A Narrative Review of Sex and Gender Differences in Sleep Disordered Breathing: Gaps and Opportunities
Abstract
1. Introduction
2. Materials and Methods
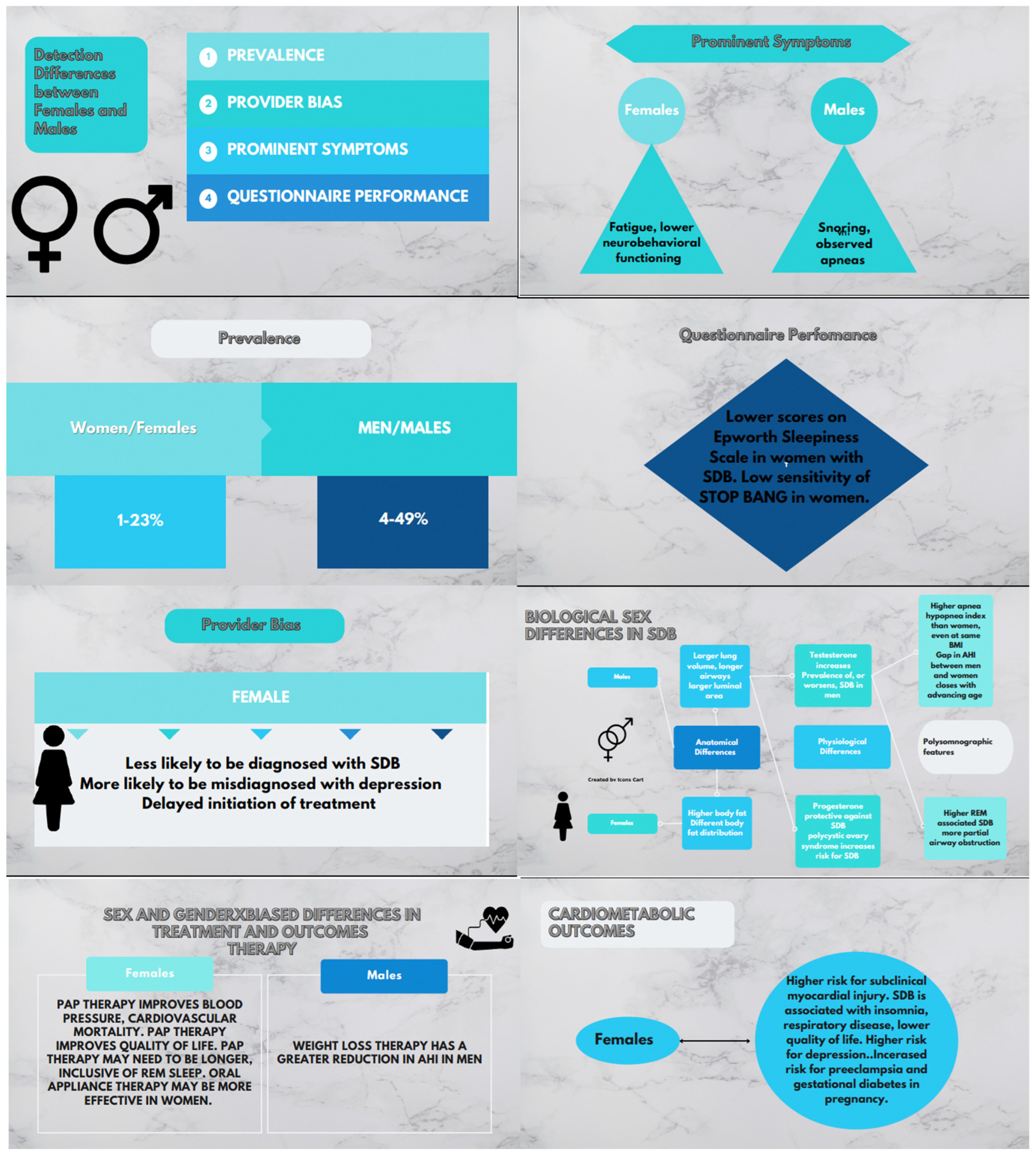
3. Gender Differences in Clinical Presentation
4. Gender Differences in Diagnostic Prediction of SDB Using Questionnaires
5. Sex Differences in Anthropometrics, Phenotype, and Pathophysiologic Mechanisms
6. Sex Differences in Polysomnographic Characteristics
7. Differences in the Association of SDB with Cardiovascular Outcomes
8. SDB in Women during Specific Life Stages
9. Treatment of SDB
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jaffe, F.; Markov, D.; Doghramji, K. Sleep-disordered breathing: In depression and schizophrenia. Psychiatry Edgmont 2006, 3, 62–68. [Google Scholar] [PubMed]
- Quan, S.F.; Gersh, B.J. Cardiovascular Consequences of Sleep-Disordered Breathing: Past, Present and Future. Circulation 2004, 109, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Anton, A.; D’Ambrosio, C.M. Sex Differences in Obstructive Sleep Apnea. Clin. Chest Med. 2021, 42, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Gallego, E.; Carmona-Bernal, C.; Capote, F.; Sánchez-Armengol, A.; Botebol-Benhamou, G.; Padillo, J.P.; Castillo-Gómez, J. Gender differences in obstructive sleep apnea syndrome: A clinical study of 1166 patients. Respir. Med. 2004, 98, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Mallampalli, M.P.; Carter, C.L. Exploring Sex and Gender Differences in Sleep Health: A Society for Women’s Health Research Report. J. Womens Health 2014, 23, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Povitz, M.; Kendzerska, T.; Hanly, P.J.; Jenkyn, K.B.; Allen, B.; George, C.F.; Shariff, S. Profile of CPAP treated patients in Ontario, Canada, 2006–2013: A population-based cohort study. Sleep Med. 2018, 51, 22–28. [Google Scholar] [CrossRef]
- Han, M.K.; Arteaga-Solis, E.; Blenis, J.; Bourjeily, G.; Clegg, D.J.; DeMeo, D.; Duffy, J.; Gaston, B.; Heller, N.M.; Hemnes, A.; et al. Female Sex and Gender in Lung/Sleep Health and Disease. Increased Understanding of Basic Biological, Pathophysiological, and Behavioral Mechanisms Leading to Better Health for Female Patients with Lung Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 850–858. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.-M.; Have, T.T.; Rein, J.; Vela-Bueno, A.; Kales, A. Prevalence of Sleep-disordered Breathing in Women. Am. J. Respir. Crit. Care Med. 2001, 163, 608–613. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Kim, J.; In, K.; Kim, J.; You, S.; Kang, K.; Shim, J.; Lee, S.; Lee, J.; Lee, S.; Park, C.; et al. Prevalence of Sleep-disordered Breathing in Middle-aged Korean Men and Women. Am. J. Respir. Crit. Care Med. 2004, 170, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Kump, K.; Tishler, P.V.; Browner, I.; Ferrette, V. Gender differences in sleep disordered breathing in a community-based sample. Am. J. Respir. Crit. Care Med. 1994, 149, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Hutton, R.; Finn, L.; Badr, S.; Palta, M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch. Intern. Med. 1996, 156, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, J.; Yang, W.; Zou, H.; Su, C.; Miao, J. Gender differences in clinical manifestations and polysomnographic findings in Chinese patients with obstructive sleep apnea. Sleep Breath. 2020, 24, 1019–1026. [Google Scholar] [CrossRef]
- Franklin, K.A.; Sahlin, C.; Stenlund, H.; Lindberg, E. Sleep apnoea is a common occurrence in females. Eur. Respir. J. 2013, 41, 610–615. [Google Scholar] [CrossRef]
- Morris, J.L.; Mazzotti, D.R.; Gottlieb, D.J.; Hall, M.H. Sex differences within symptom subtypes of mild obstructive sleep apnea. Sleep Med. 2021, 84, 253–258. [Google Scholar] [CrossRef]
- Lindberg, E.; Benediktsdottir, B.; Franklin, K.A.; Holm, M.; Johannessen, A.; Jögi, R.; Gislason, T.; Real, F.G.; Schlünssen, V.; Janson, C. Women with symptoms of sleep-disordered breathing are less likely to be diagnosed and treated for sleep apnea than men. Sleep Med. 2017, 35, 17–22. [Google Scholar] [CrossRef]
- Geer, J.H.; Hilbert, J. Focus: Health Equity: Gender Issues in Obstructive Sleep Apnea. Yale J. Biol. Med. 2021, 94, 487–496. [Google Scholar]
- Doherty, L.S.; Kiely, J.L.; Swan, V.; McNicholas, W.T. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005, 127, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Harsch, I.A.; Schahin, S.P.; Radespiel-Tröger, M.; Weintz, O.; Jahreiss, H.; Fuchs, F.S.; Wiest, G.H.; Hahn, E.G.; Lohmann, T.; Konturek, P.C.; et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2004, 169, 156–162. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Weng, J.; Mobley, D.R.; Wang, R.; Kwon, Y.; Zee, P.C.; Lutsey, P.L.; Redline, S. Effect of Manual Editing of Total Recording Time: Implications for Home Sleep Apnea Testing. J. Clin. Sleep Med. 2017, 13, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-Y.; Chen, P.-Y.; Chuang, L.-P.; Chen, N.-H.; Tu, Y.-K.; Hsieh, Y.-J.; Wang, Y.-C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, Stop-Bang, Stop, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.M.; Jossen, J.; Kröll, D.; Nett, P.C.; Baty, F.; Brill, A.-K.; Ott, S.R. Prevalence and Prediction of Obstructive Sleep Apnea Prior to Bariatric Surgery—Gender-Specific Performance of Four Sleep Questionnaires. Obes. Surg. 2018, 28, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Westlake, K.; Plihalova, A.; Pretl, M.; Lattova, Z.; Polak, J. Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus: A prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Med. 2016, 26, 71–76. [Google Scholar] [CrossRef]
