a natural product library identifies bisebromoamide and miuraenamide A as actin filament stabilizers. ACS Chem. Biol. 2011, 6, 425–431. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Kitamura, K.; Nakayama, T.; Suenaga, K. Biselyngbyaside, a macrolide glycoside from the marine cyanobacterium Lyngbya sp. Org. Lett. 2009, 11, 2421–2424. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Gross, H.; Goeger, D.; Musafija-Girt, M.; McPhail, K.; Leal, R.M.; Mooberry, S.L.; Gerwick, W.H. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org. Lett. 2005, 7, 1375–1378. [Google Scholar] [CrossRef]
- Sato, S.-I.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef]
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as natural therapeutics and pharmaceutical potential: Role in antitumor activity and as nanovectors. Molecules 2021, 26, 247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Smith, G.D.; Waring, P. Human cancer cell (Jurkat) killing by the cyanobacterial metabolite calothrixin A. J. Appl. Phycol. 2003, 15, 269–277. [Google Scholar] [CrossRef]
- Hatae, N.; Satoh, R.; Chiba, H.; Osaki, T.; Nishiyama, T.; Ishikura, M.; Abe, T.; Hibino, S.; Choshi, T.; Okada, C.; et al. N-Substituted calothrixin B derivatives inhibited the proliferation of HL-60 promyelocytic leukemia cells. Med. Chem. Res. 2014, 23, 4956–4961. [Google Scholar] [CrossRef]
- Almaliti, J.; Miller, B.; Pietraszkiewicz, H.; Glukhov, E.; Naman, C.B.; Kline, T.; Hanson, J.; Li, X.; Zhou, S.; Valeriote, F.A.; et al. Exploration of the carmaphycins as payloads in antibody-drug conjugate anticancer agents. Eur. J. Med. Chem. 2019, 161, 416–432. [Google Scholar] [CrossRef]
- Pereira, A.R.; Kale, A.J.; Fenley, A.T.; Byrum, T.; Debonsi, H.M.; Gilson, M.K.; Valeriote, F.A.; Moore, B.S.; Gerwick, W.H. The carmaphycins: New proteasome inhibitors exhibiting an α,β-epoxyketone warhead from a marine cyanobacterium. ChembioChem 2012, 13, 810–817. [Google Scholar] [CrossRef]
- Salvador, L.A.; Paul, V.J.; Luesch, H. Caylobolide B, a macrolactone from symplostatin 1-producing marine cyanobacteria Phormidium spp. from Florida. J. Nat. Prod. 2010, 73, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, J.B.; Molinski, T.F. Caylobolide A, a unique 36-membered macrolactone from a Bahamian Lyngbya majuscula. Org. Lett. 2002, 4, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Hau, A.M.; Greenwood, J.A.; Löhr, C.V.; Serrill, J.D.; Proteau, P.J.; Ganley, I.G.; McPhail, K.L.; Ishmael, J.E. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS ONE 2013, 8, e65250. [Google Scholar] [CrossRef]
- Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, A cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Symplostatin 3, a new dolastatin 10 analogue from the marine cyanobacterium Symploca sp. VP452. J. Nat. Prod. 2002, 65, 16–20. [Google Scholar] [CrossRef]
- Kwan, J.C.; Ratnayake, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Grassypeptolides A-C, cytotoxic bis-thiazoline containing marine cyclodepsipeptides. J. Org. Chem. 2010, 75, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Thimmaiah, M.; Shaala, L.A.; Hau, A.M.; Malmo, J.M.; Ishmael, J.E.; Youssef, D.T.A.; McPhail, K.L. Cyclic depsipeptides, grassypeptolides D and E and Ibu-epidemethoxylyngbyastatin 3, from a red sea Leptolyngbya cyanobacterium. J. Nat. Prod. 2011, 74, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.L.; Watts, K.S.; Yokochi, A.; Roberts, M.A.; Verdier-Pinard, P.; Jimenez, J.I.; Hamel, E.; Scheuer, P.J.; Gerwick, W.H. Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J. Nat. Prod. 2002, 65, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of hierridin B, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PLoS ONE 2013, 8, e69562. [Google Scholar] [CrossRef]
