Aptamer-Based Probes for Cancer Diagnostics and Treatment
Abstract
1. Introduction
2. Aptamer-Based Probes for Cancer Diagnosis
2.1. Cancer Surface Membrane Biomarkers
2.1.1. Receptors
2.1.2. Cell Adhesion Molecules
2.1.3. Cell Membrane-Associated Enzymes
2.1.4. Other Membrane-Associated Proteins
2.2. Extracellular Cancer Biomarkers
3. Aptamer-Based Cancer Therapy
3.1. Aptamer as Therapeutic Agent
3.1.1. Cancer Surface Membrane Biomarkers-Based Therapy
3.1.2. Other Biomarkers-Based Therapy
3.2. Aptamer as Delivery Agents
3.2.1. Cancer Surface Biomarkers-Targeted Delivery Therapy
3.2.2. Intracellular Targeted Therapy
4. Conclusions and Future Prospects
| Aptamer Name | DNA/RNA | Target | Targeted Disease | Clinical Status | Ref. |
|---|---|---|---|---|---|
| Pegaptanib (PEGylated), VEap121 | RNA | VEGF165 | Age-related macular degeneration (AMD) Diabetic macular edema Diabetic retinopathy | In market | [245] |
| AS1411 | DNA | Nucleolin | Acute myeloid leukemia Metastatic renal cell carcinoma | Phase II (NCT01034410) Phase II (NCT00740441) Phase I (NCT00881244) | [246] |
| 68Ga-Sgc8 | DNA | PTK7/ CCk-4 | Colorectal cancer | Early Phase 1 (NCT03385148) | [247] |
| ARC1905 (Zimura) | RNA | C5 | Dry AMD Idiopathic polypoidal choroidal vasculopathy (IPCV) | Phase I completed, Phase II and III recruiting (NCT02686658) Zimura in Combination with Anti-VEGF Therapy in Subjects with IPCV (NCT02397954) | [149] |
| E-10030 (Fovista) | DNA | PDGF | Neovascular AMD | Phase II (NCT02214628) Anti-PDGF Pegylated Aptamer with Lucentis (NCT01089517) for neovascular AMD Fovista in Combination with Lucentis as compared to Lucentis monotherapy (NCT01940900) | [248] |
| REG1 anticoagulation system (RB006 and RB007) (RB006) Antidote (RB007) | RNA | Coagulation factor IXa | Acute coronary syndrome Coronary artery disease Percutaneous coronary intervention | Phase I and II completed (NCT00113997, NCT00932100, NCT01872572) | [249] |
| ARC1779 | DNA | Von Willebrand factor (A1 domain) | Purpura, Thrombotic Thrombocytopenic von Willebrand disease Acute myocardial infarction | Phase II (NCT00632242) Phase II (NCT00507338) | [250] |
| NU172 | DNA | Thrombin | Heart disease | Phase II (NCT00808964) | [251] |
| NOX-A12 | RNA | CXCL12 | Chronic lymphocytic leukemia Multiple myeloma Colorectal cancer Pancreatic cancer | Phase II (NCT01486797) NOX-A12 in Combination with Bortezomib and Dexamethasone Phase II (NCT01521533) | [252] |
| NOX-E36 | RNA | MCP-1 | Chronic Inflammatory Diseases Type 2 diabetes Mellitus Systemic Lupus Erythematosus | Phase I (NCT00976729) | [253] |
| NOX-H94 (lexaptepid pegol) | RNA | Human Hepcidin | Anemia of chronic disease End-stage renal disease | Phase I and II (NCT02079896) | [254] |
| ApTOLL | DNA | Toll-like receptor (TLR4) | Acute ischemic stroke Acute myocardial infarction | Phase I (NCT04742062) | [255] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerchia, L.; Franciscis, V.D. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010, 28, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Pawel, G.T.; Pei, R.; Lu, Y. Recent progress in developing fluorescent probes for imaging cell metabolites. Biomed. Mater. 2021, 16, 044108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zu, Y. A highlight of recent advances in aptamer technology and its application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef] [PubMed]
- Mairal, T.; Özalp, V.C.; Sánchez, P.L.; Mir, M.; Katakis, I.; O’Sullivan, C.K. Aptamers: Molecular tools for analytical applications. Anal. Bioanal. Chem. 2008, 390, 989–1007. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, X.; Lake, R.J.; Pawel, G.T.; Guo, Z.; Pei, R.; Lu, Y. A photo-regulated aptamer sensor for spatiotemporally controlled monitoring of atp in the mitochondria of living cells. Chem. Sci. 2019, 11, 713–720. [Google Scholar] [CrossRef]
- Hong, S.; Ding, P.; Luo, Y.; Gao, T.; Zhang, Y.; Pei, R. Aptamer-integrated a-gal liposomes as bispecific agents to trigger immune response for killing tumor cells. J. Biomed. Mater. Res. A 2019, 107, 1176–1183. [Google Scholar] [CrossRef]
- Hong, S.; Sun, N.; Liu, M.; Wang, J.; Pei, R. Building a chimera of aptamer–antisense oligonucleotide for silencing galectin-1 gene. RSC Adv. 2016, 6, 112445–112450. [Google Scholar] [CrossRef]
- Cheng, H.; Hong, S.; Wang, Z.; Sun, N.; Wang, T.; Zhang, Y.; Chen, H.; Pei, R. Self-assembled rnai nanoflowers via rolling circle transcription for aptamer-targeted sirna delivery. J. Mater. Chem. B 2018, 6, 4638–4644. [Google Scholar] [CrossRef]
- Xu, L.; Hong, S.; Sun, N.; Wang, K.; Zhou, L.; Ji, L.; Pei, R. Berberine as a novel light-up i-motif fluorescence ligand and its application in designing molecular logic systems. Chem. Commun. 2016, 52, 179–182. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Sicence 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Radom, F.; Jurek, P.M.; Jacek Otlewski, M.P.M.; Jeleń, F. Aptamers: Molecules of great potential. Biotechnol. Adv. 2013, 31, 1260–1274. [Google Scholar] [CrossRef]
- Aquino-Jarquin, G.; Toscano-Garibay, J.D. RNA aptamer evolution: Two decades of selection. Int. J. Mol. Sci. 2011, 12, 9155–9171. [Google Scholar] [CrossRef] [PubMed]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, B. Pegaptanib for the treatment of age-related macular degeneration. Exp. Eye Res. 2006, 83, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, M.S.; Raff, M.C. Mammalian plasma membranes. Nature 1975, 258, 43–49. [Google Scholar] [CrossRef]
- Yıldırım, M.A.; Goh, K.; Cusick, M.E.; Barabási, A.L.; Vidal, M. Drug—Target network. Nat. Biotechnol. 2007, 25, 1119–1126. [Google Scholar] [CrossRef]
- Cibiel, A.; Dupont, D.M.; Ducongé, F. Methods to identify aptamers against cell surface biomarkers. Pharmaceuticals 2011, 4, 1216–1235. [Google Scholar] [CrossRef]
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; Román-Gil, M.S.; Orejana-Martín, I.; Torres-Jiménez, J.; Carrato, A.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine kinase receptors in oncology. Int. J. Mol. Sci. 2020, 21, 8529. [Google Scholar] [CrossRef]
- Zanetti-Domingues, L.C.; Bonner, S.E.; Martin-Fernandez, M.L.; Huber, V. Mechanisms of action of EGFR tyrosine kinase receptor incorporated in extracellular vesicles. Cells 2020, 9, 2505. [Google Scholar] [CrossRef]
- Ilkhani, H.; Sarparast, M.; Noori, A.; Bathaie, S.Z.; Mousavi, M.F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 2015, 74, 491–497. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Luo, J.; Xiong, Y.; Ming, T.; Liu, J.; Ma, Y.; Yan, S.; Yang, Y.; Yang, Z.; et al. Low sample volume origami-paper-based graphene-modified aptasensors for label-free electrochemical detection of cancer biomarker-EGFR. Microsyst. Nanoeng. 2020, 6, 32. [Google Scholar] [CrossRef]
- Cheng, S.; Jacobson, O.; Zhu, G.; Chen, Z.; Liang, S.H.; Tian, R.; Yang, Z.; Niu, G.; Zhu, X.; Chen, X. PET imaging of EGFR expression using an 18F-labeled RNA aptamer. Eur. J. Nucl. Med. Mol. Imaing 2019, 46, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Warram, J.M.; Boer, E.D.; Basilion, J.P.; Biel, M.A.; Bogyo, M.; Bouvet, M.; Brigman, B.E.; Colson, Y.L.; DeMeester, S.R. Successful translation of fluorescence navigation during oncologic surgery: A consensus report. J. Nucl. Med. 2016, 57, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; Susanna, W.L.; Geus, D.; Prevoo, A.J.M.H.; Lukas, J.A.C.H.; Velde, C.J.H.V.D.; Kuppen, P.J.K.; Vahrmeijer, A.L.; Sier, C.F.M. Selecting targets for tumor imaging: An overview of cancer-associated membrane proteins. Biomark. Cancer 2016, 8, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yang, J.; Hwang, M.; Choi, J.; Kim, H.; Jang, E.; Lee, J.H.; Ryu, S.; Suh, J.; Huh, Y.; et al. Aptamer-modified magnetic nanoprobe for molecular MR imaging of VEGFR2 on angiogenic vasculature. Nanoscale Res. Lett. 2013, 8, 399. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, G.; Ding, Y.; Deng, C.; Xiang, J.; Wu, H. A fluorescent aptasensor for the femtomolar detection of epidermal growth factor receptor-2 based on the proximity of G-rich sequences to Ag nanoclusters. Talanta 2019, 199, 238–243. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, W.; Zhou, W.; Lv, M.; Lu, C.; Yu, H. An amplification strategy for detecting HER2 with a quasi-targeted proteomics approach coupled with aptamer-triggered hybridization chain reaction. Talanta 2020, 215, 120918. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Rau, A.; Seifert, O.; Honer, J.; Hutt, M.; Schmid, S.; Zantow, J.; Hust, M.; Dubel, S.; Olayioye, M.A.; et al. Inhibition of HER3 activation and tumor growth with a human antibody binding to a conserved epitope formed by domain iii and iv. MAbs 2017, 9, 831–843. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Z.; Gou, X.; Shi, W.; Gong, Y.; Yi, M.; Cheng, W.; Song, F. Light up multiple protein dimers on cell surface based on proximity-induced fluorescence activation of DNA-templated sliver nanoclusters. Biosens. Bioelectron. 2021, 179, 113064. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, J.; Lee, H.W.; Kang, M.C.; Yoon, H.; Lee, S.; Park, J.H. Protein tyrosine kinase 7 plays a tumor suppressor role by inhibiting ERK and AKT phosphorylation in lung cancer. Oncol. Rep. 2014, 31, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, O.; Weiss, I.D.; Wang, L.; Wang, Z.; Yang, X.; Dewhurst, A.; Ma, Y.; Zhu, G.; Niu, G.; Kiesewetter, D.O.; et al. 18F-labeled single-stranded DNA aptamer for pet imaging of protein tyrosine kinase-7 expression. J. Nucl. Med. 2015, 56, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Z.; Xue, N.; Wu, S.; Miao, X. Electrochemical aptamer-based determination of protein tyrosine kinase-7 using toehold-mediated strand displacement amplification on gold nanoparticles and graphene oxide. Mikrochim. Acta 2019, 186, 720. [Google Scholar] [CrossRef]