- Arslan, B.O.; Uçar, Z.Z.; Batum, O.; Orman, M.N. Validation of the NoSAS score for screening sleep-disordered breathing: A sleep clinic- based study in Turkey. Turk. J. Med. Sci. 2021, 51, 319–327. [Google Scholar] [CrossRef]
- Mou, J.; Pflugeisen, B.M.; Crick, B.A.; Amoroso, P.J.; Harmon, K.T.; Tarnoczy, S.F.; Ho, S.S.; Mebust, K.A. The discriminative power of STOP-Bang as a screening tool for suspected obstructive sleep apnea in clinically referred patients: Considering gender differences. Sleep Breath. 2019, 23, 65–75. [Google Scholar] [CrossRef]
- Pataka, A.; Kotoulas, S.; Kalamaras, G.; Schiza, S.; Sapalidis, K.; Giannakidis, D.; Michalopoulos, N.; Koulouris, C.; Aidoni, Z.; Amaniti, A.; et al. Gender Differences in Obstructive Sleep Apnea: The Value of Sleep Questionnaires with a Separate Analysis of Cardiovascular Patients. J. Clin. Med. 2020, 9, 130. [Google Scholar] [CrossRef]
- Bauters, F.A.; Loof, S.; Hertegonne, K.B.; Chirinos, J.A.; De Buyzere, M.L.; Rietzschel, E.R. Sex-specific sleep apnea screening questionnaires: Closing the performance gap in women. Sleep Med. 2020, 67, 91–98. [Google Scholar] [CrossRef]
- Molgat-Seon, Y.; Peters, C.M.; Sheel, A.W. Sex-differences in the human respiratory system and their impact on resting pulmonary function and the integrative response to exercise. Curr. Opin. Physiol. 2018, 6, 21–27. [Google Scholar] [CrossRef]
- Kasai, T.; Motwani, S.S.; Yumino, D.; Mak, S.; Newton, G.E.; Bradley, T.D. Differing relationship of nocturnal fluid shifts to sleep apnea in men and women with heart failure. Circ. Heart Fail. 2012, 5, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Bourjeily, G.; Chambers, A.; Salameh, M.; Bublitz, M.H.; Kaur, A.; Coppa, B.A.; Risica, P.; Lambert-Messerlian, G. Anthropometric Measures and Prediction of Maternal Sleep-Disordered Breathing. J. Clin. Sleep Med. 2019, 15, 849–856. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Sex differences in respiratory function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Ripoll, J.G.; Cross, T.J.; Baker, S.; Wiggins, C.C.; Welch, B.T.; Joyner, M.J. Sex differences in large conducting airway anatomy. J. Appl. Physiol. 2018, 125, 960–965. [Google Scholar] [CrossRef]
- Davidson, F.E.; Matsha, T.E.; Erasmus, R.T.; Kengne, A.P.; Goedecke, J.H. Associations between body fat distribution and cardiometabolic risk factors in mixed-ancestry South African women and men. Cardiovasc. J. Afr. 2019, 30, 321–330. [Google Scholar] [CrossRef]
- Morelli, C.; Badr, M.S.; Mateika, J.H. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J. Appl. Physiol. 2004, 97, 1673–1680. [Google Scholar] [CrossRef]
- Popovic, R.M.; White, D.P. Upper airway muscle activity in normal women: Influence of hormonal status. J. Appl. Physiol. 1998, 84, 1055–1062. [Google Scholar] [CrossRef]
