Progesterone Promotes In Vitro Maturation of Domestic Dog Oocytes Leading to Successful Live Births
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Chemicals
2.3. Collection of Ovaries and Oocytes
2.4. In Vitro Maturation of COCs and Assessment of Oocyte Maturation
2.5. IVF
2.6. Detection of the Ovulation Date of Recipient Dogs
2.7. Embryo Transfer and Detection of Pregnancy
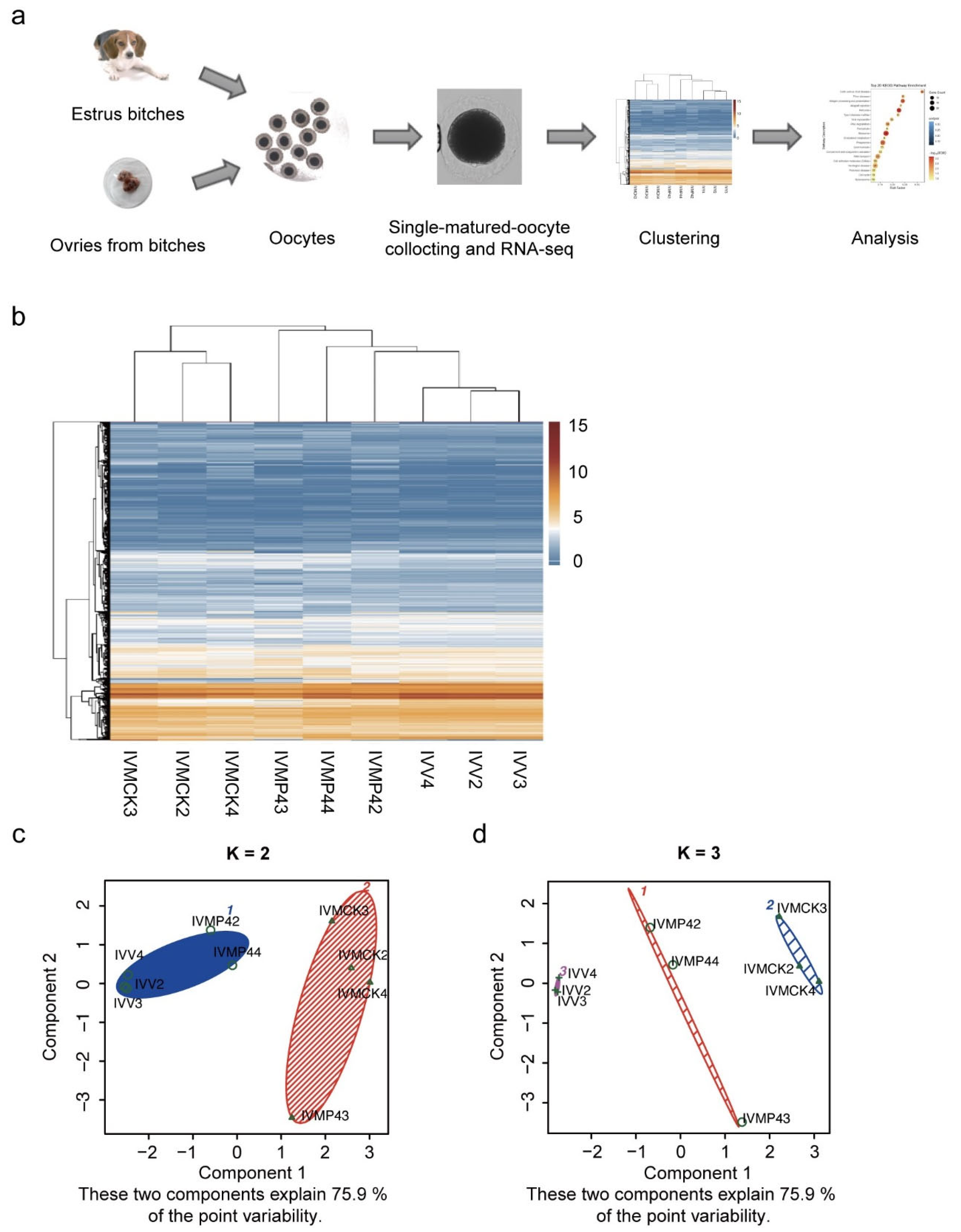
2.8. Preparation of Oocytes for scRNA-seq
2.9. Short Tandem Repeat Polymorphism Identification
2.10. Quality Control and Summary of Alignment
2.11. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Analysis
2.12. K-Means Analysis
2.13. Statistical Analysis
3. Results
3.1. Optimization of the Canine IVM Oocyte System
3.2. Birth of IVM-IVF Puppies
3.3. DEG Clustering of Mature Oocytes in Vivo and in Vitro
3.4. Differences in Gene Expression of Mature Oocytes in Vivo and in Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Wu, S.; Capecchi, M.R.; Jaenisch, R. A brief review of genome editing technology for generating animal models. Front. Agric. Sci. Eng. 2020, 7, 123–128. [Google Scholar] [CrossRef]
- Maynard, L.H.; Humbert, O.; Peterson, C.W.; Kiem, H.-P. Genome editing in large animal models. Mol. Ther. 2021, 29, 3140–3152. [Google Scholar] [CrossRef]
- Hytönen, M.K.; Lohi, H. Canine models of human rare disorders. Rare Dis. 2016, 4, e1241362. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.F. Companion Animal Medicine in the Age of Medical Genetics. J. Vet. Intern. Med. 2000, 14, 1–9. [Google Scholar] [CrossRef]
- Sargan, D.R. IDID: Inherited Diseases in Dogs: Web-based information for canine inherited disease genetics. Mamm. Genome 2004, 15, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Shearin, A.L.; Ostrander, E. Leading the way: Canine models of genomics and disease. Dis. Model. Mechanisms 2010, 3, 27–34. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Shi, H.; Yan, Q.; Zheng, M.; Li, J.; Zhang, Q.; Qin, Y.; Zhong, Y.; Mi, J.; et al. Generation of ApoE deficient dogs via combination of embryo injection of CRISPR/Cas9 with somatic cell nuclear transfer. J. Genet. Genom. 2018, 45, 47–50. [Google Scholar] [CrossRef]
- Zou, Q.J.; Wang, X.M.; Liu, Y.Z.; Ouyang, Z.; Long, H.B.; Wei, S.; Xin, J.; Zhao, B.T.; Lai, S.; Shen, J.; et al. Generation of gene-target dogs using CRISPR/Cas9 system. J. Mol. Cell Biol. 2015, 7, 580–583. [Google Scholar] [CrossRef]