- Kraus, E.; James, W.; Barclay, A.N. Cutting edge: Novel RNA ligands able to bind CD4 antigen and inhibit CD4+ T lymphocyte function. J. Immunol. 1998, 160, 5209–5212. [Google Scholar]
- Davis, K.A.; Abrams, B.; Lin, Y.; Jayasena, S.D. Staining of cell surface human CD4 with 2′-F-pyrimidine-containing RNA aptamers for flow cytometry. Nucleic Acids Res. 1998, 26, 3915–3924. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Y.; Shin, D.; Silangcruz, J.; Tuleuova, N.; Revzin, A. Aptamer-containing surfaces for selective capture of CD4 expressing cells. Langmuir 2012, 28, 12544–12549. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, N.; Zeng, Z.; Chang, C.; Zu, Y. Combination of an aptamer probe to CD4 and antibodies for multicolored cell phenotyping. Am. J. Clin. Pathol. 2010, 134, 586–593. [Google Scholar] [CrossRef]
- Zeng, Z.; Parekh, P.; Li, Z.; Shi, Z.; Tung, C.; Zu, Y. Specific and sensitive tumor imaging using biostable oligonucleotide aptamer probes. Theranostics 2014, 4, 945–952. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, N.; Zeng, Z.; Feng, Y.; Tung, C.; Chang, C.; Zu, Y. Using an RNA aptamer probe for flow cytometry detection of CD30-expressing lymphoma cells. Lab. Investig. 2009, 89, 1423–1432. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Adkins, G.B.; Shen, W.; Trinh, M.P.; Duan, L.; Jiang, J.; Zhong, W. Enhancement of the intrinsic peroxidase-like activity of graphitic carbon nitride nanosheets by ssDNAs and its application for detection of exosomes. Anal. Chem. 2017, 89, 12327–12333. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, M.; Wang, L.; Yan, A.; He, W.; Chen, M.; Lan, J.; Xu, J.; Guan, L.; Chen, J. A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens. Bioelectron. 2017, 92, 8–15. [Google Scholar] [CrossRef]
- Qiao, B.; Guo, Q.; Jiang, J.; Qi, Y.; Zhang, H.; He, B.; Cai, C.; Shen, J. An electrochemiluminescent aptasensor for amplified detection of exosomes from breast tumor Cells (MCF-7 Cells) based on G-quadruplex/hemin DNAzymes. Analyst 2019, 144, 3668–3675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct quantification of cancerous exosomes via surface plasmon resonance with dual gold nanoparticle-assisted signal amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, C.; Zhao, L.; Xu, L.; Zhou, W.; Dong, Z.; Yang, Y.; Xie, Q.; Fang, X. Aptamer-based fluorescence polarization assay for separation-free exosome quantification. Nanoscale 2019, 11, 10106–10113. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 2004, 5, 917–930. [Google Scholar] [CrossRef]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- Shi, J.; Lyu, J.; Tian, F.; Yang, M. A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 2017, 93, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shang, B.; Zhang, H.; Zhu, Z.; Chen, L.; Wang, H.; Ran, F.; Chen, Q.; Chen, J. Enzyme-free ultrasensitive fluorescence detection of epithelial cell adhesion molecules based on a toehold-aided DNA recycling amplification strategy. RSC Adv. 2018, 8, 14798–14805. [Google Scholar] [CrossRef]
- Heo, D.; Lee, E.; Ku, M.; Hwang, S.; Kim, B.; Park, Y.; Lee, Y.H.; Huh, Y.; Haam, S.; Cheong, J. Maleimidyl magnetic nanoplatform for facile molecular MRI. Nanotechnology 2014, 25, 275102. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Huang, Z.; Li, T.; Deng, A.; Kong, J. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta 2018, 184, 219–226. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, D.; Fang, Y.; Wu, W.; Wang, Y.; Xia, Y.; Wu, F.; Li, C.; Lan, J.; Chen, J. An aptasensor based on upconversion nanoparticles as LRET donors for the detection of exosomes. Sens. Actuator B Chem. 2019, 298, 126900. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, Q.; Wang, F.; Liu, Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens. Bioelectron. 2019, 124–125, 184–190. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Qiu, X.; Mei, Q.; Luo, Y.; Fu, W. Rapid and sensitive exosome detection with CRISPR/Cas12a. Anal. Bioanal. Chem. 2020, 412, 601–609. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, R.; He, P.; Zhang, X. Ultrasensitive detection of exosomes by target-triggered three-dimensional DNA walking machine and exonuclease III-assisted electrochemical ratiometric biosensing. Anal. Chem. 2019, 91, 14773–14779. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Cha, B.S.; Hwang, S.H.; Kim, J.; Park, K.S. Highly sensitive electrochemical detection of circulating EpCAM-positive tumor cells using a dual signal amplification strategy. Sens. Actuators B Chem. 2021, 343, 130087. [Google Scholar] [CrossRef]
- Lim, E.; Kim, B.; Choi, Y.; Ro, Y.; Cho, E.; Lee, J.H.; Ryu, S.; Suh, J.; Haam, S.; Huh, Y. Aptamer-conjugated magnetic nanoparticles enable efficient targeted detection of integrin Avβ3 via magnetic resonance imaging. J. Biomed. Mater. Res. 2014, 102, 49–59. [Google Scholar] [CrossRef]
- Fechter, P.; Silva, E.C.D.; Mercier, M.; Noulet, F.; Etienne-Seloum, N.; Guenot, D.; Lehmann, M.; Vauchelles, R.; Martin, R. RNA aptamers targeting integrin A5β1 as probes for cyto-and histofluorescence in glioblastoma. Mol. Ther. Nucleic Acids 2019, 17, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Heston, W.D.W. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef] [PubMed]
- Lupold, S.E.; Hicke, B.J.; Lin, Y.; Coffey, D.S. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002, 62, 4029–4033. [Google Scholar] [PubMed]
- Walter, J.G.; Petersen, S.; Stahl, F.; Scheper, T.; Barcikowski, S. Laser ablation-based one-step generation and bio-functionalization of gold nanoparticles conjugated with aptamers. J. Nanobiotechnol. 2010, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, Y.; Wang, L.; Xiong, X.; Zhu, L.; Fang, K. Diagnosis of prostate cancer using anti-PSMA aptamer A10-3.2-oriented lipid nanobubbles. Int. J. Nanomed. 2016, 11, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Hu, C.; Xia, Q.; Gong, C.; Gao, S.; Chen, Z. Aptamer-conjugated multi-walled carbon nanotubes as a new targeted ultrasound contrast agent for the diagnosis of prostate cancer. J. Nanoparticle Res. 2018, 20, 303. [Google Scholar] [CrossRef] [PubMed]
- Kue, P.F.; Taub, J.S.; Harrington, L.B.; Polakiewicz, R.D.; Ullrich, A.; Daaka, Y. Lysophosphatidic acid-regulated mitogenic erk signaling in androgen-insensitive prostate cancer PC-3 cells. J. Cancer 2002, 102, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Song, K.; Cho, M.; Chun, Y.; Shim, Y.; Ku, J.K.; Ban, C. Simultaneous electrochemical detection of both PSMA (+) and PSMA (−) prostate cancer cells using an RNA/peptide dual-aptamer probe. Chem. Commun. 2010, 46, 5566–5568. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Gomes, D.R.S.; Miguel, J.; Azéma, L.; Eimer, S.; Ries, C.; Dausse, E.; Loiseau, H.; Allard, M.; Toulmé, J. 99mTc-MAG3-aptamer for imaging human tumors associated with high level of matrix metalloprotease-9. Bioconjug. Chem. 2012, 23, 2192–2200. [Google Scholar] [CrossRef]
- Scarano, S.; Dausse, E.; Crispo, F.; Toulmé, J.J.; Minunni, M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal. Chim. Acta 2015, 897, 1–9. [Google Scholar] [CrossRef]
- Kim, J.; Yu, A.M.; Kubelick, K.P.; Emelianov, S.Y. Gold nanoparticles conjugated with DNA aptamer for photoacoustic detection of human matrix metalloproteinase-9. Photoacoustics 2022, 25, 100307. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Martínez-Sáez, N.; Peregrina, J.M.; Corzana, F. Principles of mucin structure: Implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 2017, 46, 7154–7175. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, N.; Westerlind, U.; Kunz, H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 2013, 42, 4421–4442. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Jiang, W.; Li, T.; Zhang, Z.; Qi, H.; Yang, M. Fluorescence aggregation assay for the protein biomarker mucin 1 using carbon dot-labeled antibodies and aptamers. Microchim. Acta 2015, 182, 443–447. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Chen, Y.; Fan, J.; Tong, C.; Liu, B. Aptamer-tagged silver nanoclusters for cell image and mucin1 detection in vitro. Talanta 2019, 205, 120075. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, F.; Li, X.; Jiang, B.; Yuan, R.; Xiang, Y. DNA branch migration amplification cascades for enzyme-free and non-label aptamer sensing of mucin 1. Analyst 2020, 145, 6085–6090. [Google Scholar] [CrossRef]
- Wang, K.; Fan, D.; Liu, Y.; Wang, E. Highly sensitive and specific colorimetric detection of cancer cells via dual-aptamer target binding strategy. Biosens. Bioelectron. 2015, 73, 1–6. [Google Scholar] [CrossRef]