- Driver, H.S.; McLean, H.; Kumar, D.V.; Farr, N.; Day, A.G.; Fitzpatrick, M.F. The Influence of the Menstrual Cycle on Upper Airway Resistance and Breathing During Sleep. Sleep 2005, 28, 449–456. [Google Scholar] [CrossRef]
- Stachenfeld, N.S. Hormonal Changes During Menopause and the Impact on Fluid Regulation. Reprod. Sci. 2014, 21, 555–561. [Google Scholar] [CrossRef]
- Lindberg, E.; Bonsignore, M.R.; Polo-Kantola, P. Role of menopause and hormone replacement therapy in sleep-disordered breathing. Sleep Med. Rev. 2020, 49, 101225. [Google Scholar] [CrossRef] [PubMed]
- Mirer, A.G.; Peppard, P.E.; Palta, M.; Benca, R.M.; Rasmuson, A.; Young, T. Menopausal hormone therapy and sleep-disordered breathing: Evidence for a healthy user bias. Ann. Epidemiol. 2015, 25, 779–784.e1. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.M.; Killick, R.; Yee, B.J.; Grunstein, R.R.; Liu, P.Y. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: A randomized placebo-controlled trial. Clin. Endocrinol. 2012, 77, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.M.; Sandblom, R.E.; Schoene, R.B.; Lee, K.A.; Giblin, E.C.; Pierson, D.J.; Bremner, W.J. Testosterone replacement in hypogonadal men: Effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin. Endocrinol. 1985, 22, 713–721. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Scoccia, B.; Mazzone, T.; Sam, S. Risk of obstructive sleep apnea in obese and nonobese women with polycystic ovary syndrome and healthy reproductively normal women. Fertil. Steril. 2012, 97, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Legro, R.S.; Bixler, E.O.; Grayev, A.; Kales, A.; Chrousos, G.P. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: Role of insulin resistance. J. Clin. Endocrinol. Metab. 2001, 86, 517–520. [Google Scholar] [CrossRef]
- Gopal, M.; Duntley, S.; Uhles, M.; Attarian, H. The role of obesity in the increased prevalence of obstructive sleep apnea syndrome in patients with polycystic ovarian syndrome. Sleep Med. 2002, 3, 401–404. [Google Scholar] [CrossRef]
- Tashkandi, Y.; Badr, M.S.; Rowley, J.A. Determinants of the apnea index in a sleep center population. Sleep Breath. 2005, 9, 181–186. [Google Scholar] [CrossRef]
- Won, C.H.J.; Reid, M.; Sofer, T.; Azarbarzin, A.; Purcell, S.; White, D.; Wellman, A.; Sands, S.; Redline, S. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep 2020, 43, zsz274. [Google Scholar] [CrossRef]
- Guilleminault, C.; Stoohs, R.; Kim, Y.-D.; Chervin, R.; Black, J.; Clerk, A. Upper airway sleep-disordered breathing in women. Ann. Intern. Med. 1995, 122, 493–501. [Google Scholar] [CrossRef]