- Reynaud, K.; Fontbonne, A.; Marseloo, N.; Thoumire, S.; Chebrout, M.; De Lesegno, C.V.; Chastant-Maillard, S. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction 2005, 130, 193–201. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Chebrout, M.; Thoumire, S.; Saint-Dizier, M.; Chodkiewicz, M.; Reynaud, K. Embryo biotechnology in the dog: A review. Reprod. Fertil. Dev. 2010, 22, 1049–1056. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Sylvester, S.R.; Nelson, J.L.; Cheong, S.H.; Mukai, C.; Lambo, C.; Flanders, J.A.; Meyers-Wallen, V.N.; Songsasen, N.; Travis, A.J. Live Births from Domestic Dog (Canis familiaris) Embryos Produced by In Vitro Fertilization. PLoS ONE 2015, 10, e143930. [Google Scholar] [CrossRef]
- Saikhun, J.; Sriussadaporn, S.; Thongtip, N.; Pinyopummin, A.; Kitiyanant, Y. Nuclear maturation and development of IVM/IVF canine embryos in synthetic oviductal fluid or in co-culture with buffalo rat liver cells. Theriogenology 2008, 69, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- England, G.C.; Verstegen, J.P.; Hewitt, D.A. Pregnancy following in vitro fertilisation of canine oocytes. Vet. Rec. 2001, 148, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.A.; Dos, S.L.; Rodrigues, J.L. Embryonic development of in vitro matured and in vitro fertilized dog oocytes. Mol. Reprod. Dev. 2004, 67, 215–223. [Google Scholar] [CrossRef]
- Fahiminiya, S.; Reynaud, K.; Labas, V.; Batard, S.; Chastant-Maillard, S.; Gerard, N. Steroid hormones content and proteomic analysis of canine follicular fluid during the preovulatory period. Reprod. Biol. Endocrinol. 2010, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Chastant-Maillard, S.; Viaris, D.L.C.; Chebrout, M.; Thoumire, S.; Meylheuc, T.; Fontbonne, A.; Chodkiewicz, M.; Saint-Dizier, M.; Reynaud, K. The canine oocyte: Uncommon features of in vivo and in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.Z.; Reynaud, K.; Mawa, G.; Thoumire, S.; Chastant-Maillard, S.; Saint-Dizier, M. Immunolocalization of progesterone receptors in the canine oviduct around ovulation. Reprod. Domest. Anim. 2012, 47 (Suppl. 6), 35–39. [Google Scholar] [CrossRef]
- Reynaud, K.; Saint-Dizier, M.; Tahir, M.Z.; Havard, T.; Harichaux, G.; Labas, V.; Thoumire, S.; Fontbonne, A.; Grimard, B.; Chastant-Maillard, S. Progesterone plays a critical role in canine oocyte maturation and fertilization. Biol. Reprod. 2015, 93, 87. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, C.I.; Faustino, M.; Marques, M.G.; Nichi, M.; Assumpcao, M.E.; Visintin, J.A. Effects of gonadotropin-exposed medium with high concentrations of progesterone and estradiol-17beta on in vitro maturation of canine oocytes. Vitr. Cell. Dev. Biol.-Anim. 2009, 45, 328–333. [Google Scholar] [CrossRef]