- Cao, H.; Ye, D.; Zhao, Q.; Luo, J.; Zhang, S.; Kong, J. A novel aptasensor based on MUC-1 conjugated cnss for ultrasensitive detection of tumor cells. Analyst 2014, 139, 4917–4923. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Liu, W.; Zhang, K.; Zhao, H.; Zhang, H.; Zhang, Z. A simple, specific and “ON-OFF” type MUC1 fluorescence aptasensor based on exosomes for detection of breast cancer. Sens. Actuators B Chem. 2018, 276, 552–559. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Xia, Y.; Xu, H.; Liu, C.; Xie, H.; Wang, S.; Peng, L.; Liu, Y.; Liu, Y.; et al. A sensitive aptasensor based on a hemin/G-quadruplex-assisted signal amplification strategy for electrochemical detection of gastric cancer exosomes. Small 2019, 15, 1900735. [Google Scholar] [CrossRef] [PubMed]
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bai, X.; Wang, N.; Chen, X.; Li, J.; Zhang, Z.; Tang, J. Aptamer-based microcantilever biosensor for ultrasensitive detection of tumor marker nucleolin. Talanta 2016, 146, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Bates, P.J.; Malik, T.; Li, X.; Trent, J.O.; Ng, C.K. Aptamer imaging with Cu-64 labeled AS1411: Preliminary assessment in lung cancer. Nucl. Med. Biol. 2014, 41, 179–185. [Google Scholar] [CrossRef]
- Noaparast, Z.; Hosseinimehr, S.J.; Piramoon, M.; Abedi, S.M. Tumor targeting with a 99mTc-labeled AS1411 aptamer in prostate tumor cells. J. Drug. Target. 2015, 23, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.Y.; Lee, J.H.; Kang, H.; Ryu, S.H.; Song, I.C.; Lee, D.S.; Kim, S. A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. J. Nucl. Med. 2010, 51, 98–105. [Google Scholar]
- Wu, Q.; Yuan, C.; Liu, N.; Shu, J.; Wang, J.; Qian, J.; Zeng, L.; Zhang, H.; Wang, H.; Mei, W. Fast detection, a precise and sensitive diagnostic agent for breast cancer. J. Exp. Clin. Cancer Res. 2022, 41, 201. [Google Scholar] [CrossRef]
- Zheng, J.; Li, D.; Jiao, J.; Duan, C.; Wang, Z.; Xiang, Y. Dual aptamer recognition-based G-Quadruplex nanowires to selectively analyze cancer-derived exosomes. Talanta 2021, 235, 122748. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, Z.; Yuan, L.; Liu, S. Selective collection and detection of MCF-7 breast cancer cells using aptamer-functionalized magnetic beads and quantum dots based nano-bio-probes. Anal. Chim. Acta 2013, 788, 135–140. [Google Scholar] [CrossRef]
- Ou, D.; Sun, D.; Liang, Z.; Chen, B.; Lin, X.; Chen, Z. A novel cytosensor for capture, detection and release of breast cancer cells based on metal organic framework PCN-224 and DNA tetrahedron linked dual-aptamer. Sens. Actuators B Chem. 2019, 285, 398–404. [Google Scholar] [CrossRef]
- Cai, H.; Li, L.; Guo, T.; Wang, Y.; Ma, T.; Xiao, J.; Zhao, L.; Fang, Y.; Yang, P.; Zhao, H. Cardiac shockwave therapy improves myocardial function in patients with refractory coronary artery disease by promoting VEGF and IL-8 secretion to mediate the proliferation of endothelial progenitor cells. Exp. Ther. Med. 2015, 10, 2410–2416. [Google Scholar] [CrossRef][Green Version]
- Paci, E.; Ponti, A.; Zappa, M.; Patriarca, S.; Falini, P.; Delmastro, G.; Bianchi, S.; Sapino, A.; Vezzosi, V.; Senore, C.; et al. Early diagnosis, not differential treatment, explains better survival in service screening. Eur. J. Cancer 2005, 41, 2728–2734. [Google Scholar] [CrossRef]
- Moghadam, F.M.; Rahaie, M. A signal-on nanobiosensor for VEGF165 detection based on supraparticle copper nanoclusters formed on bivalent aptamer. Biosens. Bioelectron. 2019, 132, 186–195. [Google Scholar] [CrossRef]
- Li, X.; Ding, X.; Fan, J. Nicking endonuclease-assisted signal amplification of a split molecular aptamer beacon for biomolecule detection using graphene oxide as a sensing platform. Analyst 2015, 140, 7918–7925. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, W.; Wen, H.; Yu, S.; Miao, Z.; Kang, A.; Zhang, A. Silver nanoparticles-enhanced time-resolved fluorescence sensor for VEGF165 based on Mn-doped ZnS quantum dots. Biosens. Bioelectron. 2015, 74, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- You, X.G.; Tu, R.; Peng, M.L.; Bai, Y.J.; Tan, M.; Li, H.J.; Guan, J.; Wen, L.J. Molecular magnetic resonance probe targeting VEGF165: Preparation and in vitro and in vivo evaluation. Contrast Media Mol. Imaging 2014, 9, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Lorenzo-Gómez, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Electrochemical aptamer-based assays coupled to isothermal nucleic acid amplification techniques: New tools for cancer diagnosis. Curr. Opin. Electrochem. 2019, 14, 32–43. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.; Kariya, B.; Harker, L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc. Natl. Acad. Sci. USA 1974, 71, 1207–1210. [Google Scholar] [CrossRef]
- Huang, C.; Huang, Y.; Cao, Z.; Tan, W.; Chang, H. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal. Chem. 2005, 77, 5735–5741. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Ali, M.M.; Kang, D.K.; Zhao, W.; Li, J. Colorimetric and ultrasensitive bioassay based on a dual-amplification system using aptamer and DNAzyme. Anal. Chem. 2012, 84, 4711–4717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, F.; Chen, H.; Ma, Y.; Qi, S.; Chen, X.; Zhou, L. AuNPs colorimetric sensor for detecting platelet-derived growth factor-BB based on isothermal target-triggering strand displacement amplification. Sens. Actuators B Chem. 2015, 207, 748–755. [Google Scholar] [CrossRef]
- Ye, S.; Zhai, X.; Wu, Y.; Kuang, S. Dual-primer self-generation sers signal amplification assay for PDGF-BB using label-free aptamer. Biosens. Bioelectron. 2016, 79, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Singaravadivel, S.; Manojkumar, P.; Krishnasamy, S.; Gnana kumar, G.; Rajagopal, S. Aptamer-based label-free detection of PDGF using ruthenium (II) complex as luminescent probe. Anal. Bioanal. Chem. 2013, 405, 6891–6895. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, A.; Hou, T.; Li, H.; Li, F. Enzyme-free and label-free fluorescence aptasensing strategy for highly sensitive detection of protein based on target-triggered hybridization chain reaction amplification. Biosens. Bioelectron. 2015, 70, 324–329. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Chen, L.; Zhang, X. Photoinduced electron transfer (PET) based label-free aptasensor for platelet-derived growth factor-BB and its logic gate application. Biosens. Bioelectron. 2015, 63, 552–557. [Google Scholar] [CrossRef]
- Lin, F.; Yin, B.; Li, C.; Deng, J.; Fan, X.; Yi, Y.; Liu, C.; Li, H.; Zhang, Y.; Yao, S. Fluorescence resonance energy transfer aptasensor for platelet-derived growth factor detection based on upconversion nanoparticles in 30% blood serum. Anal. Methods 2013, 5, 699–704. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Voltammetric aptasensors for protein disease biomarkers detection: A review. Biotechnol. Adv. 2016, 34, 941–953. [Google Scholar] [CrossRef]
- Razmi, N.; Baradaran, B.; Hejazi, M.; Hasanzadeh, M.; Mosafer, J.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on aptamer-based biosensors to detection of platelet-derived growth factor. Biosens. Bioelectron. 2018, 113, 58–71. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, D.; Zhang, L.; Guo, Q.; Cui, Y.; Yang, M. Dual signal amplification strategy for amperometric aptasensing using hydroxyapatite nanoparticles. Application to the sensitive detection of the cancer biomarker platelet-derived growth factor BB. Microchim. Acta 2017, 184, 4375–4381. [Google Scholar] [CrossRef]
- Bergstrand, C.G.; Czar, B. Demonstration of a new protein fraction in serum from the human fetus. Scand. J. Clin. Lab. Investig. 1956, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wei, D.; Li, G. A fluorescence turn-on biosensor based on gold nanoclusters and aptamer for alpha fetoprotein detection. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012106. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Sun, X.; Chen, M.; Lv, Y. An ultrasensitive fluorescent aptasensor for detection of cancer marker proteins based on graphene oxide–ssDNA. RSC Adv. 2018, 8, 41143–41149. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zeng, J.; Liu, H.; Ding, P.; Liang, J.; Nie, X.; Zhou, Z. A fluorometric aptamer nanoprobe for alpha-fetoprotein by exploiting the fret between 5-carboxyfluorescein and palladium nanoparticles. Microchim. Acta 2019, 186, 314. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Su, P.; Zhu, J.; Chen, J.; Xu, Y.; Gu, B.; Liu, Y.; Wang, L. Rapid aptasensor capable of simply detect tumor markers based on conjugated polyelectrolytes. Talanta 2018, 190, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ji, F.; Zhang, T.; Wang, F.; Li, Y.; Yu, Z.; Jin, X.; Ruan, B. An fluorescent aptasensor for sensitive detection of tumor marker based on the fret of a sandwich structured QDs-AFP-AuNPs. Talanta 2019, 197, 444–450. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Neville, A.M.; Carter, A.C.; Go, V.L.W.; Holyoke, E.D.; Isselbacher, K.J.; Schein, P.S.; Schwartz, M. CEA (carcinoembryonic antigen): Its role as a marker in the management of cancer. J. Cancer Res. Clin. 1981, 101, 239–242. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, M.; Liu, G.; Lu, H.; Gao, Y.; Yan, X.; Liu, F.; Sun, P.; Lu, G. A fluorescent biosensor based on molybdenum disulfide nanosheets and protein aptamer for sensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 273, 185–190. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Xu, Y. Selectively assaying CEA based on a creative strategy of gold nanoparticles enhancing silver nanoclusters’ fluorescence. Biosens. Bioelectron. 2015, 64, 345–351. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Liu, Z. An aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Sens. Actuators B Chem. 2015, 206, 531–537. [Google Scholar] [CrossRef]
