A Retrospective, Observational Study of Catheter-Associated Urinary Tract Infection Events Post-Implementation of a Novel Urinary Catheter System with Active Drain Line Clearance and Automated Intra-Abdominal Pressure Monitoring
Abstract
1. Introduction
2. Methods
2.1. Design and Patient Population
2.2. Catheter and Monitoring System
2.3. Setting
2.4. Ethical Considerations
2.5. Definition and Protocols
- Fever without evidence of another source;
- Pain or burning while urinating;
- Urgency;
- Hematuria;
- Costovertebral angle tenderness;
- (a)
- IAP measurement set-up [10,11] using the gravity urinary catheter (protocol discarded when automatic IAP measurements with the Accuryn SmartFoley® were introduced).Before performing IAP monitoring, the registered nurse (RN) is to ensure the following:
- The bedside monitor has been set up to perform IAP monitoring properly;
- The pressure bag attached to the tubing and transducer set-up for IAP monitoring is inflated to above 300 mm Hg;
- The tubing and transducer set-up has been properly primed with normal saline. Additionally, ensure that the tubing remains sterile by keeping the end covers intact;
- The following sterile supplies are at the bedside: 30 mL of sterile normal saline to instill into the bladder, a sterile 60 mL syringe, sterile gloves, sterile towels and amp, chlorhexidine, or alcohol preps for three separate cleaning steps. Nonsterile supplies needed at the bedside include clamps and amp, absorbent pads;
- The patient has an inserted Urinary Catheter with an access hub in place, and the catheter is draining urine appropriately.
- (b)
- Performing IAP monitoring in the burn ICU (adopted and slightly modified from the WSACS recommendations [10,11]:
- Ensure the patient has been placed supine;
- Clean the access hub with chlorhexidine or alcohol. Rub the hub vigorously for at least 15 s (First clean);
- Clamp the Foley Catheter below the access hub;
- Don sterile gloves and establish a sterile field with sterile towels around the access hub using sterile technique;
- Prepare a sterile syringe with 30 mL of sterile normal saline;
- Clean access hub with chlorhexidine or alcohol using sterile technique. Rub the hub vigorously for at least 15 s. Allow the hub to dry for 30 s (Second clean);
- Instill 30 mL of sterile normal saline in the bladder;
- Clean access hub with chlorhexidine or alcohol using sterile technique. Rub the hub vigorously for at least 15 s. Allow the hub to dry for 30 s (Third clean);
- Attach monitoring tubing to access the hub. The system is now considered closed;
- Zero the IAP monitoring system on the bedside monitor;
- Obtain IAP;
- Unclamp Foley Catheter. Close off IAP monitoring tubing;
- Properly position the patient. Do not leave supine.
- (c)
- Standard Operating Procedures (SOP) in the BICU concerning IAP monitoring:
- IAP monitoring is routinely performed every 4 h. Monitoring times can be increased or decreased based on the condition/or situation of the individual patient;
- It is the responsibility of the RN to promptly report IAPs of 20 mm Hg or greater to the provider;
- IAP monitoring tubing and normal saline used in the pressure bag are to be changed every 72 h;
- IAP monitoring tubing can be left attached to the Foley access hub but must be in the closed position when not in use;
- Sterile technique and proper cleaning are used when adding 30 mL of sterile saline for each IAP monitoring session.
- (d)
- Automated IAP measurement: The patient is in the supine position, and active abdominal muscle contractions are absent. IAP is measured via button press. IAP monitoring is routinely performed every 4 h. Monitoring times can be increased or decreased based on the condition/or situation of the individual patient.
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Healthcare Safety Network (NHSN) Patient Safety Component Manual. Centers for Disease Control and Prevention Website. Available online: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions (accessed on 6 June 2022).
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 2014, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeak, C.S.; Schilling, A.L. The attributable cost of catheter-associated urinary tract infections in the United States: A systematic review. Am. J. Infect. Control 2018, 46, 751–757. [Google Scholar] [CrossRef] [PubMed]
- National Healthcare Safety Network (NHSN) Definition CAUTI Criteria. Available online: https://www.ahrq.gov/hai/quality/tools/cauti-ltc/modules/resources/tools/cauti-surveillance/pocket-card.html (accessed on 12 June 2022).
