Diagnostic and Therapeutic Implications of Long Non-Coding RNAs in Leukemia
Abstract
Simple Summary
Abstract
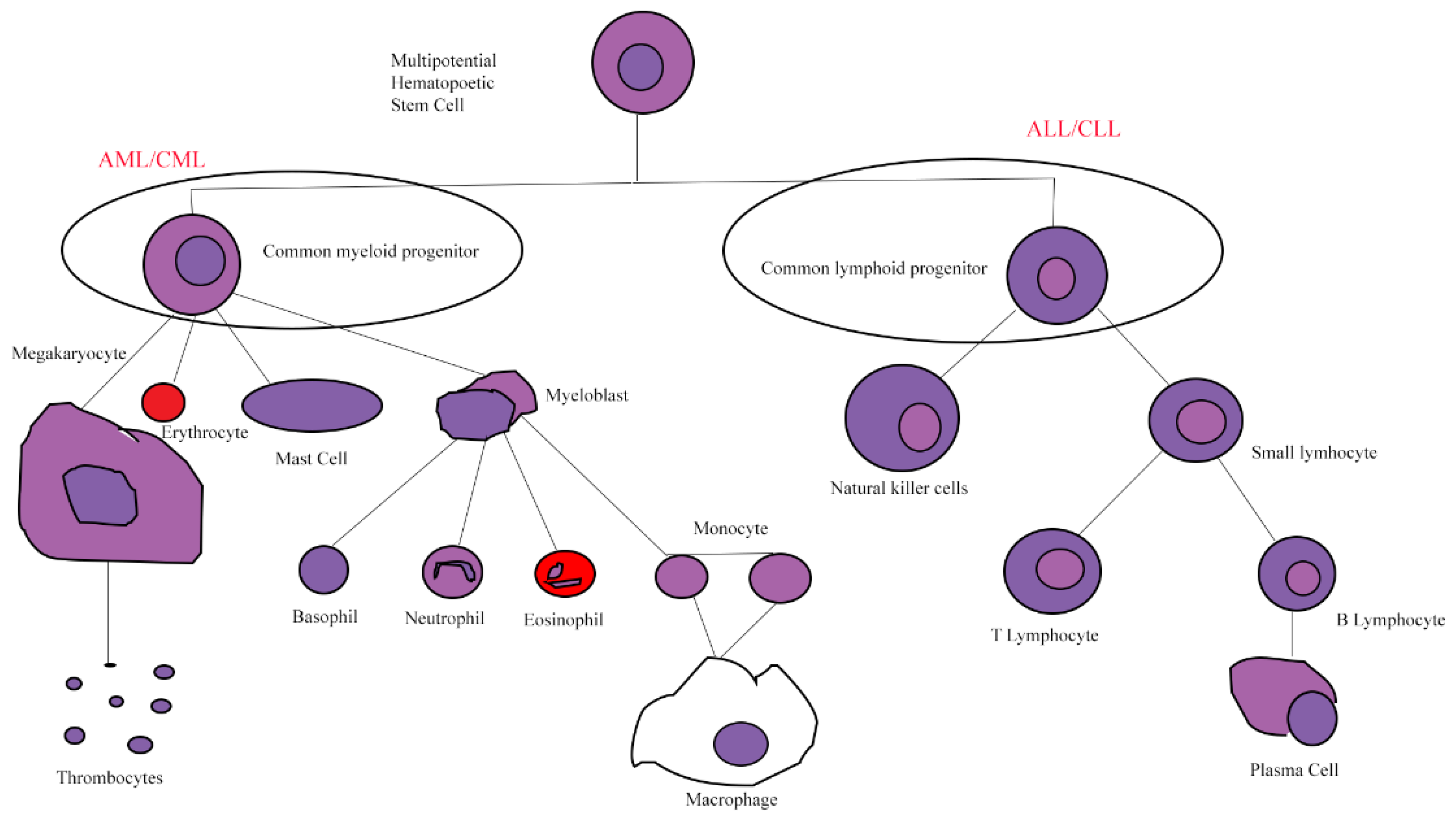
1. Leukemia
1.1. Acute Myeloid Leukemia
1.2. Acute Lymphoblastic Leukemia
1.3. Chronic Myeloid Leukemia
1.4. Chronic Lymphocytic Leukemia
2. Long Noncoding RNAs
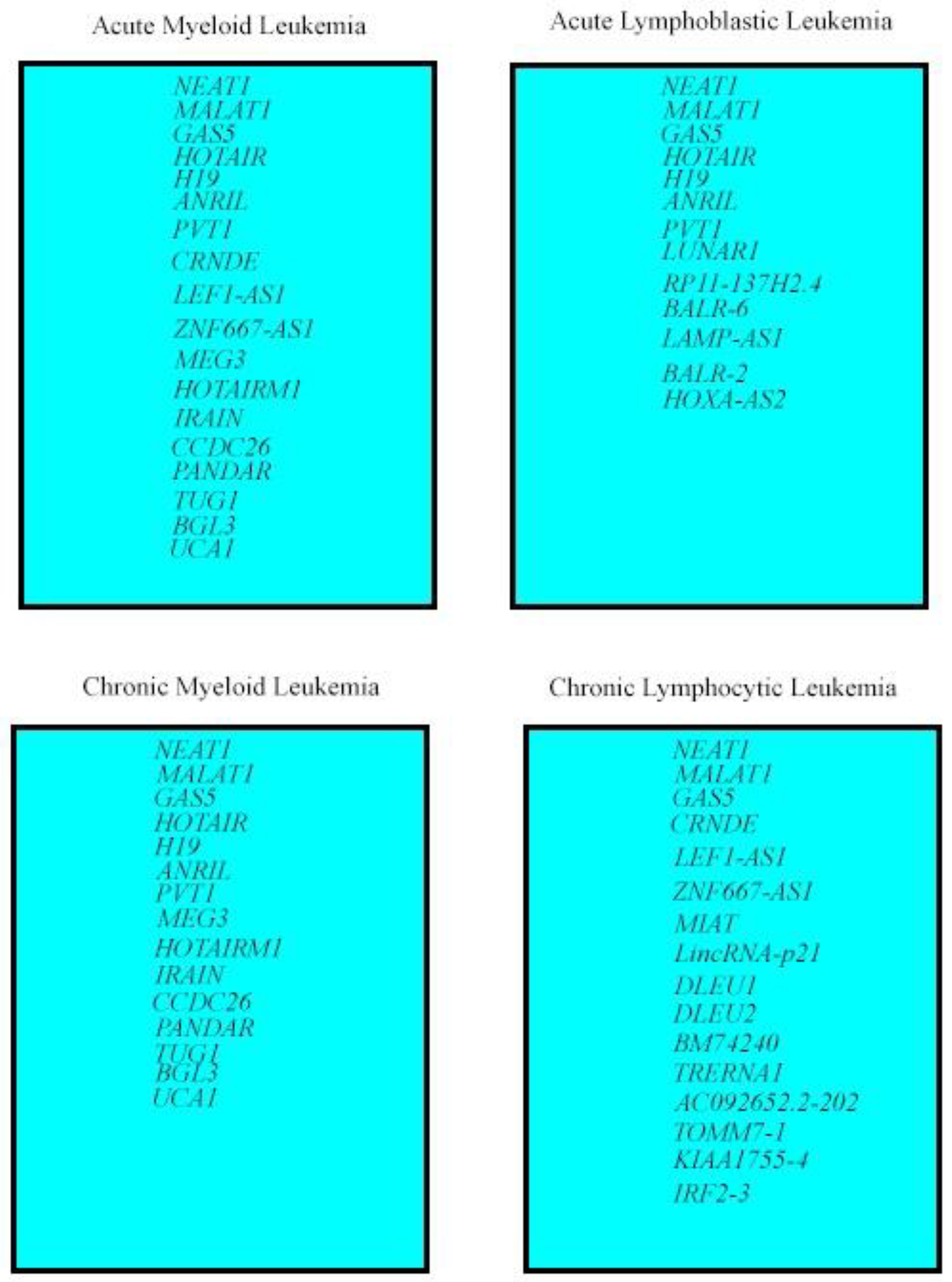
3. LncRNAs Deregulated in All Leukemia Types
3.1. LncRNA NEAT1
3.2. LncRNA MALAT1
3.3. LncRNA GAS5
4. LncRNA Deregulated in Myeloid Leukemias and ALL
4.1. LncRNA HOTAIR
4.2. LncRNA H19
4.3. LncRNA ANRIL (CDKN2B-AS1)
4.4. LncRNA PVT1
5. LncRNAs Deregulated in AML and CLL
5.1. LncRNA CRNDE
5.2. LncRNA LEF1-AS1
5.3. LncRNA ZNF667-AS1
6. LncRNAs Deregulated in Myeloid Leukemias
6.1. LncRNA MEG3
6.2. LncRNA HOTAIRM1
6.3. LncRNA IRAIN
6.4. LncRNA CCDC26
6.5. LncRNA PANDAR
6.6. LncRNA TUG1
6.7. LncRNA BGL3
6.8. LncRNA UCA1
7. LncRNAs Deregulated in ALL
7.1. Lnc RNA LUNAR1
7.2. Lnc RNA RP11-137H2.4
7.3. Linc RNA BALR-6
7.4. LncRNA LAMP-AS1
7.5. LncRNA BALR-2
7.6. LncRNA HOXA-AS2
8. LncRNAs Deregulated in CLL
8.1. LncRNA MIAT
8.2. LincRNA-p21
8.3. LncRNA DLEU1 and LncRNA DLEU2
8.4. LncRNA BM74240
8.5. LncRNA TRERNA1
8.6. LncRNA AC092652.2-202
8.7. Lnc-TOMM7-1, lnc-KIAA1755-4, lnc-IRF2-3, lncRNA ZNF667-AS1
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollyea, D.A.; Kohrt, H.E.; Medeiros, B.C. Acute myeloid leukaemia in the elderly: A review. Br. J. Haematol. 2011, 152, 524–542. [Google Scholar] [CrossRef] [PubMed]
- Elgarten, C.W.; Aplenc, R. Pediatric acute myeloid leukemia: Updates on biology, risk stratification, and therapy. Curr. Opin. Pediatrics 2020, 32, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K.; Lichtman, M.A.; Prchal, J.; Levi, M.M.; Press, O.W.; Burns, L.J.; Caligiuri, M. Williams Hematology, 9th ed.; McGraw Hill: New York, NY, USA, 2016. [Google Scholar]
- Fornerod, M.; Ma, J.; Noort, S.; Liu, Y.; Walsh, M.P.; Shi, L.; Nance, S.; Liu, Y.; Wang, Y.; Song, G.; et al. Integrative Genomic Analysis of Pediatric Myeloid-Related Acute Leukemias Identifies Novel Subtypes and Prognostic Indicators. Blood Cancer Discov. 2021, 2, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.S.; Yilmaz, M.; Kanagal-Shamanna, R.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Konopleva, M.; Pierce, S.A.; Wang, S.A.; et al. Patterns of Resistance Differ in Patients with Acute Myeloid Leukemia Treated with Type I versus Type II FLT3 inhibitors. Blood Cancer Discov. 2021, 2, 125–134. [Google Scholar] [CrossRef]
- Yan, D.; Franzini, A.; Pomicter, A.D.; Halverson, B.J.; Antelope, O.; Mason, C.C.; Ahmann, J.M.; Senina, A.V.; Vellore, N.A.; Jones, C.L.; et al. SIRT5 is a druggable metabolic vulnerability in acute myeloid leukemia. Blood Cancer Discov. 2021, 2, 266–287. [Google Scholar] [CrossRef]
- Byrd, J.C.; Mrózek, K.; Dodge, R.K.; Carroll, A.J.; Edwards, C.G.; Arthur, D.C.; Pettenati, M.J.; Patil, S.R.; Rao, K.W.; Watson, M.S.; et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002, 100, 4325–4336. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Yun, S.; Vincelette, N.D.; Yu, X.; Watson, G.W.; Fernandez, M.R.; Yang, C.; Hitosugi, T.; Cheng, C.H.; Freischel, A.R.; Zhang, L.; et al. TFEB links MYC signaling to epigenetic control of myeloid differentiation and acute myeloid leukemia. Blood Cancer Discov. 2021, 2, 162–185. [Google Scholar] [CrossRef]
- Marjanovic, I.; Karan-Djurasevic, T.; Ugrin, M.; Virijević, M.; Vidović, A.; Tomin, D.; Vuković, N.S.; Pavlovic, S.; Tosic, N. Use of Wilms Tumor 1 Gene Expression as a Reliable Marker for Prognosis and Minimal Residual Disease Monitoring in Acute Myeloid Leukemia with Normal Karyotype Patients. Clin. Lymphoma Myeloma Leuk. 2017, 17, 312–319. [Google Scholar] [CrossRef]