- Shao, K.; Wang, L.; Wen, Y.; Wang, T.; Teng, Y.; Shen, Z.; Pan, Z. Near-infrared carbon dots-based fluorescence turn on aptasensor for determination of carcinoembryonic antigen in pleural effusion. Anal. Chim. Acta 2019, 1068, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Khusbu, F.Y.; Ma, C.; Wu, K.; Zhao, H.; Chen, H.; Wang, K. A sensitive detection method of carcinoembryonic antigen based on dsDNA-templated copper nanoparticles. New J. Chem. 2018, 42, 13702–13707. [Google Scholar] [CrossRef]
- He, M.; Wang, K.; Wang, W.; Yu, Y.; Wang, J. Smart DNA machine for carcinoembryonic antigen detection by exonuclease III-assisted target recycling and DNA walker cascade amplification. Anal. Chem. 2017, 89, 9292–9298. [Google Scholar] [CrossRef]
- Wang, K.; He, M.; Zhai, F.; He, R.; Yu, Y. A label-free and enzyme-free ratiometric fluorescence biosensor for sensitive detection of carcinoembryonic antigen based on target-aptamer complex recycling amplification. Sens. Actuators B Chem. 2017, 253, 893–899. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Tang, X.; Yang, H.; Chen, S.; Zhao, H.; Liu, S.; Zhu, Y.; Wang, X.; Huang, Y. A novel fluorescence aptasensor for 8-hydroxy-2′-deoxyguanosine based on the conformational switching of K+-stabilized G-quadruplex. J. Pharmaceut. Biomed. 2016, 118, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wei, M.; Yin, L.; Pu, Y.; Liu, S. Improving the fluorometric determination of the cancer biomarker 8-hydroxy-2′-deoxyguanosine by using a 3D DNA nanomachine. Microchim. Acta 2018, 185, 494. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Y.; Xu, E.; Zhang, Y.; Wei, W.; Yin, L.; Pu, Y.; Liu, S. A label-free ultrasensitive assay of 8-hydroxy-2′-deoxyguanosine in human serum and urine samples via polyaniline deposition and tetrahedral DNA nanostructure. Anal. Chim. Acta 2016, 946, 48–55. [Google Scholar] [CrossRef]
- Gan, H.; Xu, H. A novel aptamer-based online magnetic solid phase extraction method for the selective determination of 8-hydroxy-2′-deoxyguanosine in human urine. Anal. Chim. Acta 2018, 1008, 48–56. [Google Scholar] [CrossRef]
- Mi, J.; Liu, Y.; Rabbani, Z.N.; Yang, Z.; Urban, J.H.; Sullenger, B.A.; Clary, B.M. In vivo selection of tumor-targeting RNA motifs. Nat. Chem. Biol. 2010, 6, 22–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Brekken, R.A. Direct and indirect regulation of the tumor immune microenvironment by VEGF. J. Leukoc. Biol. 2022, 111, 1269–1286. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Hayashi, M.; Oguro, T.; Kimura, K.; Wayama, F.; Furusho, H.; Yoshimoto, K. Binding and structural properties of DNA aptamers with VEGF-a-mimic activity. Mol. Ther. Nucleic Acids 2020, 19, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Šalipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.J. Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol. Oncol. 2015, 9, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.S.; Ch’ng, E.S.; Woon, P.Y.; Chen, J.; Tang, T.; Citartan, M. Unravelling the diagnostic and therapeutic potentialities of a novel RNA aptamer isolated against human pituitary tumour transforming gene 1 (PTTG1) protein. Anal. Chim. Acta 2020, 1138, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Gao, T.; Mao, Z.; Li, W.; Pei, R. Anti-PD-L1 DNA aptamer antagonizes the interaction of PD-1/PD-L1 with antitumor effect. J. Mater. Chem. B 2021, 9, 746–756. [Google Scholar] [CrossRef]
- Li, T.; Yao, F.; An, Y.; Li, X.; Duan, J.; Yang, X.D. Novel complex of PD-L1 aptamer and holliday junction enhances antitumor efficacy in vivo. Molecules 2021, 26, 1067. [Google Scholar] [CrossRef]
- Wang, L.; Liang, H.; Sun, J.; Liu, Y.; Li, J.; Li, J.; Li, J.; Yang, H. Bispecific aptamer induced artificial protein-pairing: A strategy for selective inhibition of receptor function. J. Am. Chem. Soc. 2019, 141, 12673–12681. [Google Scholar] [CrossRef]
- Chen, C.B.; Chernis, G.A.; Hoang, V.Q.; Landgraf, R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc. Natl. Acad. Sci. USA 2003, 100, 9226–9231. [Google Scholar] [CrossRef]
- Elizabeth, D.P.; Sullenger, B.A.; Nair, S.K. Identification and characterization of an agonistic aptamer against the T cell costimulatory receptor, OX40. Nucleic Acids Ther. 2013, 23, 35–43. [Google Scholar]
- McNamara, J.O., II; Kolonias, D.; Pastor, F.; Mittler, R.S.; Chen, L.; Giangrande, P.H.; Sullenger, B.; Gilboa, E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Investig. 2008, 118, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F.; Soldevilla, M.M.; Villanueva, H.; Kolonias, D.; Inoges, S.; Cerio, A.L.D.; Kandzia, R.; Klimyuk, V.; Gleba, Y.; Gilboa, E.; et al. CD28 aptamers as powerful immune response modulators. Mol. Ther. Nucleic Acids 2013, 2, e98. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, M.M.; Villaneva, H.; Bendandi, M.; Inoges, S.; Cerio, A.L.; Pastor, F. 2-fluoro-RNA oligonucleotide CD40 targeted aptamers for the control of B lymphoma and bone-marrow aplasia. Biomaterials 2015, 67, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Monsalve, A.; Sautina, L.; Segal, M.S.; Dobson, J.; Allen, J.B. DNA aptamer assembly as a vascular endothelial growth factor receptor agonist. Nucleic Acids Ther. 2015, 25, 227–234. [Google Scholar] [CrossRef]
- Yunn, N.; Koh, A.; Han, S.; Lim, J.H.; Park, S.; Lee, J.; Kim, E.; Jang, S.K.; Berggren, P.; Ryu, S.H. Agonistic aptamer to the insulin receptor leads to biased signaling and functional selectivity through allosteric modulation. Nucleic Acids Res. 2015, 43, 7688–7701. [Google Scholar] [CrossRef]
- Khedri, M.; Rafatpanah, H.; Abnous, K.; Ramezani, P.; Ramezani, M. Cancer immunotherapy via nucleic acid aptamers. Int. Immunopharmacol. 2015, 29, 926–936. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Dollins, C.M.; Nair, S.; Boczkowski, D.; Lee, J.; Layzer, J.M.; Gilboa, E.; Sullenger, B.A. Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem. Biol. 2008, 15, 675–682. [Google Scholar] [CrossRef]
- Biesecker, G.; Dihel, L.; Enney, K.; Bendele, R. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology 1999, 42, 219–230. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Nazarenko, I.; Thiele, W. Do all roads lead to rome? Routes to metastasis development. Int. J. Cancer 2011, 128, 2511–2526. [Google Scholar] [CrossRef]
- Orava, E.W.; Abdul-Wahid, A.; Huang, E.H.B.; Mallick, A.I.; Gariépy, J. Blocking the attachment of cancer cells in vivo with DNA aptamers displaying anti-adhesive properties against the carcinoembryonic antigen. Mol. Oncol. 2013, 7, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, Y.; Tao, H.; Zhou, F.; Zhang, C.; Su, F.; Wang, S.; Liu, Q.; Xu, L.; Pan, X.; et al. Inhibition of adhesion and metastasis of HepG2 hepatocellular carcinoma cells in vitro by DNA aptamer against sialyl Lewis X. J. Huazhong Uni. Sci. Technolog. Med. Sci. 2017, 37, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Quintavalle, C.; Ingenito, F.; Rotoli, D.; Roscigno, G.; Nuzzo, S.; Thomas, R.; Catuogno, S.; Franciscis, V.D.; Condorelli, G. Identification of a novel RNA aptamer that selectively targets breast cancer exosomes. Mol. Ther. Nucleic Acids 2021, 23, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Nie, H.; Zhou, Y.; Lian, S.; Mei, H.; Lu, Y.; Dong, H.; Li, F.; Li, T.; Li, B.; et al. Eliminating blood oncogenic exosomes into the small intestine with aptamer-functionalized nanoparticles. Nat. Commun. 2019, 10, 5476. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef]
- Josic, D.; Clifton, J.G.; Kovac, S.; Hixson, D.C. Membrane proteins as diagnostic biomarkers and targets for new therapies. Curr. Opin. Mol. Ther. 2008, 10, 116–123. [Google Scholar]
- Hicke, B.J.; Stephens, A.W. Escort aptamers: A delivery service for diagnosis and therapy. J. Clin. Investig. 2000, 106, 923–928. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Leach, J.C.; Wang, A.; Ye, K.; Jin, S. A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int. J. Mol. Sci. 2016, 17, 380. [Google Scholar] [CrossRef]
- Chen, Z.; Tai, Z.; Gu, F.; Hu, C.; Zhu, Q.; Gao, S. Aptamer-mediated delivery of docetaxel to prostate cancer through polymeric nanoparticles for enhancement of antitumor efficacy. Eur. J. Pharm. Biopharm. 2016, 107, 130–141. [Google Scholar] [CrossRef]