- Mevers, E.; Liu, W.-T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Sedlacek, P.; Slaninova, E.; Koller, M.; Nebesarova, J.; Marova, I.; Krzyzanek, V.; Obruca, S. PHA granules help bacterial cells to preserve cell integrity when exposed to sudden osmotic imbalances. New Biotechnol. 2018, 49, 129–136. [Google Scholar] [CrossRef]
- Raza, Z.A.; Tariq, M.R.; Majeed, M.I.; Banat, I.M. Recent developments in bioreactor scale production of bacterial polyhydroxyalkanoates. Bioprocess. Biosyst. Eng. 2019, 42, 901–919. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P.J. Cyanobacterial polyhydroxybutyrate for sustainable bioplastic production: Critical review and perspectives. J. Environ. Chem. Eng. 2020, 8, 104007. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.S.; Torzillo, G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 100, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Carpine, R.; Du, W.; Olivieri, G.; Pollio, A.; Hellingwerf, K.J.; Marzocchella, A.; dos Santos, F.B. Genetic engineering of Synechocystis sp. PCC6803 for poly-β-hydroxybutyrate overproduction. Algal Res. 2017, 25, 117–127. [Google Scholar] [CrossRef]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbüchel, A.; Maier, U.G. Microalgae as bioreactors for bioplastic production. Microb. Cell Fact. 2011, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Kaewbai-ngam, A.; Incharoensakdi, A.; Monshupanee, T. Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 2016, 212, 342–347. [Google Scholar] [CrossRef]
- Testa, R.L.; Estrada, V.; Delpino, C.; Diaz, M.S. Photosynthetic bioplastics production with cyanobacteria by coupled growth-production mutants. Comput. Aided Chem. Eng. 2021, 50, 1917–1922. [Google Scholar]
- Gopi, K.; Balaji, S.; Muthuvelan, B. Isolation purification and screening of biodegradable polymer PHB producing cyanobacteria from marine and freshwater resources. Iran. J. Energy Environ. 2014, 5, 94–100. [Google Scholar]
- Ansari, S.; Fatma, T. Cyanobacterial polyhydroxybutyrate (PHB): Screening, optimization and characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2011, 24, 803–814. [Google Scholar] [CrossRef]
- Sharma, L.; Mallick, N. Accumulation of poly-beta-hydroxybutyrate in Nostoc muscorum: Regulation by pH, light-dark cycles, N and P status and carbon sources. Bioresour. Technol. 2005, 96, 1304–1310. [Google Scholar] [CrossRef]
- Kavitha, G.; Kurinjimalar, C.; Sivakumar, K.; Aravind, R.; Shree, C.G.; Arthi, K.; Palani, P.; Kaviyarasan, V.; Rengasamy, R. Mass cultivation of UV-B adapted Arthrospira platensis RRGK under open raceway pond for the production of Poly-β-hydroxy butyrate. Int. J. Biol. Macromol. 2016, 93, 1304–1316. [Google Scholar] [CrossRef]
- Martins, R.G.; Severo Gonçalves, I.; de Morais, M.G.; Costa, J.A.V. Bioprocess engineering aspects of biopolymer production by the cyanobacterium Spirulina strain LEB 18. Int. J. Polym. Sci. 2014, 2014, 895237. [Google Scholar] [CrossRef]
- Shrivastav, A.; Mishra, S.K.; Mishra, S. Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef]
- Panda, B.; Mallick, N. Enhanced poly-beta-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007, 44, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Q.; Huang, H. Current states and challenges of salt-affected soil remediation by cyanobacteria. Sci. Total Environ. 2019, 669, 258–272. [Google Scholar] [CrossRef]
- Gupta, V.; Verma, N.K.; Choudhary, K.K. Cyanobacterial nanoparticles: Application in agriculture and allied sectors. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–40. [Google Scholar]
- Song, T.; Mårtensson, L.; Eriksson, T.; Zheng, W.; Rasmussen, U. Biodiversity and seasonal variation of the cyanobacterial assemblage in a rice paddy field in Fujian, China. FEMS Microbiol. Ecol. 2005, 54, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Priyadarshani, I.; Rath, B. Cyanobacteria—As Potential Biofertilizer. 2013. Available online: https://www.semanticscholar.org/paper/CYANOBACTERIA-AS-POTENTIAL-BIOFERTILIZER-Sahu-Priyadarshani/f35445e582f40b36cbdc9c68ea0ad0aab268fcd6 (accessed on 1 November 2022).