- Marjanovic, I.; Karan-Djurasevic, T.; Kostic, T.; Virijevic, M.; Vukovic, N.S.; Pavlovic, S.; Tosic, N. Prognostic significance of combined BAALC and MN1 gene expression level in acute myeloid leukemia with normal karyotype. Int. J. Lab. Hematol. 2021, 43, 433–440. [Google Scholar] [CrossRef]
- Handschuh, L. Not Only Mutations Matter: Molecular Picture of Acute Myeloid Leukemia Emerging from Transcriptome Studies. J. Oncol. 2019, 2019, 7239206. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, A.M.; Bertrums, E.J.M.; van Roosmalen, M.J.; Hofman, D.A.; Oka, R.; Verheul, M.; Manders, F.; Ubels, J.; Belderbos, M.E.; van Boxtel, R. Mutation signatures of pediatric acute myeloid leukemia and normal blood progenitors associated with differential patient outcomes. Blood Cancer Discov. 2021, 2, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Q.; Chen, C.J.; Jing, Y.; Qin, J.Y.; Li, Y.; Chen, G.F.; Zhou, W.; Li, Y.H.; Wang, J.; Li, D.W.; et al. Characteristics and prognostic significance of genetic mutations in acute myeloid leukemia based on a targeted next-generation sequencing technique. Cancer Med. 2020, 9, 8457–8467. [Google Scholar] [CrossRef]
- Marjanovic, I.; Kostic, J.; Stanic, B.; Pejanovic, N.; Lucic, B.; Karan-Djurasevic, T.; Janic, D.; Dokmanovic, L.; Jankovic, S.; Vukovic, N.S.; et al. Parallel targeted next generation sequencing of childhood and adult acute myeloid leukemia patients reveals uniform genomic profile of the disease. Tumour Biol. 2016, 37, 13391–13401. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.W.; Ghannam, J.; Nosiri, C.; Gui, G.; Goswami, M.; Calvo, K.R.; Lindblad, K.E.; Oetjen, K.A.; Wilkerson, M.D.; Soltis, A.R.; et al. Personalized Single-Cell Proteogenomics to Distinguish Acute Myeloid Leukemia from Non-Malignant Clonal Hematopoiesis. Blood Cancer Discov. 2021, 2, 319–325. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.M. Acute lymphoblastic leukemia. A comprehensive review with emphasis on biology and therapy. Cancer 1995, 76, 2393–2417. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Collins-Underwood, J.R.; Phillips, L.A.; Loudin, M.G.; Liu, W.; Zhang, J.; Ma, J.; Coustan-Smith, E.; Harvey, R.C.; Willman, C.L.; et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat. Genet. 2009, 41, 1243–1246. [Google Scholar] [CrossRef]
- Jeha, S.; Choi, J.; Roberts, K.G.; Pei, D.; Coustan-Smith, E.; Inaba, H.; Rubnitz, J.E.; Ribeiro, R.C.; Gruber, T.A.; Raimondi, S.C.; et al. Clinical significance of novel subtypes of acute lymphoblastic leukemia in the context of minimal residual disease-directed therapy. Blood Cancer Discov. 2021, 2, 326–337. [Google Scholar] [CrossRef]
- Cordo, V.; van der Zwet, J.C.G.; Canté-Barrett, K.; Pieters, R.; Meijerink, J.P.P. T-cell Acute Lymphoblastic Leukemia: A Roadmap to Targeted Therapies. Blood Cancer Discov. 2021, 2, 19–31. [Google Scholar] [CrossRef]
- Mullighan, C. The molecular genetic makeup of acute lymphoblastic leukemia. In Hematology 2010, the American Society of Hematology Education Program Book; American Society of Hematology: Washington, DC, USA, 2012; Volume 2012, pp. 389–396. [Google Scholar] [CrossRef]
- Iacobucci, I.; Mullighan, C.G. Genetic Basis of Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2017, 35, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Pulsipher, M.A.; Han, X.; Maude, S.L.; Laetsch, T.W.; Qayed, M.; Rives, S.; Boyer, M.W.; Hiramatsu, H.; Yanik, G.A.; Driscoll, T.; et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022, 3, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Gasic, V.; Zukic, B.; Stankovic, B.; Janic, D.; Dokmanovic, L.; Lazic, J.; Krstovski, N.; Dolzan, V.; Jazbec, J.; Pavlovic, S.; et al. Pharmacogenomic markers of glucocorticoid response in the initial phase of remission induction therapy in childhood acute lymphoblastic leukemia. Radiol. Oncol. 2018, 52, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Mughal, T.I.; Abdel-Wahab, O.; Rampal, R.; Mesa, R.; Koschmieder, S.; Levine, R.; Hehlmann, R.; Saglio, G.; Barbui, T.; Van Etten, R.A. Contemporary insights into the pathogenesis and treatment of chronic myeloproliferative neoplasms. Leuk. Lymphoma 2016, 57, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.D.; Barnes, D. Chronic myeloid leukemia as model of disease evolution in human cancer. Nat. Rev. Cancer 2007, 7, 441–453. [Google Scholar] [CrossRef]