- McNamara, J.O.; Andrechek, E.R.; Wang, Y.; Viles, K.D.; Rempel, R.E.; Gilboa, E.; Sullenger, B.A.; Giangrande, P.H. Cell type–specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006, 24, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Takahashi, Y.; Narita, S.; Yang, Y.C.; Li, X. Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget 2017, 8, 9375–9387. [Google Scholar] [CrossRef] [PubMed]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases dissemination. Nanomed. Nanotechnol. 2020, 25, 102171. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Youn, H.; Lee, S.; Ban, C. Ultra-effective photothermal therapy for prostate cancer cells using dual aptamer-modified gold nanostars. J. Mater. Chem. B 2014, 2, 4862–4867. [Google Scholar] [CrossRef] [PubMed]
- Frei, E., III. Combination cancer therapy: Presidential address. Cancer Res. 1972, 32, 2593–2607. [Google Scholar]
- Zhang, L.; Radovic-Moreno, A.F.; Alexis, F.; Gu, F.X.; Basto, P.A.; Bagalkot, V.; Jon, S.; Langer, R.S.; Farokhzad, O.C. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle–aptamer bioconjugates. ChemMedChem 2007, 2, 1268–1271. [Google Scholar] [CrossRef]
- Kim, E.; Jung, Y.; Choi, H.; Yang, J.; Suh, J.; Huh, Y.; Kim, K.; Haam, S. Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamer-conjugated polyplex. Biomaterials 2010, 31, 4592–4599. [Google Scholar] [CrossRef]
- Taghdisi, S.; Abnous, K.; Mosaffa, F.; Behravan, J. Targeted delivery of daunorubicin to T-cell acute lymphoblastic leukemia by aptamer. J. Drug Target. 2010, 18, 277–281. [Google Scholar] [CrossRef]
- Huang, Y.; Shangguan, D.; Liu, H.; Phillips, J.A.; Zhang, X.; Chen, Y.; Tan, W. Molecular assembly of an aptamer–drug conjugate for targeted drug delivery to tumor cells. Chembiochem 2009, 10, 862–868. [Google Scholar] [CrossRef]
- Zhu, G.; Zheng, J.; Song, E.; Donovan, M.; Zhang, K.; Liu, C.; Tan, W. Self-assembled, aptamer-tethered DNA nanotrains for targeted transport of molecular drugs in cancer theranostics. Proc. Natl. Acad. Sci. USA 2013, 110, 7998–8003. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; Tan, W.; Lai, Z.; Fang, D.; Jiang, L.; Zuo, C.; Yang, N.; Lai, Y. An efficient cell-targeting drug delivery system based on aptamer-modified mesoporous silica nanoparticles. Nanoscale Res. Lett. 2019, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Y.; Liu, J.; Yang, X.; Xu, Y.; Shi, J.; Liu, W.; Li, J. Y-shaped circular aptamer DNAzyme conjugates for highly efficient in vivo gene silencing. CCS Chem. 2020, 2, 631–641. [Google Scholar] [CrossRef]
- Zong, S.; Wang, L.; Yang, Z.; Wang, H.; Wang, Z.; Cui, Y. Black phosphorus-based drug nanocarrier for targeted and synergetic chemophotothermal therapy of acute lymphoblastic leukemia. ACS Appl. Mater. Inter. 2019, 11, 5896–5902. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.L.; Zhu, G.; Xiao, X.; Puszyk, W.; Sefah, K.; Wu, Q.; Tan, W.; Liu, C. A synthetic aptamer-drug adduct for targeted liver cancer therapy. PLoS ONE 2015, 10, e0136673. [Google Scholar] [CrossRef]
- Rajabnejad, S.H.; Mokhtarzadeh, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Razavi, B.M. Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev. Ind. Pharm. 2018, 44, 982–987. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, S.; Wu, W.; Kankala, R.K.; Chen, A.; Liu, Y.; Fan, J. Co-delivery of doxorubicin and AS1411 aptamer by poly(ethylene glycol)-poly(β-amino esters) polymeric micelles for targeted cancer therapy. J. Nanoparticle Res. 2017, 19, 224. [Google Scholar] [CrossRef]
- Yang, S.; Ren, Z.; Chen, M.; Wang, Y.; You, B.; Chen, W.; Qu, C.; Liu, Y.; Zhang, X. Nucleolin-targeting AS1411-aptamer-modified graft polymeric micelle with dual pH/redox sensitivity designed to enhance tumor therapy through the codelivery of doxorubicin/TLR4 siRNA and suppression of invasion. Mol. Pharmaceut. 2018, 15, 314–325. [Google Scholar] [CrossRef]
- Yang, D.; Van, S.; Jiang, X.; Yu, L. Novel free paclitaxel-loaded poly(L-γ-glutamylglutamine)-paclitaxel nanoparticles. Int. J. Nanomed. 2011, 6, 85–91. [Google Scholar]
- Luo, Z.; Yan, Z.; Jin, K.; Pang, Q.; Jiang, T.; Lu, H.; Liu, X.; Pang, Z.; Yu, L.; Jiang, X. Precise glioblastoma targeting by AS1411 aptamer-functionalized poly (L-γ-glutamylglutamine)–paclitaxel nanoconjugates. J. Colloid Interface Sci. 2017, 490, 783–796. [Google Scholar] [CrossRef]
- Aravind, A.; Jeyamohan, P.; Nair, R.; Veeranarayanan, S.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol. Bioeng. 2012, 109, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Vandghanooni, S.; Eskandani, M.; Barar, J.; Omidi, Y. AS1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine 2018, 13, 2729–2758. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Hu, X.; Shanmugam, S.; Chelliah, R.; Sekar, P.; Oh, D.; Vijayakumar, S.; Kathiresan, K.; Wang, M. Enhanced cancer therapy with pH-dependent and aptamer functionalized doxorubicin loaded oolymeric (poly D, L-lactic-co-glycolic acid) nanoparticles. Arch. Biochem. Biophys. 2019, 671, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Ramezani, M.; Abnous, K.; Hadizadeh, F. AS1411 aptamer-decorated biodegradable polyethylene glycol–poly(lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non–small cell lung cancer in vitro. J. Pharm. Sci. 2016, 105, 1741–1750. [Google Scholar] [CrossRef]
- Hofman, J.; Buncek, M.; Haluza, R.; Streinz, L.; Ledvina, M.; Cigler, P. In vitro transfection mediated by dendrigraft poly(L-lysines): The effect of structure and molecule size. Macromol. Biosci. 2013, 13, 167–176. [Google Scholar] [CrossRef]
- Chen, H.; Tian, J.; Liu, D.; He, W.; Guo, Z. Dual aptamer modified dendrigraft poly-L-lysine nanoparticles for overcoming multi-drug resistance through mitochondrial targeting. J. Mater. Chem. B 2017, 5, 972–979. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohyd. Polym. 2017, 155, 218–229. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Chen, L.; Yu, S.; Cao, Y.; He, C.; Chen, X. Intracellular pH-sensitive peg-block-acetalated-dextrans as efficient drug delivery platforms. ACS Appl. Mater. Inter. 2013, 5, 10760–10766. [Google Scholar] [CrossRef]
- Xing, H.; Tang, L.; Yang, X.; Hwang, K.; Wang, W.; Yin, Q.; Wong, N.Y.; Dobrucki, L.W.; Yasui, N.; Katzenellenbogen, J.A.; et al. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar] [CrossRef]
- Li, L.; Hou, J.; Liu, X.; Guo, Y.; Wu, Y.; Zhang, L.; Yang, Z. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014, 35, 3840–3850. [Google Scholar] [CrossRef]
- Liao, Z.; Chuang, E.; Lin, C.; Ho, Y.; Lin, K.; Cheng, P.; Chen, K.; Wei, H.; Sung, H. An AS1411 aptamer-conjugated liposomal system containing a bubble-generating agent for tumor-specific chemotherapy that overcomes multidrug resistance. J. Control. Release 2015, 208, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-delivery of paclitaxel and PLK1-targeted siRNA using aptamer-functionalized cationic liposome for synergistic anti-breast cancer effects in vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted DOX-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharmacother. 2019, 117, 109072. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, L.; Wang, L.; Jiang, W. A dual-targeting DNA tetrahedron nanocarrier for breast cancer cell imaging and drug delivery. Talanta 2018, 179, 356–363. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Ramezani, M.; Jalalian, S.H.; Abnous, K.; Taghdisi, S.M. Targeted delivery of epirubicin to cancer cells by polyvalent aptamer system in vitro and in vivo. Pharm. Res. 2016, 33, 2289–2297. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Lavaee, P.; Jalalian, S.H.; Robati, R.Y.; Abnous, K. Double targeting and aptamer-assisted controlled release delivery of epirubicin to cancer cells by aptamers-based dendrimer in vitro and in vivo. Eur. J. Pharm. Biopharm. 2016, 102, 152–158. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Yazdian-Robati, R.; Abnous, K. A novel AS1411 aptamer-based three-way junction pocket DNA nanostructure loaded with doxorubicin for targeting cancer cells in vitro and in vivo. Mol. Pharmaceut. 2018, 15, 1972–1978. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Charbgoo, F.; Bahreyni, A.; Taghdisi, S.M. Targeted delivery of doxorubicin to cancer cells by a cruciform DNA nanostructure composed of AS1411 and FOXM1 aptamers. Expert Opin. Drug Deliv. 2018, 15, 1045–1052. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Li, Y.; Duo, Y.; Bi, J.; Zeng, X.; Mei, L.; Bao, S.; He, L.; Shan, A.; Zhang, Y.; Yu, X. Targeted delivery of anti-miR-155 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Int. J. Nanomed. 2018, 13, 1241–1256. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Darvishi, B.; Mirzaie, Z.; Dorkoosh, F.; Shanehsazzadeh, S.; Dinarvand, R. Pegylated magnetic mesoporous silica nanoparticles decorated with AS1411 aptamer as a targeting delivery system for cytotoxic agents. Pharm. Dev. Technol. 2019, 24, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ding, B.; Zhao, Z.; Zhang, H.; Sun, B.; Zhao, Y.; Jiang, L.; Zhou, J.; Ding, Y. Immune lipoprotein nanostructures inspired relay drug delivery for amplifying antitumor efficiency. Biomaterials 2018, 185, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, J.; Zhang, Y.; Zhang, J.; Tang, A.; Kong, D. A ZnO-gated porphyrinic metal–organic framework-based drug delivery system for targeted bimodal cancer therapy. J. Mater. Chem. B 2018, 6, 7898–7907. [Google Scholar] [CrossRef] [PubMed]