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, J.; Chen, C.; Zhao, L.; Wu, Z.; Liu, L.; Cai, D. Promoting desert biocrust formation using aquatic cyanobacteria with the aid of MOF-based nanocomposite. Sci. Total. Environ. 2020, 708, 134824. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, C.; Gao, Y.; Wang, B.; Wang, D.; Du, Y.; Liu, L.; Wu, Z.; Cai, D. Synergistic effect of cyanobacteria and nano-sand-stabilizer on biocrust formation and sand fixation. J. Environ. Chem. Eng. 2021, 9, 104887. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Yang, H.; Zhang, D.; Hu, C. A new biofilm-based microalgal cultivation approach on shifting sand surface for desert cyanobacterium Microcoleus vaginatus. Bioresour. Technol. 2017, 238, 602–608. [Google Scholar] [CrossRef]
- Román, J.R.; Roncero-Ramos, B.; Chamizo, S.; Rodríguez-Caballero, E.; Cantón, Y. Restoring soil functions by means of cyanobacteria inoculation: Importance of soil conditions and species selection. Land Degrad. Dev. 2018, 29, 3184–3193. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, D.; Hu, C.; Rao, B. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol. BioChem. 2009, 41, 926–929. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J. Mechanical sand fixing is more beneficial than chemical sand fixing for artificial cyanobacteria crust colonization and development in a sand desert. Appl. Soil Ecol. 2019, 140, 115–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, R.L.; Wang, J. Towards stopping land degradation in drylands: Water-saving techniques for cultivating biocrusts in situ. Land Degrad. Dev. 2019, 30, 2336–2346. [Google Scholar] [CrossRef]
- Meng, X.; Liang, L.; Liu, B. Synthesis and sand-fixing properties of cationic poly(vinyl acetate-butyl acrylate-2-hydroxyethyl acrylate-DMC) copolymer emulsions. J. Polym. Environ. 2016, 25, 487–498. [Google Scholar] [CrossRef]
- Fick, S.E.; Barger, N.N.; Duniway, M.C. Hydrological function of rapidly induced biocrusts. Ecohydrology 2019, 12, e2089. [Google Scholar] [CrossRef]
- Park, C.H.; Li, X.R.; Jia, R.L.; Hur, J.S. Combined application of cyanobacteria with soil fixing chemicals for rapid induction of biological soil crust formation. Arid. Land Res. Manag. 2016, 31, 81–93. [Google Scholar] [CrossRef]
- Zaady, E.; Katra, I.; Barkai, D.; Knoll, Y.; Sarig, S. The coupling effects of using coal fly-ash and bio-inoculant for rehabilitation of disturbed biocrusts in active sand dunes. Land Degrad. Dev. 2017, 28, 1228–1236. [Google Scholar] [CrossRef]