- Bezerra, F.; Paulino, L.; Silva, B.R.; Silva, A.; Souza, B.A.; Silva, J. Effects of epidermal growth factor and progesterone on oocyte meiotic resumption and the expression of maturation-related transcripts during prematuration of oocytes from small and medium-sized bovine antral follicles. Reprod. Fertil. Dev. 2020, 32, 1190–1199. [Google Scholar] [CrossRef]
- Fair, T.; Lonergan, P. The role of progesterone in oocyte acquisition of developmental competence. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 142–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, X.; Yin, S.; Yan, J.; Lv, P.; Shan, H.; Cui, K.; Liu, H.; Liu, Q. Single-Cell RNA-Seq Revealed the Gene Expression Pattern during the In Vitro Maturation of Donkey Oocytes. Genes 2021, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Meng, T.G.; Li, A.; Schatten, H.; Wang, Z.B.; Sun, Q.Y. RNA-Seq transcriptome reveals different molecular responses during human and mouse oocyte maturation and fertilization. BMC Genom. 2020, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Qu, Y.; Li, R.; Qiao, J. In Vivo and In Vitro Matured Oocytes from Mice of Advanced Reproductive Age Exhibit Alternative Splicing Processes for Mitochondrial Oxidative Phosphorylation. Front. Endocrinol. 2022, 13, 816606. [Google Scholar] [CrossRef] [PubMed]
- De Los, R.M.; Palomino, J.; Parraguez, V.H.; Hidalgo, M.; Saffie, P. Mitochondrial distribution and meiotic progression in canine oocytes during in vivo and in vitro maturation. Theriogenology 2011, 75, 346–353. [Google Scholar]
- Songsasen, N.; Wildt, D.E. Oocyte biology and challenges in developing in vitro maturation systems in the domestic dog. Anim. Reprod. Sci. 2007, 98, 2–22. [Google Scholar] [CrossRef]
- Singh, M.; Al-Eryani, G.; Carswell, S.; Ferguson, J.M.; Blackburn, J.; Barton, K.; Roden, D.; Luciani, F.; Phan, T.G.; Junankar, S.; et al. High-throughput targeted long-read single cell sequencing reveals the clonal and transcriptional landscape of lymphocytes. Nat. Commun. 2019, 10, 3120. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Ghosh, S.; Chan, C.K. Analysis of RNA-Seq Data Using TopHat and Cufflinks. Methods Mol. Biol. 2016, 1374, 339–361. [Google Scholar]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Li, M.; Wang, J.; Pan, Y. Essential Protein Discovery Based on Network Motif and Gene Ontology. In Proceedings of the 2011 IEEE International Conference on Bioinformatics and Biomedicine, Atlanta, GA, USA, 12–15 November 2011; pp. 470–475. [Google Scholar]