- Dudeck, M.A.; Edwards, J.R.; Allen-Bridson, K.; Gross, C.; Malpiedi, P.J.; Peterson, K.D.; Pollock, D.A.; Weiner, L.M.; Sievert, D.M. National Healthcare Safety Network report, data summary for 2013, Device-associated Module. Am. J. Infect. Control 2015, 43, 206–221. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F.; et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: Device-associated module. Am. J. Infect. Control 2016, 44, 1495–1504. [Google Scholar] [CrossRef]
- Burton, D.C.; Edwards, J.R.; Srinivasan, A.; Fridkin, S.K.; Gould, C.V. Trends in Catheter-Associated Urinary Tract Infections in Adult Intensive Care Units—United States, 1990–2007. Infect. Control Hosp. Epidemiol. 2011, 32, 748–756. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Balogh, Z.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensiv. Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; The Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome; Roberts, D.J.; De Waele, J.; Jaeschke, R.; Malbrain, M.L.N.G.; De Keulenaer, B.; Duchesne, J.; Bjorck, M.; Leppaniemi, A.; et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensiv. Care Med. 2013, 39, 1190–1206. [Google Scholar] [CrossRef]
- Desie, N.; Willems, A.; De Laet, I.; Dits, H.; Van Regenmortel, N.; Schoonheydt, K.; Van De Vyvere, M.; Malbrain, M.L. Intra-abdominal pressure measurement using the FoleyManometer does not increase the risk for urinary tract infection in critically ill patients. Ann. Intensiv. Care 2012, 2 (Suppl. 1), S10. [Google Scholar] [CrossRef]
- Cheatham, M.L.; Sagraves, S.G.; Johnson, J.L.; White, M.W. Intravesicular pressure monitoring does not cause urinary tract infection. Intensiv. Care Med. 2006, 32, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Duane, T.M.; Brown, H.; Wolfe, L.G.; Malhotra, A.K.; Aboutanos, M.B.; Ivatury, R.R. Bladder Pressure Measurements Are an Independent Predictor of Urinary Tract Infection in Trauma Patients. Surg. Infect. 2011, 12, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.C.; Luxon, E.; Wolf, J.; Burnett, D.R.; Nanduri, D.; Friedman, B.C. Inaccuracy of Urine Output Measurements due to Urinary Retention in Catheterized Patients in the Burn ICU. J. Burn Care Res. 2017, 38, e409–e417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wise, R.; Jacobs, J.; Pilate, S.; Jacobs, A.; Peeters, Y.; Vandervelden, S.; Van Regenmortel, N.; De Laet, I.; Schoonheydt, K.; Dits, H.; et al. Incidence and prognosis of intra-abdominal hypertension and abdominal compartment syndrome in severely burned patients: Pilot study and review of the literature. Anaesthesiol. Intensiv. Ther. 2016, 48, 95–109. [Google Scholar] [CrossRef]
- Peeters, Y.; Lebeer, M.; Wise, R.; Malbrain, M.L. An overview on fluid resuscitation and resuscitation endpoints in burns: Past, present and future. Part 2—Avoiding complications by using the right endpoints with a new personalized protocolized approach. Anaesthesiol. Intensive Ther. 2015, 47, 15–26. [Google Scholar] [CrossRef]
- Peeters, Y.; Vandervelden, S.; Wise, R.; Malbrain, M.L.N.G. An overview on fluid resuscitation and resuscitation endpoints in burns: Past, present and future. Part 1—Historical background, resuscitation fluid and adjunctive treatment. Anaesthesiol. Intensive Ther. 2015, 47, 6–14. [Google Scholar] [CrossRef]
- National Healthcare Safety Network-CAUTI Update. 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (accessed on 23 September 2022).