- Peng, C.; Zheng, J.; Huang, S.; Li, S.; Li, D.; Cheng, M.; Liu, Y. Application of sodium alginate in induced biological soil crusts: Enhancing the sand stabilization in the early stage. J. Appl. Phycol. 2017, 29, 1421–1428. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Varman, A.M.; Yu, Y.; You, L.; Tang, Y.J. Photoautotrophic production of D-lactic acid in an engineered cyanobacterium. Microb. Cell Fact. 2013, 12, 117. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, F.; Ni, J.; Li, C.; Xu, P. Production of C3 platform chemicals from CO2 by genetically engineered cyanobacteria. Green Chem. 2015, 17, 3100–3110. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Liu, H.-L.; Chen, Y.-J.; Cheng, Y.-H.; Lin, W.-L.; Yeh, C.-H.; Chang, C.-H. Enhancing CO2 bio-mitigation by genetic engineering of cyanobacteria. Energy Environ. Sci. 2012, 5, 8318–8327. [Google Scholar] [CrossRef]
- Gao, X.; Sun, T.; Pei, G.; Chen, L.; Zhang, W. Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl. Microbiol. Biotechnol. 2016, 100, 3401–3413. [Google Scholar] [CrossRef]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Danon, A.; Marchand, C.H.; Fermani, S.; Trost, P.; et al. Redox regulation of the Calvin-Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef]
- Bentley, F.K.; Zurbriggen, A.; Melis, A. Heterologous expression of the mevalonic acid pathway in cyanobacteria enhances endogenous carbon partitioning to isoprene. Mol. Plant 2014, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.N.; Raines, C.A.; Kerfeld, C.A. Comparative analysis of 126 cyanobacterial genomes reveals evidence of functional diversity among homologs of the redox-regulated CP12 protein. Plant Physiol. 2013, 161, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Lan, E.I.; Liao, J.C. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab. Eng. 2011, 13, 353–363. [Google Scholar] [CrossRef]
- Robertson, D.E.; Jacobson, S.A.; Morgan, F.; Berry, D.; Church, G.M.; Afeyan, N.B. A new dawn for industrial photosynthesis. Photosynth. Res. 2011, 107, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rajneesh Maurya, P.K.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacterial farming for environment-friendly sustainable agriculture practices: Innovations and perspectives. Front. Environ. Sci. 2018, 6, 7. [Google Scholar] [CrossRef]
- Quiroz-Arita, C.; Sheehan, J.J.; Bradley, T.H. Life cycle net energy and greenhouse gas emissions of photosynthetic cyanobacterial biorefineries: Challenges for industrial production of biofuels. Algal Res. 2017, 26, 445–452. [Google Scholar] [CrossRef]
- Nilsson, A.; Shabestary, K.; Brandão, M.; Hudson, E.P. Environmental impacts and limitations of third-generation biobutanol: Life cycle assessment of n-butanol produced by genetically engineered cyanobacteria. J. Ind. Ecol. 2020, 24, 205–216. [Google Scholar] [CrossRef]