- Koo, B.B.; Patel, S.R.; Strohl, K.; Hoffstein, V. Rapid eye movement-related sleep-disordered breathing: Influence of age and gender. Chest 2008, 134, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Youn, M.; Kwon, J.Y.; Lee, K.S.; Park, J.H.; Lee, H.W. Gender Differences in Rapid Eye Movement-Related Sleep Disordered Breathing. Health 2015, 7, 106–111. [Google Scholar] [CrossRef][Green Version]
- Mano, M.; Hoshino, T.; Sasanabe, R.; Murotani, K.; Nomura, A.; Hori, R.; Konishi, N.; Baku, M.; Shiomi, T. Impact of Gender and Age on Rapid Eye Movement-Related Obstructive Sleep Apnea: A Clinical Study of 3234 Japanese OSA Patients. Int. J. Environ. Res. Public Health 2019, 16, 1068. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Finn, L.A.; Hagen, E.W.; Young, T.; Hla, K.M.; Van Cauter, E.; Peppard, P.E. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 2014, 190, 1158–1167. [Google Scholar] [CrossRef]
- Acosta-Castro, P.; Hirotsu, C.; Marti-Soler, H.; Marques-Vidal, P.; Tobback, N.; Andries, D.; Waeber, G.; Preisig, M.; Vollenweider, P.; Haba-Rubio, J.; et al. REM-associated sleep apnoea: Prevalence and clinical significance in the HypnoLaus cohort. Eur. Respir. J. 2018, 52, 1702484. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; de la Cruz-Moron, I.; Almeida-Gonzalez, C.; Catalan-Serra, P.; Montserrat, J.M. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: A cohort study. Ann. Intern. Med. 2012, 156, 115–122. [Google Scholar] [CrossRef]
- Roca, G.Q.; Shah, A.M. Sleep Disordered Breathing: Hypertension and Cardiac Structure and Function. Curr. Hypertens. Rep. 2015, 17, 91. [Google Scholar] [CrossRef]
- Appleton, S.; Gill, T.; Taylor, A.; McEvoy, D.; Shi, Z.; Hill, C.; Reynolds, A.; Adams, R. Influence of Gender on Associations of Obstructive Sleep Apnea Symptoms with Chronic Conditions and Quality of Life. Int. J. Environ. Res. Public Health 2018, 15, 930. [Google Scholar] [CrossRef]
- Donovan, L.M.; Feemster, L.C.; Billings, M.E.; Spece, L.J.; Griffith, M.F.; Rise, P.J.; Parsons, E.C.; Palen, B.N.; O’Hearn, D.J.; Redline, S.; et al. Risk of Cardiovascular Disease Related to Smoking Is Greater Among Women with Sleep-Disordered Breathing. J. Clin. Sleep Med. 2018, 14, 1929–1935. [Google Scholar] [CrossRef]
- Cunningham, J.; Hunter, M.; Budgeon, C.; Murray, K.; Knuiman, M.; Hui, J.; Hillman, D.; Singh, B.; James, A. The prevalence and comorbidities of obstructive sleep apnea in middle-aged men and women: The Busselton Healthy Ageing Study. J. Clin. Sleep Med. 2021, 17, 2029–2039. [Google Scholar] [CrossRef]
- Boukari, R.; Laouafa, S.; Ribon-Demars, A.; Bairam, A.; Joseph, V. Ovarian steroids act as respiratory stimulant and antioxidant against the causes and consequences of sleep-apnea in women. Respir. Physiol. Neurobiol. 2017, 239, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Laouafa, S.; Ribon-Demars, A.; Marcouiller, F.; Roussel, D.; Bairam, A.; Pialoux, V.; Joseph, V. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep 2017, 40, zsx104. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Palomer, X.; Montserrat, J.M.; Vázquez-Carrera, M.; Farré, R. Effect of ovariectomy on inflammation induced by intermittent hypoxia in a mouse model of sleep apnea. Respir. Physiol. Neurobiol. 2014, 202, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Faulx, M.D.; Larkin, E.K.; Hoit, B.D.; Aylor, J.E.; Wright, A.T.; Redline, S. Sex Influences Endothelial Function in Sleep-Disordered Breathing. Sleep 2004, 27, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Bourjeily, G.; Danilack, V.A.; Bublitz, M.H.; Lipkind, H.; Muri, J.; Caldwell, D.; Tong, I.; Rosene-Montella, K. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: A national cohort. Sleep Med. 2017, 38, 50–57. [Google Scholar] [CrossRef]
- Facco, F.L.; Parker, C.B.; Reddy, U.M.; Silver, R.M.; Koch, M.A.; Louis, J.; Basner, R.C.; Chung, J.H.; Nhan-Chang, C.-L.; Pien, G.W.; et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet. Gynecol. 2017, 129, 31–41. [Google Scholar] [CrossRef]
- Fung, A.M.; Wilson, D.L.; Lappas, M.; Howard, M.; Barnes, M.; O’Donoghue, F.; Tong, S.; Esdale, H.; Fleming, G.; Walker, S.P. Effects of maternal obstructive sleep apnoea on fetal growth: A prospective cohort study. PLoS ONE 2013, 8, e68057. [Google Scholar] [CrossRef]
- Telerant, A.; Dunietz, G.L.; Many, A.; Tauman, R. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci. Rep. 2018, 8, 10768. [Google Scholar] [CrossRef]
- Bourjeily, G.; Danilack, V.A.; Bublitz, M.H.; Muri, J.; Rosene-Montella, K.; Lipkind, H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2020, 66, 233–240. [Google Scholar] [CrossRef]
- Sanapo, L.; Bublitz, M.H.; Bourjeily, G. Sleep Disordered Breathing, a Novel, Modifiable Risk Factor for Hypertensive Disorders of Pregnancy. Curr. Hypertens. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Pengo, M.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef] [PubMed]
- De Campos, H.H.; Brandão, L.C.; D’Almeida, V.; Grego, B.H.C.; Bittencourt, L.R.; Tufik, S.; Baracat, E.C. Sleep disturbances, oxidative stress and cardiovascular risk parameters in postmenopausal women complaining of insomnia. Climacteric 2006, 9, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Redline, S.; Curhan, G.C.; Hu, F.B.; Tworoger, S.S. Type of Menopause, Age at Menopause, and Risk of Developing Obstructive Sleep Apnea in Postmenopausal Women. Am. J. Epidemiol. 2018, 187, 1370–1379. [Google Scholar] [PubMed]
- Campos-Rodriguez, F.; Gonzalez-Martinez, M.; Sanchez-Armengol, A.; Jurado-Gamez, B.; Cordero-Guevara, J.; Reyes-Nuñez, N.; Troncoso, M.F.; Abad-Fernandez, A.; Teran-Santos, J.; Caballero-Rodriguez, J.; et al. Effect of continuous positive airway pressure on blood pressure and metabolic profile in women with sleep apnoea. Eur. Respir. J. 2017, 50, 1700257. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Pien, G.W.; Ratcliffe, S.J.; Weaver, T.E. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J. Clin. Sleep Med. 2009, 5, 512–518. [Google Scholar] [CrossRef]