- Petegrosso, R.; Li, Z.; Kuang, R. Machine learning and statistical methods for clustering single-cell RNA-sequencing data. Brief Bioinform. 2020, 21, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Karre, I.; Meyer-Lindenberg, A.; Urhausen, C.; Beineke, A.; Meinecke, B.; Piechotta, M.; Beyerbach, M.; Günzel-Apel, A.-R. Distribution and viability of spermatozoa in the canine female genital tract during post-ovulatory oocyte maturation. Acta Vet. Scand. 2012, 54, 49. [Google Scholar] [CrossRef]
- Dieleman, S.J.; Bevers, M.M.; Poortman, J.; van Tol, H.T. Steroid and pituitary hormone concentrations in the fluid of preovulatory bovine follicles relative to the peak of LH in the peripheral blood. J. Reprod. Fertil. 1983, 69, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.M.; Garcia-Herreros, M.; O’Shea, L.C.; Hensey, C.; Lonergan, P.; Fair, T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol. Reprod. 2011, 84, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Fibrianto, Y.H.; Oh, H.J.; Jang, G.; Kim, H.J.; Lee, K.S.; Kang, S.K.; Lee, B.C.; Hwang, W.S. Effects of estradiol-17beta and progesterone supplementation on in vitro nuclear maturation of canine oocytes. Theriogenology 2005, 63, 1342–1353. [Google Scholar] [CrossRef]
- Willingham-Rocky, L.A.; Hinrichs, K.; Westhusin, M.E.; Kraemer, D.C. Effects of stage of oestrous cycle and progesterone supplementation during culture on maturation of canine oocytes in vitro. Reproduction 2003, 126, 501–508. [Google Scholar] [CrossRef]
- Kim, J.J.; Park, K.B.; Choi, E.J.; Hyun, S.H.; Kim, N.H.; Jeong, Y.W.; Hwang, W.S. Relationship between time post-ovulation and progesterone on oocyte maturation and pregnancy in canine cloning. Anim. Reprod. Sci. 2017, 185, 75–82. [Google Scholar] [CrossRef]
- Moawad, A.R.; Salama, A.; Badr, M.R.; Fathi, M. Beneficial Effects of L-Carnitine Supplementation during IVM of Canine Oocytes on Their Nuclear Maturation and Development In Vitro. Animals 2021, 11, 581. [Google Scholar] [CrossRef]
- Fathi, M.; Salama, A.; Badr, M.R. Improvement of the developmental competence of canine oocyte using caffeine supplementation during IVM at different maturation time. Zygote 2018, 26, 162–167. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Kim, G.A.; Choi, Y.B.; Jo, Y.K.; Setyawan, E.; Lee, B.C. Oocyte maturation-related gene expression in the canine oviduct, cumulus cells, and oocytes and effect of co-culture with oviduct cells on in vitro maturation of oocytes. J. Assist. Reprod. Genet. 2017, 34, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Aspee, K.; Ramirez, G.; Dettleff, P.; Palomino, J.; Peralta, O.A.; Parraguez, V.H.; De Los, R.M. Influence of growth differentiation factor 9 and bone morphogenetic protein 15 on in vitro maturation of canine oocytes. Reprod. Domest. Anim. 2019, 54, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.X.; Gilchrist, R.B.; Thompson, J.G.; Lane, M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum. Reprod. 2008, 23, 67–73. [Google Scholar] [CrossRef]