- Henley, W.J.; Litaker, R.W.; Novoveská, L.; Duke, C.S.; Quemada, H.D.; Sayre, R.T. Initial risk assessment of genetically modified (GM) microalgae for commodity-scale biofuel cultivation. Algal Res. 2013, 2, 66–77. [Google Scholar] [CrossRef]
- Legere, E.; Roessler, P.; Miller, H.; Belicka, L.; Yuan, Y.; Chance, R.; Dalrymple, K.; Porubsky, W.; Coleman, J.; Sweeney, K.; et al. Recovery Act—Integrated Pilot-Scale Biorefinery for Producing Ethanol from Hybrid Algae; Algenol Biotech LLC: Golden, CO, USA, 2017. [Google Scholar]
- Zhou, Y.; Sun, T.; Chen, Z.; Song, X.; Chen, L.; Zhang, W. Development of a new biocontainment strategy in model Cyanobacterium Synechococcus strains. ACS Synth. Biol. 2019, 8, 2576–2584. [Google Scholar] [CrossRef]
- González-Morales, S.I.; Pacheco-Gutiérrez, N.B.; Ramírez-Rodríguez, C.A.; Brito-Bello, A.A.; Estrella-Hernández, P.; Herrera-Estrella, L.; López-Arredondo, D.L. Metabolic engineering of phosphite metabolism in Synechococcus elongatus PCC 7942 as an effective measure to control biological contaminants in outdoor raceway ponds. Biotechnol. Biofuels 2020, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Selão, T.T.; Włodarczyk, A.; Nixon, P.J.; Norling, B. Growth and selection of the cyanobacterium Synechococcus sp. PCC 7002 using alternative nitrogen and phosphorus sources. Metab. Eng. 2019, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Sano, K.; Watanabe, S.; Kanbara, A.; Nasser, A.-H.G.; Ikeda, T.; Ishida, T.; Funabashi, H.; Kuroda, A.; Hirota, R. Synthetic phosphorus metabolic pathway for biosafety and contamination management of cyanobacterial cultivation. ACS Synth. Biol. 2018, 7, 2189–2198. [Google Scholar] [CrossRef] [PubMed]

| S.No | Species | Metal | Incubation | pH | Temperature | Bioremediation Value | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Synechococcus sp. and Aphanocapsa sp. | Chromium (Cr), lead (Pb) | 240 h | 7.8 | 27 °C | Cr−(63.8 to 56.2 μg/L), Pb− (418 to 239 μg/L) | [39] |
| 2 | Nostoc muscorum | Cu(II), Zn(II), Pb(II) and Cd(II) | 72-h | 8.0 | 25–30 °C | Pb (II) (96.3 %) and Cu (II) (96.42 %) were the most abundant, closely by Cd(II) (80.04 %) and Zn(II) (71.3 %). | [40] |
| 3 | Phormidium bohneri | NO3−, PO43− | 14 h light–10 h dark. | _ | 15 °C | 80 to 350 μmol photon m−2 s−1. | [41] |
| 4 | Phormidium laminosum | Arsenic, | 24 h | 7.8 | _ | 37.17 μg g−1 | [42] |
| 5 | Nostoc sphaeroides | Cr3+, Pb2+ | 4 h | 5.0 | 25 °C | 116.28 and 22.37 mg g−1 | [43] |
| 6 | Arthrospira platensis | Cu2+ and Ni2+ | 48 h | 5.0–6.0 | 30 ± 2 °C | Cu2+-(2.33–3.08 mg/g), Ni2+- (2.14–2.84 mg/g). | [44] |
| 7 | Spirulina sp. | Cr3+, Cd2+, Cu2+ | 100 h | 7.0 | 35 °C | Cr3+-(185 mg g−1), Cu2+-(196 mg g−1), Cd2+-(159 mg g−1) | [45,46] |