- Greenhalgh, D.G. Sepsis in the burn patient: A different problem than sepsis in the general population. Burns Trauma 2017, 5, 23. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, B.; Liou, Y.-C.; Huang, C. The pathogenesis and diagnosis of sepsis post burn injury. Burns Trauma 2021, 9, tkaa047. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Clarke, K.; Hall, C.L.; Wiley, Z.; Tejedor, S.C.; Kim, J.S.; Reif, L.; Witt, L.; Jacob, J.T. Catheter-Associated Urinary Tract Infections in Adults: Diagnosis, Treatment, and Prevention. J. Hosp. Med. 2019, 15, 552–556. [Google Scholar] [CrossRef]
- Wiley, Z.; Jacob, J.T.; Burd, E.M. Targeting Asymptomatic Bacteriuria in Antimicrobial Stewardship: The Role of the Microbiology Laboratory. J. Clin. Microbiol. 2020, 58, e00518-18. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Choe, H.-S.; Son, S.-W.; Choi, H.-A.; Kim, H.-J.; Ahn, S.-G.; Bang, J.-H.; Lee, S.-J.; Lee, J.-Y.; Cho, Y.-H.; Lee, S.-S. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am. J. Infect. Control 2012, 40, e249–e254. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B.; et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: A multicentre randomised controlled trial. Lancet 2012, 380, 1927–1935. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Oon, J. Catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 2012, 25, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.; Nicolle, L.E.; Coffin, S.E.; Gould, C.; Maragakis, L.L.; Meddings, J.; Pegues, D.A.; Pettis, A.M.; Saint, S.; Yokoe, D.S. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35 (Suppl. 2), S32–S47. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25376068 (accessed on 29 August 2022). [CrossRef]
- Agency for Healthcare Research and Quality: Technical Interventions to Prevent CAUTI. Available online: https://www.ahrq.gov/hai/cauti-tools/guides/implguide-pt3.html (accessed on 10 July 2022).
- Wenzler-Röttele, S.; Dettenkofer, M.; Schmidt-Eisenlohr, E.; Gregersen, A.; Schulte-Mönting, J.; Tvede, M. Comparison in a Laboratory Model between the Performance of a Urinary Closed System Bag with Double Non-return Valve and that of a Single Valve System. Infection 2006, 34, 214–218. [Google Scholar] [CrossRef]
- Tandogdu, Z.; Wagenlehner, F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef]
- Gandhi, T.; Flanders, S.A.; Markovitz, E.; Saint, S.; Kaul, D.R. Importance of Urinary Tract Infection to Antibiotic Use Among Hospitalized Patients. Infect. Control Hosp. Epidemiol. 2009, 30, 193–195. [Google Scholar] [CrossRef]
- Van, C.; Smeltzer, J.; Che, J. Reducing CAUTIS with a novel catheter technology in the ICU. Crit. Care Med. 2022, 50, 603. Available online: https://journals.lww.com/ccmjournal/Fulltext/2022/01001/1207__REDUCING_CAUTIS_WITH_A_NOVEL_CATHETER.1173.aspx (accessed on 23 September 2022). [CrossRef]