- Nabavinia, M.S.; Gholoobi, A.; Charbgoo, F.; Nabavinia, M.; Ramezani, M.; Abnous, K. Anti-MUC1 aptamer: A potential opportunity for cancer treatment. Med. Res. Rev. 2017, 37, 1518–1539. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; He, L.; Yao, Y.; Zhou, Y.; Wu, J.; Liu, J.; Ding, J. Aptamer density dependent cellular uptake of lipid-capped polymer nanoparticles for polyvalent targeted delivery of vinorelbine to cancer cells. RSC Adv. 2015, 5, 16931–16939. [Google Scholar] [CrossRef]
- Chang, M.; Yang, C.; Huang, D. Aptamer-conjugated DNA icosahedral nanoparticles as a carrier of doxorubicin for cancer therapy. ACS Nano 2011, 5, 6156–6163. [Google Scholar] [CrossRef]
- Razis, E.D.; Fountzilas, G. Paclitaxel: Epirubicin in metastatic breast cancer—A review. Ann. Oncol. 2001, 12, 593–598. [Google Scholar] [CrossRef]
- Dai, B.; Hu, Y.; Duan, J.; Yang, X.D. Aptamer-guided DNA tetrahedron as a novel targeted drug delivery system for MUC1-expressing breast cancer cells in vitro. Oncotarget 2016, 7, 38257–38269. [Google Scholar] [CrossRef]
- Savla, R.; Taratula, O.; Garbuzenko, O.; Minko, T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J. Control. Release 2011, 153, 16–22. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Taghdisi, S.M.; Hamedani, N.S.; Kalat, S.A.M.; Lavaee, P.; ZandKarimi, M.; Ghows, N.; Jaafari, M.R.; Naghibi, S.; Danesh, N.M.; et al. Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for combined colon cancer therapy and imaging in vivo. Eur. J. Pharm. Sci. 2013, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sayari, E.; Dinarvand, M.; Amini, M.; Azhdarzadeh, M.; Mollarazi, E.; Ghasemi, Z.; Atyabi, F. MUC1 aptamer conjugated to chitosan nanoparticles, an efficient targeted carrier designed for anticancer SN38 delivery. Int. J. Pharmaceut. 2014, 473, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, Z.; Dinarvand, R.; Mottaghitalab, F.; Esfandyari-Manesh, M.; Sayari, E.; Atyabi, F. Aptamer decorated hyaluronan/chitosan nanoparticles for targeted delivery of 5-fluorouracil to MUC1 overexpressing adenocarcinomas. Carbohyd. Polym. 2015, 121, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari-Manesh, M.; Mohammadi, A.; Atyabi, F.; Nabavi, S.M.; Ebrahimi, S.M.; Shahmoradi, E.; Varnamkhasti, B.S.; Ghahremani, M.H.; Dinarvand, R. Specific targeting delivery to MUC1 overexpressing tumors by albumin-chitosan nanoparticles conjugated to DNA aptamer. Int. J. Pharmaceut. 2016, 515, 607–615. [Google Scholar] [CrossRef]
- Stecker, J.R.; Savage, A.A.; Bruno, J.G.; García, D.M.; Koke, J.R. Dynamics and visualization of MCF7 adenocarcinoma cell death by aptamer-C1Q-mediated membrane attack. Nucleic Acids Ther. 2012, 22, 275–282. [Google Scholar] [CrossRef]
- Borbas, K.E.; Ferreira, C.S.M.; Perkins, A.; Bruce, J.I.; Missailidis, S. Design and synthesis of mono- and multimeric targeted radiopharmaceuticals based on novel cyclen ligands coupled to anti-MUC1 aptamers for the diagnostic imaging and targeted radiotherapy of cancer. Bioconjug. Chem. 2007, 18, 1205–1212. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef]
- Kurosaki, T.; Higuchi, N.; Kawakami, S.; Higuchi, Y.; Nakamura, T.; Kitahara, T.; Hashida, M.; Sasaki, H. Self-assemble gene delivery system for molecular targeting using nucleic acid aptamer. Gene 2012, 491, 205–209. [Google Scholar] [CrossRef]
- Wang, C.; Lin, B.; Chen, C. An aptamer targeting shared tumor-specific peptide antigen of MEGA-A3 in multiple cancers. Int. J. Cancer 2016, 138, 918–926. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Abnous, K.; Badiee, A.; Jaafari, M.R. Improvement in the drug delivery and anti-tumor efficacy of pegylated liposomal doxorubicin by targeting RNA aptamers in mice bearing breast tumor model. Colloids Surface B 2016, 139, 228–236. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Liu, T.; Liu, J.; Xie, Y.; Zhang, J.; Xu, S.; Liu, H. A dual-functional HER2 aptamer-conjugated, pH-activated mesoporous silica nanocarrier-based drug delivery system provides in vitro synergistic cytotoxicity in HER2-positive breast cancer cells. Int. J. Nanomed. 2019, 14, 4029–4044. [Google Scholar] [CrossRef]
- Zhou, G.; Bae, S.D.W.; Nguyen, R.; Huo, X.; Han, S.; Zhang, Z.; Hebbard, L.; Duan, W.; Eslam, M.; Liddle, C.; et al. An aptamer-based drug delivery agent (CD133-Apt-DOX) selectively and effectively kills liver cancer stem-like cells. Cancer Lett. 2021, 501, 124–132. [Google Scholar] [CrossRef]
- Ma, J.; Zhuang, H.; Zhuang, Z.; Lu, Y.; Xia, R.; Gan, L.; Wu, Y. Development of docetaxel liposome surface modified with CD133 aptamers for lung cancer targeting. Artif. Cells Nanomed. B 2018, 46, 1864–1871. [Google Scholar] [CrossRef]
- Xie, X.; Li, F.; Zhang, H.; Lu, Y.; Lian, S.; Lin, H.; Gao, Y.; Jia, L. EPCAM aptamer-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. Eur. J. Pharm. Sci. 2016, 83, 28–35. [Google Scholar] [CrossRef]
- Das, M.; Duan, W.; Sahoo, S.K. Multifunctional nanoparticle–EPCAM aptamer bioconjugates: A paradigm for targeted drug delivery and imaging in cancer therapy. Nanomed. Nanotechnol. 2015, 11, 379–389. [Google Scholar] [CrossRef]
- Li, L.; Xiang, D.; Shigdar, S.; Yang, W.; Li, Q.; Lin, J.; Liu, K.; Duan, W. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int. J. Nanomed. 2014, 9, 1083–1096. [Google Scholar]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Ghavami, M.; Shanehsazzadeh, S.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surface B 2016, 143, 224–232. [Google Scholar] [CrossRef]
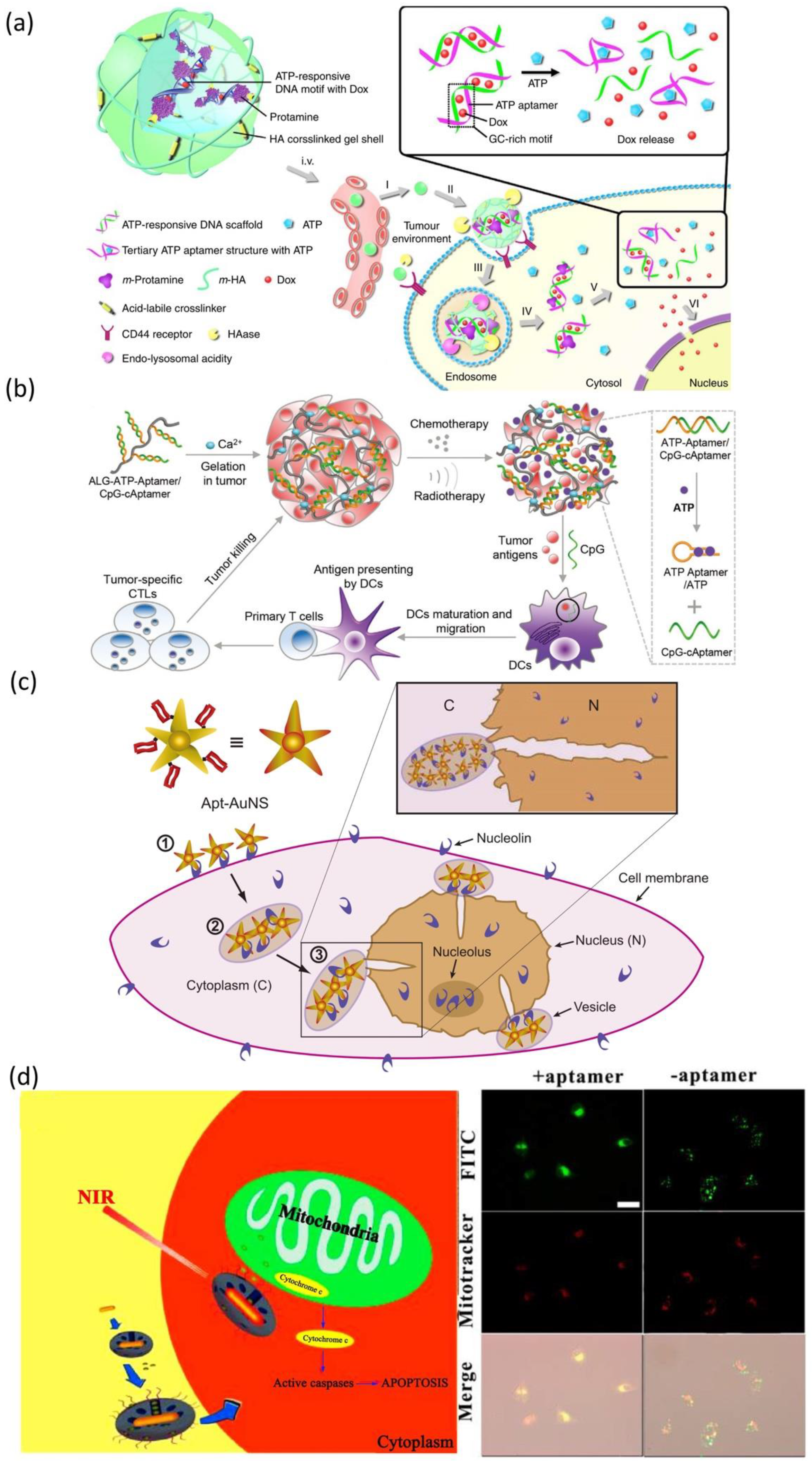
- Sun, W.; Gu, Z. ATP-responsive drug delivery systems. Expert Opin. Drug Del. 2016, 13, 311–314. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; DiSanto, R.; Tai, W.; Gu, Z. ATP-triggered anticancer drug delivery. Nat. Commun. 2014, 5, 3364. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; Gu, Z. Enhanced anticancer efficacy by ATP-mediated liposomal drug delivery. Angew. Chem. Int. Ed. Engl. 2014, 53, 5815–5820. [Google Scholar] [CrossRef]
- He, X.; Zhao, Y.; He, D.; Wang, K.; Xu, F.; Tang, J. ATP-responsive controlled release system using aptamer-functionalized mesoporous silica nanoparticles. Langmuir 2012, 28, 12909–12915. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Sun, W.; Gu, Z. ATP-responsive DNA-graphene hybrid nanoaggregates for anticancer drug delivery. Biomaterials 2015, 50, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lu, C.; Hartmann, R.; Wang, F.; Sohn, Y.S.; Parak, W.J.; Willner, I. Adenosine triphosphate-triggered release of macromolecular and nanoparticle loads from aptamer/DNA-cross-linked microcapsules. ACS Nano 2015, 9, 9078–9086. [Google Scholar] [CrossRef] [PubMed]
- Dam, D.H.M.; Lee, J.H.; Sisco, P.N.; Co, D.T.; Zhang, M.; Wasielewski, M.R.; Odom, T.W. Direct observation of nanoparticle–cancer cell nucleus interactions. ACS Nano 2012, 6, 3318–3326. [Google Scholar] [CrossRef]
- Chen, W.; Luo, G.; Zhang, X. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv. Mater. 2019, 31, 1802725. [Google Scholar] [CrossRef]
- Ju, E.; Li, Z.; Liu, Z.; Ren, J.; Qu, X. Near-infrared light-triggered drug-delivery vehicle for mitochondria-targeted chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2014, 6, 4364–4370. [Google Scholar] [CrossRef]
- Zamay, T.N.; Kolovskaya, O.S.; Glazyrin, Y.E.; Zamay, G.S.; Kuznetsova, S.A.; Spivak, E.A.; Wehbe, M.; Savitskaya, A.G.; Zubkova, O.A.; Kadkina, A.; et al. DNA-aptamer targeting vimentin for tumor therapy in vivo. Nucleic Acids Ther. 2014, 24, 160–170. [Google Scholar] [CrossRef]
- Gening, L.V.; Klincheva, S.A.; Reshetnjak, A.; Grollman, A.P.; Miller, H. RNA aptamers selected against DNA polymerase B inhibit the polymerase activities of DNA polymerases B and K. Nucleic Acids Res. 2006, 34, 2579–2586. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in selex technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef]
- Bruno, J.G. Predicting the uncertain future of aptamer-based diagnostics and therapeutics. Molecules 2015, 20, 6866–6887. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, J.; Liu, J. Clinical applications of nucleic acid aptamers in cancer. Mol. Clin. Oncol. 2014, 2, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamers, the nucleic acid antibodies, in cancer therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Wang, Z.; Ke, P.; Zhu, W.; Yuan, Z.; Feng, S.; Chen, Y.; Lin, C.; Liu, X.; Li, Y.; et al. Development of a novel ssDNA aptamer targeting cardiac troponin I and its clinical applications. Anal. Bioanal. Chem. 2021, 413, 7043–7053. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.J. Pegaptanib sodium for neovascular age-related macular degeneration: Two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology 2006, 113, 992–1001.e6. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.M.; Bates, P.J. Characterizing oligonucleotide uptake in cultured cells: A case study using AS1411 aptamer. Methods Mol. Biol. 2019, 2036, 173–186. [Google Scholar]
- Shangguan, D.; Cao, Z.; Meng, L.; Mallikaratchy, P.; Sefah, K.; Wang, H.; Li, Y.; Tan, W. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 2008, 7, 2133–2139. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Eliott, D.; Wells, J.A.; Prenner, J.L.; Papp, A.; Patel, S. A phase 1 study of intravitreous E10030 in combination with ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2016, 123, 78–85. [Google Scholar] [CrossRef]