| 8 | Anabaena torulosa | Cd2+ | 4–5 h | 7.0 | 18.5Â °C | 8 mg L−1 | [47,48] |
| 9 | Synechocysis sp. PCC6803 | Arsenite | 72 h | 6.2 | 25 °C/20 °C day/night | 0.9 and 1.0 mg kg−1 DW | [49] |
| S.No | Strains | Bioactive Compounds | Structure | Potential Activities | Reference |
|---|---|---|---|---|---|
| 1 | Spirulina | c-phycocyanin | 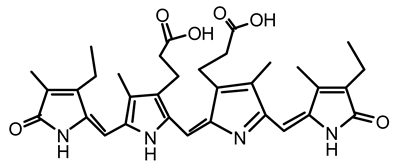 | Neuroprotective action through cyclooxygenase 2 inhibition (COX-2) | [85,86] |
| 2 | S. platensis (synonym Arthrospira platensis) | Gamma linolenic acid (GLA) | 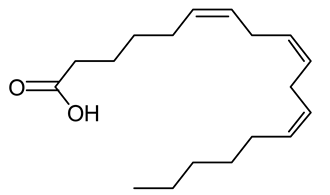 | Reduces blood pressure and improves lipid metabolism | [87] |
| 3 | Lyngbya sp. | Apratoxin A |  | Inhibits the JAK/STAT signaling pathway by downregulating IL-6 signal transducer (gp130) | [88] |
| 4 | Lyngbya sp. | Bisebromoamide |  | Actin stabilizing property | [89] |
| 5 | Lyngbya sp. | Biselyngbyaside |  | Cytotoxic/anti-proliferative | [90] |
| 6 | Geitlerinema sp. | Ankaraholide A |  | Loss of F-actin | [91] |
| 7 | Dolabella auricularia | Aurilide |  | It increases mitochondrial-induced apoptosis by binding PHB1 and initiating OA1 proteolysis (OPA1) | [92] |
| 8 | Nostoc linckia and N. spongiaeforme var. tenue | Borophycin |  | Cytotoxic against colorectal cancer | [93] |
| 9 | Calothrix sp. | Calothrixins A |  | Triggered apoptosis and G2/M cell cycle arrest in all cancer cell lines | [94,95] |
| 10 | Symploca sp. | Carmaphycins A and B |  | High levels of activity against proteasomes | [96,97] |
| 11 | Lyngbya majuscule and Phormidium sp. | Caylobolide A and B |  | Produce cytotoxicity in cancer cells. Caylobolide A fights HCT-116 colon cancer | [98,99] |
| 12 | Leptolyngbya sp. | Coibamide A | 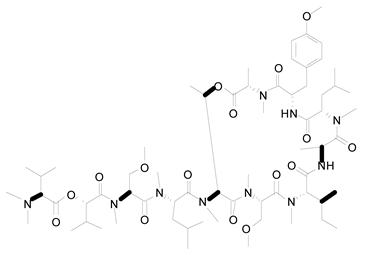 | mTOR-independent autophagy and glioblastoma cell death | [100] |
| 13 | Nostoc sp. var. ATCC 53789 | Cryptophycin | 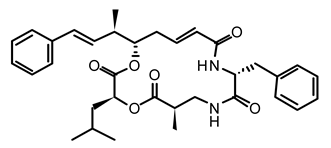 | Suppress microtubule synthesis; anti-tumorigenic | [101] |
| 14 | Lyngbya majuscula | Desmethoxymajusculamide C | 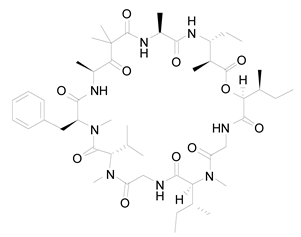 | Depolymerizes actin cytoskeleton, disrupting cell microfibril network | [102] |
| 15 | Dolabella auricularia | Dolastatin | 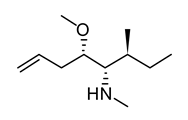 | Interference with microtubule assembly arrests the cell cycle in G2/M, causing apoptosis | [103] |
| 16 | Symploca sp. VP64 | Symplostatin 3 | 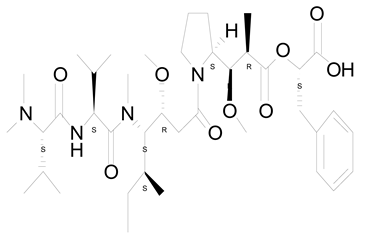 | Microtubules disruption | [104] |
| 17 | Lyngbya confervoides | Grassypeptolide | 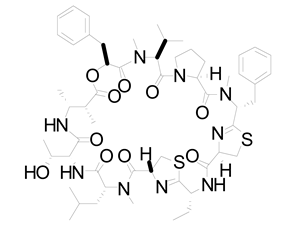 | G1 cell cycle arrest | [105,106] |
| 18 | L. majuscula | Hectochlorin |  | Increases actin polymerization | [107] |