- Schindler, T.; Bornmann, W.; Pellicena, P.; Miller, W.T.; Clarkson, B.; Kuriyan, J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 2000, 289, 1938–1942. [Google Scholar] [CrossRef]
- Wei, G.; Rafiyath, S.; Liu, D. First-line treatment for chronic myeloid leukemia: Dasatinib, nilotinib, or imatinib. J. Hematol. Oncol. 2010, 3, 47. [Google Scholar] [CrossRef]
- Chereda, B.; Melo, J.V. Natural course and biology of CML. Ann. Hematol. 2015, 94 (Suppl. 2), S107–S121. [Google Scholar] [CrossRef]
- Poudel, G.; Tolland, M.G.; Hughes, T.P.; Pagani, I.S. Mechanisms of Resistance and Implications for Treatment Strategies in Chronic Myeloid Leukaemia. Cancers 2022, 14, 3300. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef]
- Delgado, J.; Nadeu, F.; Colomer, D.; Campo, E. Chronic lymphocytic leukemia: From molecular pathogenesis to novel therapeutic strategies. Haematologica 2020, 105, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.K.; Forconi, F.; Packham, G. The meaning and relevance of B-cell receptor structure and function in chronic lymphocytic leukemia. Semin. Hematol. 2014, 51, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, L.; Wierzbinska, J.A.; Plass, C.; Rosenquist, R. Epigenetic deregulation in chronic lymphocytic leukemia: Clinical and biological impact. Semin. Cancer Biol. 2018, 51, 1–11. [Google Scholar] [CrossRef]
- Puente, X.S.; Beà, S.; Valdés-Mas, R.; Villamor, N.; Gutiérrez-Abril, J.; Martín-Subero, J.I.; Munar, M.; Rubio-Pérez, C.; Jares, P.; Aymerich, M.; et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 2015, 526, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Juracek, J.; Calin, G. Non-Coding RNAs as Cancer Hallmarks in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2020, 21, 6720. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: An updated database dedicated to long non-coding RNA annotation in both animals and plants. Nucleic Acids Res. 2021, 49, D165–D171. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- St Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of long noncoding RNA classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef]
- Ørom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Núñez-Martínez, H.N.; Recillas-Targa, F. Emerging Functions of lncRNA Loci beyond the Transcript Itself. Int. J. Mol. Sci. 2022, 23, 6258. [Google Scholar] [CrossRef]
- Bhan, A.; Mandal, S.S. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 2015, 1856, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef]
- Nandwani, A.; Rathore, S.; Datta, M. LncRNAs in cancer: Regulatory and therapeutic implications. Cancer Lett. 2021, 501, 162–171. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Wu, P.; Chen, Y.; Jia, Y. Aberrant LncRNA Expression in Leukemia. J. Cancer 2020, 11, 4284–4296. [Google Scholar] [CrossRef]
- Sasaki, Y.T.; Ideue, T.; Sano, M.; Mituyama, T.; Hirose, T. MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc. Natl. Acad. Sci. USA 2009, 106, 2525–2530. [Google Scholar] [CrossRef]
- Souquere, S.; Beauclair, G.; Harper, F.; Fox, A.; Pierron, G. Highly ordered spatial organization of the structural long noncoding NEAT1 RNAs within paraspeckle nuclear bodies. Mol. Biol. Cell 2010, 21, 4020–4027. [Google Scholar] [CrossRef]