- McArdle, N.; King, S.; Shepherd, K.; Baker, V.; Ramanan, D.; Ketheeswaran, S.; Bateman, P.; Wimms, A.; Armitstead, J.; Richards, G.; et al. Study of a Novel APAP Algorithm for the Treatment of Obstructive Sleep Apnea in Women. Sleep 2015, 38, 1775–1781. [Google Scholar] [CrossRef]
- May, A.M.; Gharibeh, T.; Wang, L.; Hurley, A.; Walia, H.; Strohl, K.P.; Mehra, R. CPAP Adherence Predictors in a Randomized Trial of Moderate-to-Severe OSA Enriched with Women and Minorities. Chest 2018, 154, 567–578. [Google Scholar] [CrossRef]
- Jayaraman, G.; Majid, H.; Surani, S.; Kao, C.; Subramanian, S. Influence of gender on continuous positive airway pressure requirements in patients with obstructive sleep apnea syndrome. Sleep Breath. 2010, 15, 781–784. [Google Scholar] [CrossRef]
- Vecchierini, M.-F.; Attali, V.; Collet, J.-M.; d’Ortho, M.-P.; Goutorbe, F.; Kerbrat, J.-B.; Leger, D.; Lavergne, F.; Monaca, C.; Monteyrol, P.-J. Sex differences in mandibular repositioning device therapy effectiveness in patients with obstructive sleep apnea syndrome. Sleep Breath. 2019, 23, 837–848. [Google Scholar] [CrossRef]
- Newman, A.B.; Foster, G.; Givelber, R.; Nieto, F.J.; Redline, S.; Young, T. Progression and Regression of Sleep-Disordered Breathing with Changes in Weight. Arch. Intern. Med. 2005, 165, 2408–2413. [Google Scholar] [CrossRef]
- Madsen, T.E.; Bourjeily, G.; Hasnain, M.; Jenkins, M.; Morrison, M.F.; Sandberg, K.; Tong, I.L.; Trott, J.; Werbinski, J.L.; McGregor, A.J. Sex and Gender-Based Medicine: The Need for Precise Terminology. Gend. Genome 2017, 1, 122–128. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bublitz, M.; Adra, N.; Hijazi, L.; Shaib, F.; Attarian, H.; Bourjeily, G. A Narrative Review of Sex and Gender Differences in Sleep Disordered Breathing: Gaps and Opportunities. Life 2022, 12, 2003. https://doi.org/10.3390/life12122003
Bublitz M, Adra N, Hijazi L, Shaib F, Attarian H, Bourjeily G. A Narrative Review of Sex and Gender Differences in Sleep Disordered Breathing: Gaps and Opportunities. Life. 2022; 12(12):2003. https://doi.org/10.3390/life12122003
Chicago/Turabian StyleBublitz, Margaret, Nour Adra, Leen Hijazi, Fidaa Shaib, Hrayr Attarian, and Ghada Bourjeily. 2022. "A Narrative Review of Sex and Gender Differences in Sleep Disordered Breathing: Gaps and Opportunities" Life 12, no. 12: 2003. https://doi.org/10.3390/life12122003
APA StyleBublitz, M., Adra, N., Hijazi, L., Shaib, F., Attarian, H., & Bourjeily, G. (2022). A Narrative Review of Sex and Gender Differences in Sleep Disordered Breathing: Gaps and Opportunities. Life, 12(12), 2003. https://doi.org/10.3390/life12122003