| 19 | Symploca sp | Hoiamide D | 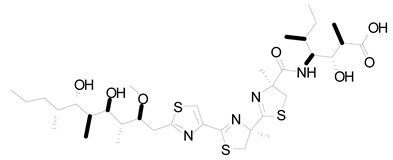 | Blocking p53 and high-fidelity minichromosome 2 (p53/HDM2) | [108] |
| 20 | Oscillatoria margaritifera | Veraguamides A | 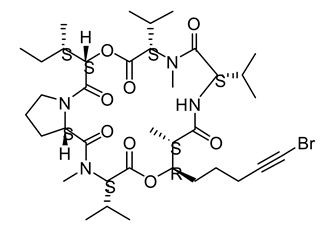 | Cytotoxic effect | [109] |
| S.No | Species | Approach/Analysis | Culture Condition | Source | Biomass Conc. | Ref. |
|---|---|---|---|---|---|---|
| 1 | Synechocystis sp. PCC 6803 | Systems Metabolic Engineering | Photoautotrophic | Glucose | 1.37 gDW/L | [119] |
| 2 | Anabaena sp. | HPLC and FTIR | Photoautotrophic | Fresh Water, Marine water | 2.314 ± 0.012 mg/L | [120] |
| 3 | N. muscorum NCCU-442 | FTIR, NMR, and GC MS | Heteroautotrophic | Glucose, maltose, fructose, sucrose, lactose and starch | Accumulation of 26.37% PHB | [121] |
| 4 | Aulosira fertilissima | Composite rotary design (CCRD)-statistical package, Pyris Diamond Differential Scanning Calorimeter | Mixotrophy (Chemoheterotrophic and photoautotrophic) | Sucrose, fructose, glucose, maltose | 1.59 g L−1 | [122] |
| 5 | Calothrix scytonemicola TISTR 8095 | HPLC, NMR | Photoautotrophic | Atmospheric carbon dioxide (CO2) | 25.2% (w/w DW | [118] |
| 6 | Nostoc muscorum | Spectro- Photometer | Photoautotrophic | Acetate, glucose, maltose, fructose, and ethane | 8.6%, w/w, of the dry cell | [123] |
| 7 | Arthrospira platensis RRGK | FTIR, DSC, TGA, and XRD | Photoautotrophic | Sodium bicarbonate | 1.101 g L−1 | [124] |
| 8 | Cyanobacterium Spirulina LEB 18 | Photobioreactor | Mixotrophic | Glucose or sodium acetate | 44.2 | [125] |
| 9 | Spirulina subsalsa | IR, NMR, TGA, and DS | Phototrophic | Atmospheric carbon dioxide (CO2) | 1.97 g L−1 | [126] |
| 10 | Synechocystis sp. PCC 6803 | Propanolysis method | Photoautotrophic | Glucose, maltose, fructose | 38 | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life 2022, 12, 2013. https://doi.org/10.3390/life12122013
Govindasamy R, Gayathiri E, Sankar S, Venkidasamy B, Prakash P, Rekha K, Savaner V, Pari A, Thirumalaivasan N, Thiruvengadam M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life. 2022; 12(12):2013. https://doi.org/10.3390/life12122013
Chicago/Turabian StyleGovindasamy, Rajakumar, Ekambaram Gayathiri, Sathish Sankar, Baskar Venkidasamy, Palanisamy Prakash, Kaliaperumal Rekha, Varsha Savaner, Abirami Pari, Natesan Thirumalaivasan, and Muthu Thiruvengadam. 2022. "Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications" Life 12, no. 12: 2013. https://doi.org/10.3390/life12122013
APA StyleGovindasamy, R., Gayathiri, E., Sankar, S., Venkidasamy, B., Prakash, P., Rekha, K., Savaner, V., Pari, A., Thirumalaivasan, N., & Thiruvengadam, M. (2022). Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life, 12(12), 2013. https://doi.org/10.3390/life12122013