- Malbrain, M.L.; De Keulenaer, B.L.; Oda, J.; De Laet, I.; De Waele, J.J.; Roberts, D.J.; Kirkpatrick, A.W.; Kimball, E.; Ivatury, R. Intra-abdominal hypertension and abdominal compartment syndrome in burns, obesity, pregnancy, and general medicine. Anaesthesiol. Intensiv. Ther. 2015, 47, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Blaser, A.R.; Regli, A.; De Keulenaer, B.; Kimball, E.J.; Starkopf, L.; Davis, W.; Greiffenstein, P.; Starkopf, J. Incidence, Risk Factors, and Outcomes of Intra-Abdominal Hypertension in Critically Ill Patients—A Prospective Multicenter Study (IROI Study). Crit. Care Med. 2019, 47, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Strang, S.G.; Van Lieshout, E.M.; Breederveld, R.S.; Van Waes, O.J. A systematic review on intra-abdominal pressure in severely burned patients. Burns 2014, 40, 9–16. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; De Keulenaer, B.L.; Khanna, A.K. Continuous intra-abdominal pressure: Is it ready for prime time? Intensiv. Care Med. 2022, 48, 1501–1504. [Google Scholar] [CrossRef]
- Khanna, A.K.; Minear, S.; Kurz, A.; Moll, V.; Stanton, K.; Essakalli, L.; Prabhakar, A.; Harris, L.C.; Sweatt, N.; Flores, K.; et al. Intra-abdominal hypertension in cardiac surgery patients: A multicenter observational sub-study of the Accuryn registry. Int. J. Clin. Monit. Comput. 2022. [Google Scholar] [CrossRef]

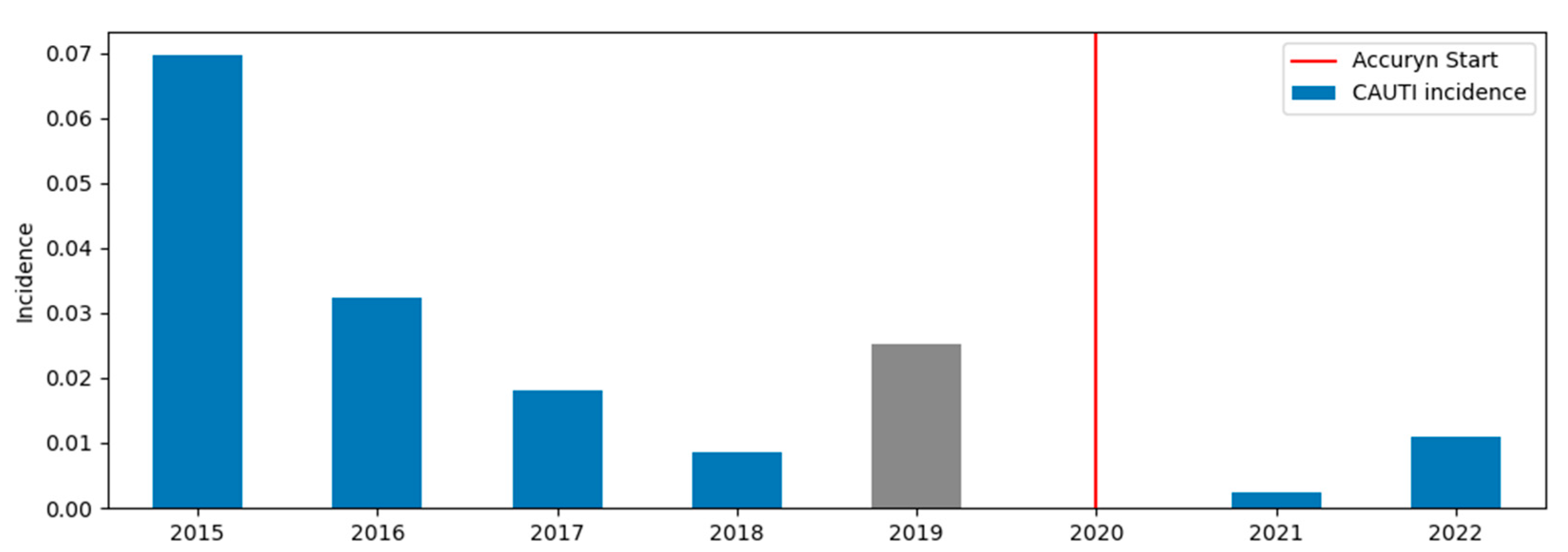
| Characteristic | Category | Period 1, Median [Q25, Q75] or No. (%) | Period 2, Median [Q25, Q75] or No. (%) |
|---|---|---|---|
| Total patients (n) | 2243 | 1317, 40 CAUTIs | 926, 2 CAUTIs |
| Age (years) | 49.0 [33.0, 61.5] | 43.5 [34.2, 52.8] | |
| BMI (kg/m2) | 26.3 [23.4, 29.7] | 24.1 [24.0, 24.2] | |
| Gender | Male | 52.5% (21) | 100.0% (2) |
| Female | 47.5% (19) | 0.0% (0) | |
| Race | Caucasian | 57.5% (23) | 50.0% (1) |
| African American | 32.5% (13) | 50.0% (1) | |
| Unknown | 7.5% (3) | 0.0% (0) | |
| Hispanic | 2.5% (1) | 0.0% (0) | |
| Catheter day of CAUTI | 4.5 [2.0, 6.2] | 6.0 [5.5, 6.5] | |
| Type of injury | Burn | 85.0% (34) | 100.0% (2) |
| TBSA% | 24.2 [12.2, 70.0] | 19.8 [18.4, 21.1] | |
| Smoke inhalation | 5.0% (2) | 0.0% (0) | |
| Electrical injury | 2.5% (1) | 0.0% (0) | |
| Frostbite | 2.5% (1) | 0.0% (0) | |
| Unknown | 2.5% (1) | 0.0% (0) | |
| Comorbidities | Hypertension | 13.4% (11) | 16.7% (1) |
| Smoker | 11.0% (9) | 0 | |
| IDDM | 8.5% (7) | 0 | |
| Anxiety | 7.3% (6) | 0 | |
| Depression | 6.1% (5) | 0 | |
| Cerebrovascular accident | 4.9% (4) | 0 | |
| Hyperlipidemia | 4.9% (4) | 0 | |
| Polysubstance abuse | 3.7% (3) | 16.7% (1) | |
| Hepatitis | 3.7% (3) | 0 | |
| Coronary artery disease | 3.7% (3) | 0 | |
| COPD | 3.7% (3) | 0 | |
| Dementia | 2.4% (2) | 16.7% (1) | |
| Hypothyroidism | 2.4% (2) | 0 | |