- Christians, E.; Davis, A.A.; Thomas, S.D.; Benjamin, I.J. Maternal effect of Hsf1 on reproductive success. Nature 2000, 407, 693–694. [Google Scholar] [CrossRef]
- Dayalan, N.S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627. [Google Scholar] [CrossRef]
- Wu, X.; Viveiros, M.M.; Eppig, J.J.; Bai, Y.; Fitzpatrick, S.L.; Matzuk, M.M. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat. Genet. 2003, 33, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.H.; Viveiros, M.M.; Ren, Y.; Wang, P.; DeMayo, F.J.; Frail, D.E.; Eppig, J.J.; Matzuk, M.M. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 2003, 300, 633–636. [Google Scholar] [CrossRef]
- Oh, B.; Hwang, S.; Solter, D.; Knowles, B.B. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 1997, 124, 493–503. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Litscher, E.S. The Mouse Egg’s Zona Pellucida. Curr. Top. Dev. Biol. 2018, 130, 331–356. [Google Scholar]
- Wang, Y.; Li, Y.; Zhang, B.; Zhang, F. The preclinical evaluation of immunocontraceptive vaccines based on canine zona pellucida 3 (cZP3) in a mouse model. Reprod. Biol. Endocrinol. 2018, 16, 47. [Google Scholar] [CrossRef]
- Avella, M.A.; Baibakov, B.A.; Jimenez-Movilla, M.; Sadusky, A.B.; Dean, J. ZP2 peptide beads select human sperm in vitro, decoy mouse sperm in vivo, and provide reversible contraception. Sci. Transl. Med. 2016, 8, 336ra60. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. The Human Egg’s Zona Pellucida. Curr. Top. Dev. Biol. 2018, 130, 379–411. [Google Scholar] [PubMed]
- Rankin, T.; Familari, M.; Lee, E.; Ginsberg, A.; Dwyer, N.; Blanchette-Mackie, J.; Drago, J.; Westphal, H.; Dean, J. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 1996, 122, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.L.; O’Brien, M.; Lee, E.; Wigglesworth, K.; Eppig, J.; Dean, J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 2001, 128, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Sadler, S.E.; Maller, J.L. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J. Biol. Chem. 1981, 256, 6368–6373. [Google Scholar]
- Ferrell, J.J. Xenopus oocyte maturation: New lessons from a good egg. BioEssays 1999, 21, 833–842. [Google Scholar] [CrossRef]
- Andersen, C.B.; Roth, R.A.; Conti, M. Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J. Biol. Chem. 1998, 273, 18705–18708. [Google Scholar] [CrossRef]