- Mao, Y.S.; Sunwoo, H.; Zhang, B.; Spector, D.L. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat. Cell Biol. 2011, 13, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, C.; Rambow, F.; Bervoets, G.; Silla, T.; Mito, M.; Chiba, T.; Asahara, H.; Hirose, T.; Nakagawa, S.; Jensen, T.H.; et al. The long noncoding RNA NEAT1_1 is seemingly dispensable for normal tissue homeostasis and cancer cell growth. RNA 2019, 25, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Hayashi, T.; Agata, K.; Takeichi, M.; Nakagawa, S. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J. Cell Sci. 2007, 120, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Carmichael, G.G. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: Functional role of a nuclear noncoding RNA. Mol. Cell 2009, 35, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Yamazaki, T.; Hirose, T. Molecular dissection of nuclear paraspeckles: Towards understanding the emerging world of the RNP milieu. Open Biol. 2018, 8. [Google Scholar] [CrossRef]
- Blume, C.J.; Hotz-Wagenblatt, A.; Hüllein, J.; Sellner, L.; Jethwa, A.; Stolz, T.; Slabicki, M.; Lee, K.; Sharathchandra, A.; Benner, A.; et al. p53-dependent non-coding RNA networks in chronic lymphocytic leukemia. Leukemia 2015, 29, 2015–2023. [Google Scholar] [CrossRef]
- Adriaens, C.; Standaert, L.; Barra, J.; Latil, M.; Verfaillie, A.; Kalev, P.; Boeckx, B.; Wijnhoven, P.W.; Radaelli, E.; Vermi, W.; et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat. Med. 2016, 22, 861–868. [Google Scholar] [CrossRef]
- Li, K.; Yao, T.; Zhang, Y.; Li, W.; Wang, Z. NEAT1 as a competing endogenous RNA in tumorigenesis of various cancers: Role, mechanism and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 3428–3440. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; Zhao, Y.; Du, F.; Wang, W.; Lv, P.; Qi, L. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J. Cell Physiol. 2019, 234, 6161–6172. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, S.; Lu, S.; Yu, X.; Lai, J.; Wu, Y.; Chen, S.; Wang, L.; Yu, Z.; Luo, G.; et al. The c-Myc-regulated lncRNA NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML cells. Mol. Cancer 2018, 17, 130. [Google Scholar] [CrossRef]
- Rostami, M.; Kharajo, R.S.; Parsa-Kondelaji, M.; Ayatollahi, H.; Sheikhi, M.; Keramati, M.R. Altered expression of NEAT1 variants and P53, PTEN, and BCL-2 genes in patients with acute myeloid leukemia. Leuk. Res. 2022, 115, 106807. [Google Scholar] [CrossRef] [PubMed]
- Pouyanrad, S.; Rahgozar, S.; Ghodousi, E.S. Dysregulation of miR-335-3p, targeted by NEAT1 and MALAT1 long non-coding RNAs, is associated with poor prognosis in childhood acute lymphoblastic leukemia. Gene 2019, 692, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, J.; Wang, Q.; Ren, C. Overexpression of lncRNA NEAT1 mitigates multidrug resistance by inhibiting ABCG2 in leukemia. Oncol. Lett. 2016, 12, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Reavie, L.; Buckley, S.M.; Loizou, E.; Takeishi, S.; Aranda-Orgilles, B.; Ndiaye-Lobry, D.; Abdel-Wahab, O.; Ibrahim, S.; Nakayama, K.I.; Aifantis, I. Regulation of c-Myc ubiquitination controls chronic myelogenous leukemia initiation and progression. Cancer Cell 2013, 23, 362–375. [Google Scholar] [CrossRef]
- Ronchetti, D.; Favasuli, V.; Monti, P.; Cutrona, G.; Fabris, S.; Silvestris, I.; Agnelli, L.; Colombo, M.; Menichini, P.; Matis, S.; et al. NEAT1 Long Isoform Is Highly Expressed in Chronic Lymphocytic Leukemia Irrespectively of Cytogenetic Groups or Clinical Outcome. Noncoding RNA 2020, 6, 11. [Google Scholar] [CrossRef]
- Hutchinson, J.N.; Ensminger, A.W.; Clemson, C.M.; Lynch, C.R.; Lawrence, J.B.; Chess, A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genom. 2007, 8, 39. [Google Scholar] [CrossRef]