- Vavalle, J.P.; Cohen, M.G. The Reg1 anticoagulation system: A novel actively controlled factor Ix inhibitor using RNA aptamer technology for treatment of acute coronary syndrome. Future Cardiol. 2012, 8, 371–382. [Google Scholar] [CrossRef]
- Cosmi, B. Arc-1779, a pegylated aptamer antagonist of von willebrand factor for ootential use as an anticoagulant or antithrombotic agent. Curr. Opin. Mol. Ther. 2009, 11, 322–328. [Google Scholar]
- Bunka, D.H.J.; Platonova, O.; Stockley, P.G. Development of aptamer therapeutics. Curr. Opin. Pharmacol. 2010, 10, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The spiegelmer Nox-A12, a novel Cxcl12 inhibitor, Interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Ninichuk, V.; Clauss, S.; Kulkarni, O.; Schmid, H.; Segerer, S.; Radomska, E.; Eulberg, D.; Buchner, K.; Selve, N.; Klussmann, S.; et al. Late onset of CCL2 blockade with the spiegelmer Mnox-E36–3′Peg prevents glomerulosclerosis and improves glomerular filtration rate in Db/Db mice. Am. J. Pathol. 2008, 172, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Schwoebel, F.; Eijk, L.T.V.; Zboralski, D.; Sell, S.; Buchner, K.; Maasch, C.; Purschke, W.G.; Humphrey, M.; Zöllner, S.; Eulberg, D.; et al. The effects of the anti-hepcidin spiegelmer Nox-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood 2013, 121, 2311–2315. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Martín-Vílchez, S.; Ochoa, D.; Mejía-Abril, G.; Román, M.; Camargo-Mamani, P.; Luquero-Bueno, S.; Jilma, B.; Moro, M.A.; Fernández, G.; et al. First-in-human phase I clinical trial of a T1R4-binding DNA aptamer, aptoll: Safety and pharmacokinetics in healthy volunteers. Mol. Ther. Nucleic Acids 2022, 28, 124–135. [Google Scholar] [CrossRef]



| Biomarkers | Aptamer Name | Diagnosis Mechanism | DNA/RNA | Limit of Detection (LOD) | Cancer Type | Ref. |
|---|---|---|---|---|---|---|
| EGFR | Anti-EGFR aptamer | Aptamer/antibody based immunosensor. | DNA | 50 pg/mL | Human epidermal squamous cell carcinoma | [22] |
| Anti-EGFR aptamer | Origami-paper-based graphene-modified aptasensor. | DNA | 5 pg/mL | Nonsmall-cell lung cancer | [23] | |
| MinE07 | 18F-labeled RNA aptamer for PET imaging. | RNA | - | Lung cancer | [24] | |
| VEGFR2 | Anti-VEGFR2 aptamer | Aptamers-modified magnetic nanoparticles for MR imaging | DNA | - | Breast cancer | [27] |
| HER2 | HB5 | Proximity-induced fluorescence activation of aptamers and G-rich sequences templated AgNCs. | DNA | 0.0904 fM | Breast cancer | [28] |
| LC-MS/MS-based quasi-targeted proteomics strategy coupled with aptamers-triggered hybridization chain reactions. | DNA | - | Breast cancer | [29] | ||
| Proximity-induced fluorescence activation of aptamer-templated AgNCs. | DNA | 220 pM | Breast cancer | [32] | ||
| PTK7 | Sgc8 | 18F labeled aptamer for PET imaging. | DNA | - | Malignant melanoma | [33] |
| Electrochemical aptamer-based determination by toehold-mediated strand displacement amplification on AuNPs and GOs. | DNA | 1.8 fM | Colon cancer | [34] | ||
| CD4 | Anti-CD4 aptamer | Aptamers containing surfaces for capture of CD4 expressing cells. | RNA | - | HIV/AIDS | [37] |
| CD30 | CD30 | Aptamer conjugated IRD800CW reporter for imaging. | RNA | - | Lymphoma | [39] |
| Cyanine dye (Cy5) labeled aptamers for flow cytometry detection of CD30 -expressing cells. | RNA | 0.3 nM | Lymphoma | [40] | ||
| CD63 | CD63 | Aptamer accelerated intrinsic peroxidase-like activity of g-C3N4 nanosheets for detection of exosomes. | DNA | 13.52 × 105 particles/μL | Breast cancer | [41] |
| Aptamer capped single-wall carbon nanotubes based colorimetric aptasensor for detection of exosomes. | DNA | 5.2 × 102 particles/μL | Breast cancer | [42] | ||
| Aptamer and G-quadruplex/hemin DNAzyme modified ECL sensor for detection of exoxomes. | DNA | 7.41 × 104 particles/mL | Breast cancer | [43] | ||
| Aptamer linked dual AuNPs based sensor for detection of exosomes by surface plasmon resonance. | DNA | 5 × 103 particles/mL | Breast cancer | [44] | ||
| Aptamer-based fluorescence polarization for separation of free exosomes quantification. | DNA | 500 particles/μL | Breast cancer | [45] |
| Biomarkers | Aptamer Name | Diagnosis Mechanism | DNA/RNA | LOD | Cancer Type | Ref. |
|---|---|---|---|---|---|---|
| EpCAM | EpCAM | Aptamer-based graphene quantum dots and MoS2 nanosheets for detection of target through FRET. | DNA | 450 pM | Breast cancer | [48] |
| Enzyme-free fluorescence detection of EpCAM by a combined aptamer-based recognition and toehold-aided DNA recycling amplification strategy. | DNA | 0.1 ng/mL | Breast cancer | [49] | ||
| Aptamer conjugated maleimidyl magnetic nanoplatform for facile MRI. | DNA | - | Breast cancer | [50] | ||
| Graphene oxide and fluorescent labeled aptamers for detection of exosomes. | DNA | 2.1 × 104 particles/µL | Colorectal cancer | [51] | ||
| Aptamer modified Ti3C2 MXenes nanosheets based chemiluminescence biosensor for detection of exosomes. | DNA | 125 particles/µL | Breast cancer | [53] | ||
| EpCAM and CD63 aptamer-based 3D DNA walker amplification and Exo III-assisted electrochemical ratiometric detection of exosomes. | DNA | 1.3 × 104 particles/mL | Breast cancer | [55] | ||
| Aptamer-based gold nanostars for detection of EpCAM overexpressed-CTCs by RCA coupled with the hemin/G-quadruplex complex. | DNA | 1 cell/mL | Breast cancer | [56] | ||
| Integrins | Aptαvβ3 | Aptamer conjugated magnetic nanoparticles for detection of integrin ανβ3 by MRI. | DNA | - | Glioblastoma | [57] |
| H02 | Aptamer as probes for cyto- and histofluorescence. | RNA | - | Glioblastoma | [58] |
| Biomarkers | Aptamer Name | Diagnosis Mechanism | DNA/RNA | LOD | Cancer Type | Ref. |
|---|---|---|---|---|---|---|
| PSMA | xPSM-A9 xPSM-A10 | Aptamer as probes and processed the ability to inhibit N-acetyl-α-inked acid dipeptidase. | RNA | 2.1 nM 11.9 nM | Prostatic cancer | [61] |
| xPSM-A10 | Aptamer conjugated AuNPs for detection of target by utilizing tissue microarrays. | RNA | - | Prostatic cancer | [62] | |
| A10-3.2 | Aptamer-oriented lipid nanobubbles as ultrasound contrast agent. | RNA | - | Prostatic cancer | [63] | |
| A10 | Aptamer conjugated multi-walled carbon nanotubes as ultrasound contrast agent. | RNA | - | Prostatic cancer | [64] | |
| RNA/peptide dual-aptamer probe based electrochemical detection. | RNA | - | Prostatic cancer | [66] | ||
| MMP-9 | F3B | 99mTc labeled aptamer for ex vivo imaging slices of human brain tumors. | RNA | 20 nM | Glioblastoma | [68] |
| Dual aptamer-based piezoelectric biosensor for the detection of target. | RNA | 1.2 pM | - | [69] | ||
| Programmed hybridization/dehybridization of aptamers on the surface of AuNSs based photoacoustic contrast agent. | RNA | - | Breast cancer | [70] |
| Biomarkers | Aptamer Name | Diagnosis Mechanism | DNA/RNA | LOD | Cancer Type | Ref. |
|---|---|---|---|---|---|---|
| MUC1 | MUC1 | Fluorescence aggregation assay by carbon dot-labeled antibodies and aptamers. | DNA | 2 nM | - | [74] |
| Aptamer-tagged AgNCs for fluorescent imaging. | DNA | 0.05 nM | Breast cancer | [75] | ||
| Four-way branch migration-based strategy for amplified aptamer detection of MUC1. | DNA | 2.8 nM | - | [76] | ||
| Dual-aptamer (VEGF and MUC1 aptamers) nanoparticle-mediated signal amplification strategy for cancer cells colorimetric detection. | DNA | 10 cells/mL | Breast cancer | [77] | ||
| Aptamer conjugated carbon nanospheres for electrochemical detection of target. | DNA | 40 cells/mL | Colon cancer | [78] | ||
| Label free aptasensor for electrochemical detection of target by combining hemin/G-quadruplex system and RCA. | DNA | 9.54 × 102 particles/mL | Gastric cancer | [80] | ||
| Nucleolin | AS1411 | Aptamer-based microcantilever biosensor for the detection of target. | DNA | 1.0 nM | - | [82] |
| 64Cu labeled aptamer for tumor-targeted imaging by microPET/CT. | DNA | - | Lung cancer | [83] | ||
| Aptamer conjugated with HYNIC and 99mTc for thin layer chromatography. | DNA | - | Prostate cancer | [84] | ||
| Cobalt-ferrite nanoparticle surrounded by fluorescent rhodamine within silica shell matrix conjugated with aptamer for multimodal cancer-targeted imaging. | DNA | - | Glioma | [85] | ||
| AS1411 aptamer-based phosphorescent nanoprobe for tumor imaging. | DNA | - | Breast cancer | [86] | ||
| Dual aptamer (AS1411 and CD63 aptamer) recognition-based G-quadruplex nanowires for the detection of exosomes. | DNA | 1.85 × 103 particles/mL | Cervical carcinoma | [87] | ||
| MUC1 aptamer functionalized magnetic beads and AS1411 modified quantum dots based nano-bio-probes for the multimode detection of target. | DNA | 201 cells/mL 85 cells/mL | Breast cancer | [88] | ||
| Sandwich-type cytosensor based on MOF and DNA tetrahedron linked dual aptamer (AS1411 and MUC1) for electrochemical detection of target. | DNA | 6 cells/mL | Breast cancer | [89] | ||
| VEGF165 | VEGF165 | Bivalent aptamer-Cu nanocluster for fluorescent detection of target. | DNA | 12 pM | Colorectal cancer | [92] |