| Myocardial infarction | 2.4% (2) | 0 | |
| Arthritis | 2.4% (2) | 0 | |
| Seizures | 2.4% (2) | 0 | |
| Asthma | 2.4% (2) | 0 | |
| Post-traumatic stress disorder | 1.2% (1) | 0 | |
| Alcohol abuse | 1.2% (1) | 16.7% (1) | |
| Degenerative disc disease | 1.2% (1) | 0 | |
| Back pain | 1.2% (1) | 0 | |
| GERD | 1.2% (1) | 0 | |
| Anemia | 1.2% (1) | 0 | |
| Congestive heart failure | 1.2% (1) | 0 | |
| HIV | 1.2% (1) | 0 | |
| Hyperthyroidism | 1.2% (1) | 0 | |
| Autism | 1.2% (1) | 0 | |
| Bipolar disorder | 1.2% (1) | 0 | |
| Renal insufficiency | 1.2% (1) | 0 | |
| Pancytopenia | 0 | 16.7% (1) | |
| Cholelithiasis | 0 | 16.7% (1) |
| Organism | Period 1, % (N) | Period 2, % (N) |
|---|---|---|
| Pseudomonas aeruginosa | 45.2% (19) | 0 |
| Escherichia coli | 19.0% (8) | 50.0% (1) |
| VRE | 4.8% (2) | 0 |
| Klebsiella pneumoniae | 4.8% (2) | 0 |
| Enterococcus species | 4.8% (2) | 0 |
| Achromobacter xylosoxidans | 4.8% (2) | 0 |
| Acinetobacter Baumannii | 4.8% (2) | 0 |
| Staphylococcus | 2.4% (1) | 0 |
| Lactobacillus | 2.4% (1) | 0 |
| Klebsiella oxytoca | 2.4% (1) | 0 |
| Proteus vulgaris | 2.4% (1) | 0 |
| Enterobacter cloacae | 2.4% (1) | 0 |
| Enterococcus faecalis | 0 | 50.0% (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockway, P.; Hill, D.M.; Moll, V.; Stanton, K.; Malbrain, M.L.N.G.; Velamuri, S.R. A Retrospective, Observational Study of Catheter-Associated Urinary Tract Infection Events Post-Implementation of a Novel Urinary Catheter System with Active Drain Line Clearance and Automated Intra-Abdominal Pressure Monitoring. Life 2022, 12, 1950. https://doi.org/10.3390/life12121950
Brockway P, Hill DM, Moll V, Stanton K, Malbrain MLNG, Velamuri SR. A Retrospective, Observational Study of Catheter-Associated Urinary Tract Infection Events Post-Implementation of a Novel Urinary Catheter System with Active Drain Line Clearance and Automated Intra-Abdominal Pressure Monitoring. Life. 2022; 12(12):1950. https://doi.org/10.3390/life12121950
Chicago/Turabian StyleBrockway, Patrick, David M. Hill, Vanessa Moll, Kelly Stanton, Manu L. N. G. Malbrain, and Sai R. Velamuri. 2022. "A Retrospective, Observational Study of Catheter-Associated Urinary Tract Infection Events Post-Implementation of a Novel Urinary Catheter System with Active Drain Line Clearance and Automated Intra-Abdominal Pressure Monitoring" Life 12, no. 12: 1950. https://doi.org/10.3390/life12121950
APA StyleBrockway, P., Hill, D. M., Moll, V., Stanton, K., Malbrain, M. L. N. G., & Velamuri, S. R. (2022). A Retrospective, Observational Study of Catheter-Associated Urinary Tract Infection Events Post-Implementation of a Novel Urinary Catheter System with Active Drain Line Clearance and Automated Intra-Abdominal Pressure Monitoring. Life, 12(12), 1950. https://doi.org/10.3390/life12121950