- Tokai, N.; Fujimoto-Nishiyama, A.; Toyoshima, Y.; Yonemura, S.; Tsukita, S.; Inoue, J.; Yamamota, T. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J. 1996, 15, 457–467. [Google Scholar] [CrossRef]
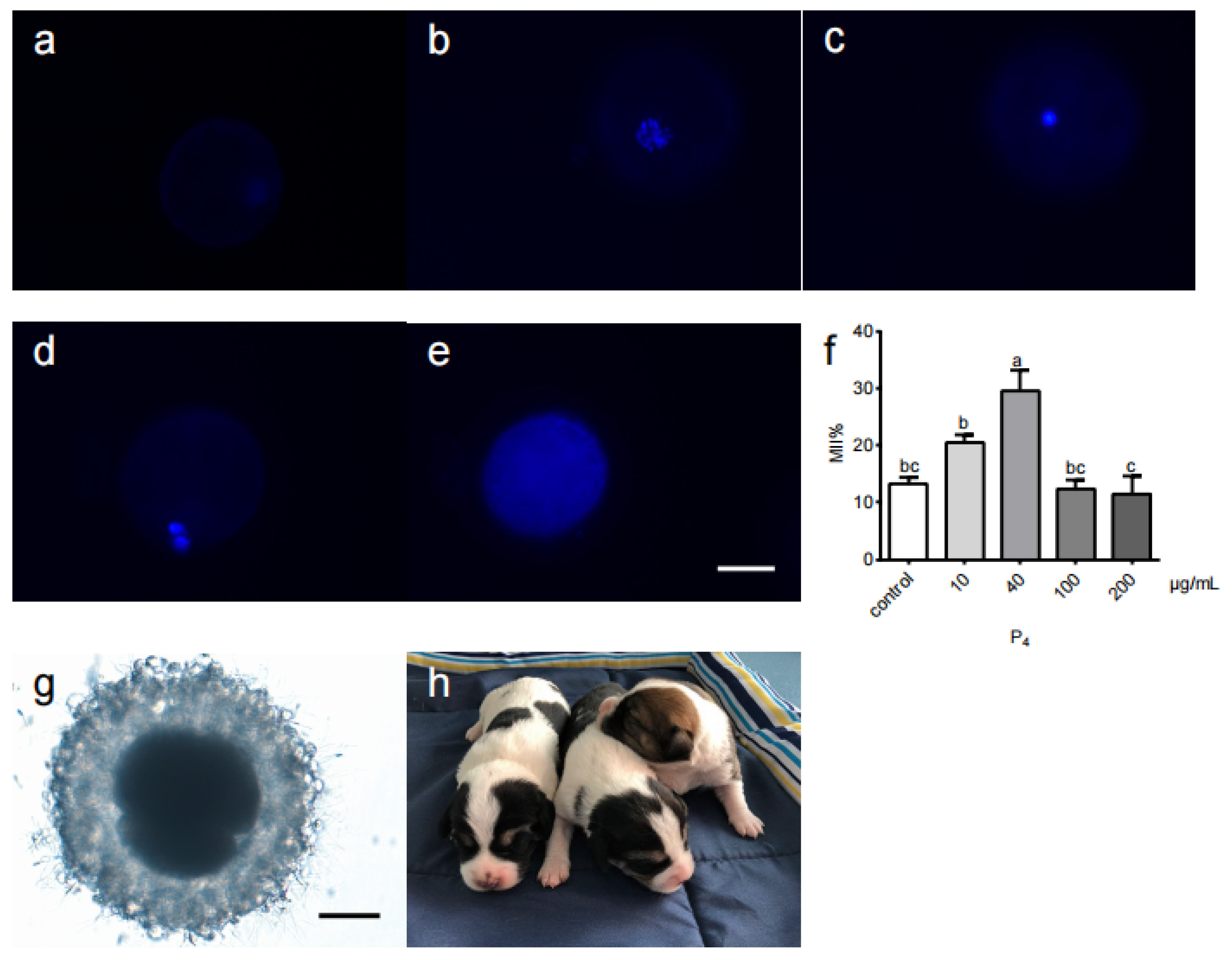

| P4 Concentration (μg/mL) | No. of Oocytes Examined | No. of Oocytes at Various Meiotic Stages | ||||
|---|---|---|---|---|---|---|
| GV% | GVBD% | MI% | MII% | DE% | ||
| 0 | 118 | 3.8 ± 3.3 | 6.2 ± 5.6 | 19.4 ± 12.8 | 13.2 ± 2.1 bc | 57.5 ± 5.4 |
| 10 | 71 | 10.8 ± 4.1 | 17.4 ± 4.3 | 19.7 ± 11.2 | 20.4 ± 2.7 b | 29.7 ± 1.4 |
| 40 | 90 | 9.9 ± 2.7 | 10.7 ± 6.1 | 21.9 ± 6.7 | 29.7 ± 7.1 a | 27.8 ± 7.3 |
| 100 | 84 | 16.7 ± 7.3 | 18.0 ± 7.0 | 21.7 ± 8.8 | 12.3 ± 4.0 bc | 30.9 ± 5.7 |
| 200 | 224 | 8.7 ± 4.7 | 12.0 ± 6.8 | 25.7 ± 7.0 | 11.5 ± 7.7 c | 44.9 ± 5.0 |
| Group | Treatment (n) | Cleavage (n) | 2-Cell (n) | 4-Cell to 8-Cell (n) | 8-Cell (n) |
|---|---|---|---|---|---|
| In vivo | 29 | 26 | 6 | 13 | 7 |
| In vitro (40 μg/mL P4) | 29 | 22 | 6 | 12 | 4 |
| Group | Stage | Transplant Location | Embryo (n) | Pregnant (25 d) (n) | Birth (n) |
|---|---|---|---|---|---|
| In vivo | 2-8 cell | oviduct | 7 | 6 | 6 |
| 2-4 cell | oviduct | 2 | 0 | 0 | |
| In vitro | 2-4 cell | oviduct | 6 | 0 | 0 |
| 2-8 cell | oviduct | 10 | 4 | 3 |
| A_vs_B | NO_DEG | NO_DEG_UpInA | NO_DEG_UpInB |
|---|---|---|---|
| IVMCK_vs_IVV | 4366 | 2365 | 2000 |
| IVMP4_vs_IVMCK | 1233 | 701 | 531 |
| IVMP4_vs_IVV | 2590 | 1443 | 1146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Feng, S.; Zheng, M.; Liu, X.; Zhao, J.; Zhao, Q.; Ye, J.; Mi, J.; Zhong, Y. Progesterone Promotes In Vitro Maturation of Domestic Dog Oocytes Leading to Successful Live Births. Life 2022, 12, 1778. https://doi.org/10.3390/life12111778
Qin Y, Feng S, Zheng M, Liu X, Zhao J, Zhao Q, Ye J, Mi J, Zhong Y. Progesterone Promotes In Vitro Maturation of Domestic Dog Oocytes Leading to Successful Live Births. Life. 2022; 12(11):1778. https://doi.org/10.3390/life12111778
Chicago/Turabian StyleQin, Yumin, Shenjiong Feng, Min Zheng, Xiaojuan Liu, Jianping Zhao, Qintao Zhao, Junhua Ye, Jidong Mi, and Yougang Zhong. 2022. "Progesterone Promotes In Vitro Maturation of Domestic Dog Oocytes Leading to Successful Live Births" Life 12, no. 11: 1778. https://doi.org/10.3390/life12111778
APA StyleQin, Y., Feng, S., Zheng, M., Liu, X., Zhao, J., Zhao, Q., Ye, J., Mi, J., & Zhong, Y. (2022). Progesterone Promotes In Vitro Maturation of Domestic Dog Oocytes Leading to Successful Live Births. Life, 12(11), 1778. https://doi.org/10.3390/life12111778