| Nicking endonuclease-assisted signal amplification of a split aptamer beacon for detection of target. | DNA | 1 pM | Breast cancer | [93] | ||
| AgNPs-enhanced time resolved fluorescence sensor for VEGF165 detection by using long-lived fluorescent Mn-doped ZnS QDs. | DNA | - | - | [94] | ||
| Aptamer conjugated USPIO nanoparticles for MRI imaging of VEGF165-expressing tumors in vivo. | DNA | - | Liver cancer | [95] |
| Biomarkers | Aptamer Name | Diagnosis Mechanism | DNA/RNA | LOD | Cancer Type | Ref. |
|---|---|---|---|---|---|---|
| PDGF-BB | PDGF-BB | Aptamer modified AuNPs for colorimetric detection of target. | DNA | 32 nM | - | [100] |
| Aptamer-based DNA enzyme-catalyzed colorimetric reaction coupled with RCA for colorimetric detection of target. | DNA | 8.2 fM | Breast cancer | [101] | ||
| AuNPs labeled and target-triggered strand displacement amplification for colorimetric detection of target. | DNA | 1.1 nM | Breast cancer | [102] | ||
| Aptamer-based cascade amplification SERS method for the detection of target. | DNA | 0.42 pM | - | [103] | ||
| Aptamer conjugated hydrophobic Ru (II) complex for label-free luminescent detection of target. | DNA | 0.8 pM | - | [104] | ||
| Target-triggered hybridization chain reaction amplification and GO-based selective fluorescence quenching. | DNA | 1.25 pM | - | [105] | ||
| Photo-induced electron transfer between aptamer-AgNCs and G-quadruplex/hemin complexes. | DNA | 1 × 10−13 M | - | [106] | ||
| FRET based aptasensor using UCNPs as donor and AuNPs as acceptor for the detection of target. | DNA | 10 nM | Lymphoma | [107] | ||
| The precipitates’ electrochemical aptasensing between the reaction of phosphate group in both HAP-NPs and the aptamer reacted with molybdate. | DNA | 50 fg/mL | - | [110] | ||
| AFP | AFP | Target-induced aptamer switched mode for label-free fluorescent detection of target. | DNA | 1.76 nM | - | [115] |
| Sandwich binding type based fluorescent aptasensor for detection of target. | DNA | 400 pM | Hepatocellular carcinoma | [116] | ||
| CEA | CEA | Fluorophore-labeled aptamer-absorbed MoS2 nanosheets for detection of target. | DNA | 34 pg/mL | Gastrointestinal neoplasms | [118] |
| Aptamer linking AgNCs with AuNPs for fluorescent detection of target. | DNA | 3 pg/mL | - | [119] | ||
| Aptamer-based FRET sensor between NIR-CDs and AuNRs for fluorescent detection of target. | DNA | 0.02 pg/mL | Lung cancer | [121] | ||
| Aptamer-based dsDNA templated copper nanoparticles for label-free fluorescent detection of target. | DNA | 6.5 pg/mL | - | [122] | ||
| Exonuclease III-assisted target recycling and DNA walker amplification strategy for fluorescent detection of target. | DNA | 1.2 pg/mL | - | [123] | ||
| 8-OHdG | 8-OHdG | Aptamer combined with NMM fluorophore to form a fluorescent switch to detect target. | DNA | 1.19 nM | - | [126] |
| Aptamer-based 3D DNA nanomachine for fluorescent detection of target. | DNA | 4 pM | - | [127] | ||
| Target-triggered polyaniline deposition on aptamer-based tetrahedral DNA nanostructure for electrochemical detection of target. | DNA | 1 pM | Bladder cancer | [128] | ||
| Fe3O4-aptamer magnetic nanoparticles for the detection of target by HPLC-MS. | DNA | 0.01 nM | - | [129] |
| Biomarkers | Aptamer Name | Delivery Mechanism | DNA/RNA | Cancer Type | Ref. |
|---|---|---|---|---|---|
| PSMA | A10 | Dtxl-encapsulated nanoparticles formulated with PLGA-b-PEG copolymer and surface functionalized with aptamers. | RNA | Prostate cancer | [158] |
| DNA-RNA hybrid aptamer coupled SPION to delivery DOX. | RNA | Prostate cancer | [159] | ||
| Aptamers anchored nanoparticles to encapsulate docetaxel for tumor delivery | RNA | Prostate cancer | [160] | ||
| Aptamers-siRNA chimera for in vivo delivery for targeted gene silencing therapy | RNA | Prostate cancer | [161] | ||
| Aptamer-conjugated cationic liposomes to deliver therapeutic CRISPR/Cas9 | RNA | Prostate cancer | [162] | ||
| Dual aptamer-modified gold nanostars for generating heat to induce apoptosis. | RNA | Prostate cancer | [164] | ||
| DOX carried aptamer conjugated with DTX loaded PLGA-b-PEG nanoparticles for targeted chemotherapy. | RNA | Prostate cancer | [166] | ||
| DOX carried aptamer conjugated with shRNA loaded PEG-PEI polyplexes for targeted chemogene therapy. | RNA | Prostate cancer | [167] | ||
| PTK7 | Sgc8 | Daunorubicin-carried aptamer for targeted chemotherapy. | DNA | Lymphoblastic leukemia | [168] |
| DOX-carried aptamer for targeted chemotherapy. | DNA | Lymphoblastic leukemia | [169] | ||
| DOX-loaded aptamer-tethered DNA nanotrains for targeted chemotherapy. | DNA | Lymphoblastic leukemia | [170] | ||
| Aptamers modified MSN to deliver DOX to tumors. | DNA | Lymphoblastic leukemia | [171] | ||
| Aptamer-DNAzyme conjugate for targeted gene therapy. | DNA | Cervical carcinoma | [172] | ||
| Aptamer modified composite drug nanocarrier based on black phosphorus nanosheets for targeted chemophotothermal therapy. | DNA | Lymphoblastic leukemia | [173] | ||
| Nucleolin | AS1411 | DOX-carried aptamer for targeted chemotherapy. | DNA | Hepatocellular carcinoma | [174] |
| Melittin conjugated aptamer for targeted chemotherapy. | DNA | Human non-small cell lung cancer | [175] | ||
| Dual aptamer modified dendrigraft poly-L-lysin nanoparticles for targeted delivery of DOX. | DNA | Cervical carcinoma | [186] | ||
| Aptamer-decorated dextran coated nano-graphene oxide for targeted dextran delivery. | DNA | Breast cancer | [187] | ||
| Aptamer-functionalized liposomes loaded with DOX for in vitro and in vivo targeted delivery. | DNA | Breast cancer | [189] | ||
| Dual targeting DNA tetrahedron nanocarrier (MUC1-Td-AS1411) for targeted delivery of DOX. | DNA | Breast cancer | [194] | ||
| Anti-miR-155-loaded MSNs modified with polymerized dopamine and aptamer for targeted gene therapy. | DNA | Rectal cancer | [200] | ||
| Lipophilic aptamer-CpG fused sequences conjugated lipoprotein for targeted delivery of DOX. | DNA | Lung cancer | [202] | ||
| Aptamer modified ZnO-gated porMOF-based drug delivery system for targeted delivery for targeted bimodal cancer therapy. | DNA | Cervical carcinoma | [203] | ||
| MUC1 | MUC1 | PEGylated aptamer-DOX complex for targeted chemotherapy. | DNA | Breast cancer | [204] |
| Aptamer modified and vinorelbine loaded lipid-polymer hybrid nanoparticles for targeted chemotherapy. | DNA | Breast cancer | [206] | ||
| Aptamer-conjugated DNA icosahedral nanoparticles as a DOX carrier for targeted chemotherapy. | DNA | Epithelial cell carcinoma | [207] | ||
| Quantum dots-aptamer -DOX conjugates for targeted chemotherapy. | RNA | Ovarian cancer | [210] | ||
| Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for targeted chemotherapy. | DNA | Colorectal cancer | [211] | ||
| Aptamer conjugated chitosan nanoparticles as an anticancer SN38 carrier for targeted chemotherapy. | DNA | Colon cancer | [212] | ||
| Aptamer-C1q protein conjugates for targeted immunotherapy. | DNA | Breast cancer | [215] | ||
| Mono- and multimeric targeted radiopharmaceuticals based on cyclen ligands coupled with aptamer for targeted radiotherapy. | DNA | Breast cancer | [216] | ||
| Aptamer conjugated gold coated superparamagnetic iron oxide nanoparticles for targeted photothermal therapy. | DNA | Colon cancer | [227] | ||
| Aptamer coated pDNA/PEI complexes for targeted gene therapy. | DNA | Lung cancer | [218] | ||
| MAGE | Ap52 | Phosphonothioate-modified aptamer complexed with DOX for targeted chemotherapy. | DNA | Pancreatic cancer | [219] |
| HER2 | HB5 | Aptamer conjugated mesoporous silica nanocarrier-based DOX delivery system for targeted chemotherapy. | DNA | Breast cancer | [221] |
| Aptamer functionalized curcumin-loaded human serum albumin nanoparticles for targeted chemotherapy. | DNA | Breast cancer | [220] | ||
| CD133 | CD133 | DOX-loaded aptamer for targeted chemotherapy. | DNA | Hepatocellular carcinoma | [222] |
| Aptamer-modified docetaxel liposome for targeted chemotherapy. | DNA | Lung cancer | [223] | ||
| EpCAM | EpCAM | Aptamer-functionalized mesoporous silica nanoparticles as a DOX carrier for targeted chemotherapy. | DNA | Colon cancer | [224] |
| Aptamer and quantum dots-functionalized nutlin-3a loaded poly (lactide-co-glycolide) nanoparticles for targeted chemotherapy. | DNA | Breast cancer | [225] | ||
| Aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted chemotherapy. | DNA | Colorectal cancer | [226] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhang, D.; Zeng, Z.; Huang, L.; Lin, X.; Hong, S. Aptamer-Based Probes for Cancer Diagnostics and Treatment. Life 2022, 12, 1937. https://doi.org/10.3390/life12111937
Hu X, Zhang D, Zeng Z, Huang L, Lin X, Hong S. Aptamer-Based Probes for Cancer Diagnostics and Treatment. Life. 2022; 12(11):1937. https://doi.org/10.3390/life12111937
Chicago/Turabian StyleHu, Xueqi, Dongdong Zhang, Zheng Zeng, Linjie Huang, Xiahui Lin, and Shanni Hong. 2022. "Aptamer-Based Probes for Cancer Diagnostics and Treatment" Life 12, no. 11: 1937. https://doi.org/10.3390/life12111937
APA StyleHu, X., Zhang, D., Zeng, Z., Huang, L., Lin, X., & Hong, S. (2022). Aptamer-Based Probes for Cancer Diagnostics and Treatment. Life, 12(11), 1937. https://doi.org/10.3390/life12111937