- Brown, J.A.; Bulkley, D.; Wang, J.; Valenstein, M.L.; Yario, T.A.; Steitz, T.A.; Steitz, J.A. Structural insights into the stabilization of MALAT1 noncoding RNA by a bipartite triple helix. Nat. Struct. Mol. Biol. 2014, 21, 633–640. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Amodio, N.; Raimondi, L.; Juli, G.; Stamato, M.A.; Caracciolo, D.; Tagliaferri, P.; Tassone, P. MALAT1: A druggable long non-coding RNA for targeted anti-cancer approaches. J. Hematol. Oncol. 2018, 11, 63. [Google Scholar] [CrossRef]
- Tripathi, V.; Shen, Z.; Chakraborty, A.; Giri, S.; Freier, S.M.; Wu, X.; Zhang, Y.; Gorospe, M.; Prasanth, S.G.; Lal, A.; et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013, 9, e1003368. [Google Scholar] [CrossRef]
- Huang, J.L.; Liu, W.; Tian, L.H.; Chai, T.T.; Liu, Y.; Zhang, F.; Fu, H.Y.; Zhou, H.R.; Shen, J.Z. Upregulation of long non-coding RNA MALAT-1 confers poor prognosis and influences cell proliferation and apoptosis in acute monocytic leukemia. Oncol. Rep. 2017, 38, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Q.; Zhu, J.; Fu, J.; Chen, Y.-X. Prognostic value of miR-96 in patients with acute myeloid leukemia. Diagn. Pathol. 2014, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Chen, L.; Wang, C.; Zhao, H. MALAT1 knockdown inhibits proliferation and enhances cytarabine chemosensitivity by upregulating miR-96 in acute myeloid leukemia cells. Biomed. Pharm. 2019, 112, 108720. [Google Scholar] [CrossRef]
- Song, Y.; Guo, N.H.; Zheng, J.F. LncRNA-MALAT1 regulates proliferation and apoptosis of acute lymphoblastic leukemia cells via miR-205-PTK7 pathway. Pathol. Int. 2020, 70, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Cao, Y.X.; Luo, Z.Y.; Liao, P.; Lu, Z.W. LncRNA MALAT1 promotes cell proliferation and imatinib resistance by sponging miR-328 in chronic myelogenous leukemia. Biochem. Biophys. Res. Commun. 2018, 507, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Kaviani, S.; Yaghmaie, M.; Pashaiefar, H.; Ahmadvand, M.; Jalili, M.; Alimoghaddam, K.; Eslamijouybari, M.; Ghavamzadeh, A. Altered expression of MALAT1 lncRNA in chronic lymphocytic leukemia patients, correlation with cytogenetic findings. Blood Res. 2018, 53, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, X.; Zhu, Q.; Li, Q.; Liu, Y.; Yao, Y.; Song, Y. The growth arrest-specific transcript 5 (GAS5): A pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol. 2016, 37, 1437–1444. [Google Scholar] [CrossRef]
- Williams, G.T.; Mourtada-Maarabouni, M.; Farzaneh, F. A critical role for non-coding RNA GAS5 in growth arrest and rapamycin inhibition in human T-lymphocytes. Biochem. Soc. Trans. 2011, 39, 482–486. [Google Scholar] [CrossRef]
- Pickard, M.R.; Williams, G.T. Molecular and Cellular Mechanisms of Action of Tumour Suppressor GAS5 LncRNA. Genes 2015, 6, 484–499. [Google Scholar] [CrossRef]
- Mazar, J.; Rosado, A.; Shelley, J.; Marchica, J.; Westmoreland, T.J. The long non-coding RNA GAS5 differentially regulates cell cycle arrest and apoptosis through activation of BRCA1 and p53 in human neuroblastoma. Oncotarget 2017, 8, 6589–6607. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.Y. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, D.-Y.; Li, X.; Yuan, X.-Q.; Yang, Y.-L.; Zhu, K.-W.; Zeng, H.; Li, X.-L.; Cao, S.; Zhou, H.-H.; et al. Long non-coding RNA GAS5 polymorphism predicts a poor prognosis of acute myeloid leukemia in Chinese patients via affecting hematopoietic reconstitution. Leuk. Lymphoma 2016, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Tosic, N.; Zukic, B.; Pravdic, Z.; Vukovic, N.S.; Pavlovic, S.; Gasic, V. Expression Profiles of Long Non-Coding RNA GAS5 and MicroRNA-222 in Younger AML Patients. Diagnostics 2021, 12, 86. [Google Scholar] [CrossRef]
- Gasic, V.; Stankovic, B.; Zukic, B.; Janic, D.; Dokmanovic, L.; Krstovski, N.; Lazic, J.; Milosevic, G.; Lucafò, M.; Stocco, G.; et al. Expression Pattern of Long Non-coding RNA Growth Arrest-specific 5 in the Remission Induction Therapy in Childhood Acute Lymphoblastic Leukemia. J. Med. Biochem. 2019, 38, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, M.; Marmarinos, A.; Kossiva, L.; Baka, M.; Doganis, D.; Servitzoglou, M.; Tsolia, M.; Scorilas, A.; Avgeris, M.; Gourgiotis, D. Overexpression of the GR Riborepressor LncRNA GAS5 Results in Poor Treatment Response and Early Relapse in Childhood B-ALL. Cancers 2021, 13, 6064. [Google Scholar] [CrossRef]
- Jing, Z.; Gao, L.; Wang, H.; Chen, J.; Nie, B.; Hong, Q. Long non-coding RNA GAS5 regulates human B lymphocytic leukaemia tumourigenesis and metastasis by sponging miR-222. Cancer Biomark. 2019, 26, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Frenquelli, M.; Muzio, M.; Scielzo, C.; Fazi, C.; Scarfò, L.; Rossi, C.; Ferrari, G.; Ghia, P.; Caligaris-Cappio, F. MicroRNA and proliferation control in chronic lymphocytic leukemia: Functional relationship between miR-221/222 cluster and p27. Blood 2010, 115, 3949–3959. [Google Scholar] [CrossRef]
- Bomben, R.; Roisman, A.; D’Agaro, T.; Castellano, G.; Baumann, T.; Delgado, J.; López-Guillermo, A.; Zucchetto, A.; Dal-Bo, M.; Bravin, V.; et al. Expression of the transcribed ultraconserved region 70 and the related long non-coding RNA AC092652.2-202 has prognostic value in Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2019, 184, 1045–1050. [Google Scholar] [CrossRef]
- Yap, K.L.; Li, S.; Muñoz-Cabello, A.M.; Raguz, S.; Zeng, L.; Mujtaba, S.; Gil, J.; Walsh, M.J.; Zhou, M.M. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell 2010, 38, 662–674. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, K.; Yin, Y.; Luo, Z. Long non-coding RNA ANRIL is a potential indicator of disease progression and poor prognosis in acute myeloid leukemia. Mol. Med. Rep. 2021, 23, 112. [Google Scholar] [CrossRef]
- Sun, L.Y.; Li, X.J.; Sun, Y.M.; Huang, W.; Fang, K.; Han, C.; Chen, Z.H.; Luo, X.Q.; Chen, Y.Q.; Wang, W.T. LncRNA ANRIL regulates AML development through modulating the glucose metabolism pathway of AdipoR1/AMPK/SIRT1. Mol. Cancer 2018, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Chattopadhyay, M.; Pospishil, L.; Cieciura, L.Z.; Goswami, S.; Kodela, R.; Saavedra, J.E.; Keefer, L.K.; Kashfi, K. JS-K, a nitric oxide-releasing prodrug, modulates ß-catenin/TCF signaling in leukemic Jurkat cells: Evidence of an S-nitrosylated mechanism. Biochem. Pharm. 2010, 80, 1641–1649. [Google Scholar] [CrossRef]
- Fernando, T.R.; Rodriguez-Malave, N.I.; Waters, E.V.; Yan, W.; Casero, D.; Basso, G.; Pigazzi, M.; Rao, D.S. LncRNA Expression Discriminates Karyotype and Predicts Survival in B-Lymphoblastic Leukemia. Mol. Cancer Res. 2015, 13, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Malavé, N.I.; Fernando, T.R.; Patel, P.C.; Contreras, J.R.; Palanichamy, J.K.; Tran, T.M.; Anguiano, J.; Davoren, M.J.; Alberti, M.O.; Pioli, K.T.; et al. BALR-6 regulates cell growth and cell survival in B-lymphoblastic leukemia. Mol. Cancer 2015, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Kang, Q.; Chen, Q.; Chen, Z.; Wang, J.; Tan, L.; Chen, J.L. High expression of long non-coding RNA H19 is required for efficient tumorigenesis induced by Bcr-Abl oncogene. FEBS Lett. 2014, 588, 1780–1786. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wong, K.Y.; Li, Z.H.; Chim, C.S. Epigenetic silencing of tumor suppressor long non-coding RNA BM742401 in chronic lymphocytic leukemia. Oncotarget 2016, 7, 82400–82410. [Google Scholar] [CrossRef]
- Izadifard, M.; Pashaiefar, H.; Yaghmaie, M.; Montazeri, M.; Sadraie, M.; Momeny, M.; Jalili, M.; Ahmadvand, M.; Ghaffari, S.H.; Mohammadi, S.; et al. Expression Analysis of PVT1, CCDC26, and CCAT1 Long Noncoding RNAs in Acute Myeloid Leukemia Patients. Genet. Test. Mol. Biomark. 2018, 22, 593–598. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Mo, W.; Zhang, Y.; Zhou, W.; Deng, T.; Zhou, M.; Chen, X.; Wang, S.; Wang, C. lncRNA-CCDC26, as a novel biomarker, predicts prognosis in acute myeloid leukemia. Oncol. Lett. 2019, 18, 2203–2211. [Google Scholar] [CrossRef]
- Hirano, T.; Yoshikawa, R.; Harada, H.; Harada, Y.; Ishida, A.; Yamazaki, T. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol. Cancer 2015, 14, 90. [Google Scholar] [CrossRef]
- Ni, J.; Hong, J.; Li, Q.; Zeng, Q.; Xia, R. Long non-coding RNA CRNDE suppressing cell proliferation is regulated by DNA methylation in chronic lymphocytic leukemia. Leuk. Res. 2021, 105, 106564. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Ma, J.J. High expression of lnc-CRNDE presents as a biomarker for acute myeloid leukemia and promotes the malignant progression in acute myeloid leukemia cell line U937. Eur. Rev. Med. Pharm. Sci. 2018, 22, 763–770. [Google Scholar] [CrossRef]
- Hola, M.A.M.; Ali, M.A.M.; ElNahass, Y.; Salem, T.A.E.; Mohamed, M.R. Expression and prognostic relevance of long noncoding RNAs CRNDE and AOX2P in adult acute myeloid leukemia. Int. J. Lab. Hematol. 2021, 43, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, W.; Zhao, M.; Li, S.; Jin, W.; Wang, K. Oncogenic role of lncRNA CRNDE in acute promyelocytic leukemia and NPM1-mutant acute myeloid leukemia. Cell Death Discov. 2020, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhong, L.; Shen, C.; Chu, X.; Luo, X.; Yu, L.; Ye, J.; Xiong, L.; Dan, W.; Li, J.; et al. CRNDE enhances the expression of MCM5 and proliferation in acute myeloid leukemia KG-1a cells by sponging miR-136-5p. Sci. Rep. 2021, 11, 16755. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, S.; Cao, W.; Wan, D.; Sun, L. Knockdown of LncRNA CRNDE suppresses proliferation and P-glycoprotein-mediated multidrug resistance in acute myelocytic leukemia through the Wnt/β-catenin pathway. Biosci. Rep. 2020, 40, BSR20193450. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.; Harada, M.; Lovén, J.; Castro, J.; Davis, Z.; Oscier, D.; Henriksson, M.; Sangfelt, O.; Grandér, D.; Corcoran, M.M. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16-1. Exp. Cell Res. 2009, 315, 2941–2952. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Zimta, A.A.; Tomuleasa, C.; Sahnoune, I.; Calin, G.A.; Berindan-Neagoe, I. Long Non-coding RNAs in Myeloid Malignancies. Front. Oncol. 2019, 9, 1048. [Google Scholar] [CrossRef]
- Zhang, T.J.; Zhou, J.D.; Zhang, W.; Lin, J.; Ma, J.C.; Wen, X.M.; Yuan, Q.; Li, X.X.; Xu, Z.J.; Qian, J. H19 overexpression promotes leukemogenesis and predicts unfavorable prognosis in acute myeloid leukemia. Clin. Epigenet. 2018, 10, 47. [Google Scholar] [CrossRef]
- Zhao, T.F.; Jia, H.Z.; Zhang, Z.Z.; Zhao, X.S.; Zou, Y.F.; Zhang, W.; Wan, J.; Chen, X.F. LncRNA H19 regulates ID2 expression through competitive binding to hsa-miR-19a/b in acute myelocytic leukemia. Mol. Med. Rep. 2017, 16, 3687–3693. [Google Scholar] [CrossRef]
- Zhao, T.T.; Liu, X. LncRNA-H19 inhibits apoptosis of acute myeloid leukemia cells via targeting miR-29a-3p. Eur. Rev. Med. Pharm. Sci. 2019, 23, 224–231. [Google Scholar] [CrossRef]
- Zhou, J.D.; Lin, J.; Zhang, T.J.; Ma, J.C.; Li, X.X.; Wen, X.M.; Guo, H.; Xu, Z.J.; Deng, Z.Q.; Zhang, W.; et al. Hypomethylation-mediated H19 overexpression increases the risk of disease evolution through the association with BCR-ABL transcript in chronic myeloid leukemia. J. Cell Physiol. 2018, 233, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, M.; Rahgozar, S.; Pouyanrad, S. Increased level of long non coding RNA H19 is correlated with the downregulation of miR-326 and BCL-2 genes in pediatric acute lymphoblastic leukemia, a possible hallmark for leukemogenesis. Mol. Biol. Rep. 2021, 48, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, B.; Li, H.; Huang, X.; Wu, Y.; Xing, C.; Yu, X.; Ji, Y. Long noncoding RNA HOTAIR promotes the self-renewal of leukemia stem cells through epigenetic silencing of p15. Exp. Hematol. 2018, 67, 32–40.e33. [Google Scholar] [CrossRef]
- Xing, C.Y.; Hu, X.Q.; Xie, F.Y.; Yu, Z.J.; Li, H.Y.; Bin, Z.; Wu, J.B.; Tang, L.Y.; Gao, S.M. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015, 589, 1981–1987. [Google Scholar] [CrossRef]
- Hao, S.; Shao, Z. HOTAIR is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int. J. Clin. Exp. Pathol. 2015, 8, 7223–7228. [Google Scholar]
- Wang, H.; Li, Q.; Tang, S.; Li, M.; Feng, A.; Qin, L.; Liu, Z.; Wang, X. The role of long noncoding RNA HOTAIR in the acquired multidrug resistance to imatinib in chronic myeloid leukemia cells. Hematology 2017, 22, 208–216. [Google Scholar] [CrossRef]
- Raveh, E.; Matouk, I.J.; Gilon, M.; Hochberg, A. The H19 Long non-coding RNA in cancer initiation, progression and metastasis—A proposed unifying theory. Mol. Cancer 2015, 14, 184. [Google Scholar] [CrossRef]
- Zhang, X.; Weissman, S.M.; Newburger, P.E. Long intergenic non-coding RNA HOTAIRM1 regulates cell cycle progression during myeloid maturation in NB4 human promyelocytic leukemia cells. RNA Biol. 2014, 11, 777–787. [Google Scholar] [CrossRef]
- Jing, Y.; Jiang, X.; Lei, L.; Peng, M.; Ren, J.; Xiao, Q.; Tao, Y.; Tao, Y.; Huang, J.; Wang, L.; et al. Mutant NPM1-regulated lncRNA HOTAIRM1 promotes leukemia cell autophagy and proliferation by targeting EGR1 and ULK3. J. Exp. Clin. Cancer Res. 2021, 40, 312. [Google Scholar] [CrossRef]
- Hu, N.; Chen, L.; Li, Q.; Zhao, H. LncRNA HOTAIRM1 is involved in the progression of acute myeloid leukemia through targeting miR-148b. RSC Adv. 2019, 9, 10352–10359. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, W.T.; Huang, W.; Fang, K.; Sun, Y.M.; Liu, S.R.; Luo, X.Q.; Chen, Y.Q. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017, 24, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Ozaki, K.; Sato, H.; Mizuno, H.; Susumu, S.; Takahashi, A.; Miyamoto, Y.; Ikegawa, S.; Kamatani, N.; Hori, M.; et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J. Hum. Genet. 2006, 51, 1087–1099. [Google Scholar] [CrossRef]
- Pashaiefar, H.; Izadifard, M.; Yaghmaie, M.; Montazeri, M.; Gheisari, E.; Ahmadvand, M.; Momeny, M.; Ghaffari, S.H.; Kasaeian, A.; Alimoghaddam, K.; et al. Low Expression of Long Noncoding RNA IRAIN Is Associated with Poor Prognosis in Non-M3 Acute Myeloid Leukemia Patients. Genet. Test. Mol. Biomark. 2018, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, F.; Hertweck, M.; Filipovich, A.; Uhrmacher, S.; Kreuzer, K.A. High lymphoid enhancer-binding factor-1 expression is associated with disease progression and poor prognosis in chronic lymphocytic leukemia. Hematol. Rep. 2010, 2, e3. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yang, C.; Shi, X.; Cao, L.; Zhao, X.; Miao, Y.; Zhu, H.; Wang, L.; Xu, W.; et al. LncRNA landscape analysis identified LncRNA LEF-AS1 as an oncogene that upregulates LEF1 and promotes survival in chronic lymphocytic leukemia. Leuk. Res. 2021, 110, 106706. [Google Scholar] [CrossRef]
- Chen, S.; Liang, H.; Yang, H.; Zhou, K.; Xu, L.; Liu, J.; Lai, B.; Song, L.; Luo, H.; Peng, J.; et al. LincRNa-p21: Function and mechanism in cancer. Med. Oncol. 2017, 34, 98. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, N.; Zamudio, J.R.; Jong, R.M.; Soukup, D.; Resnick, R.; Sarma, K.; Ward, A.J.; Raj, A.; Lee, J.T.; Sharp, P.A.; et al. LincRNA-p21 Activates p21 In cis to Promote Polycomb Target Gene Expression and to Enforce the G1/S Checkpoint. Mol. Cell 2014, 54, 777–790. [Google Scholar] [CrossRef]
- El-Khazragy, N.; Abdel Aziz, M.A.; Hesham, M.; Matbouly, S.; Mostafa, S.A.; Bakkar, A.; Abouelnile, M.; Noufal, Y.; Mahran, N.A.; Abd Elkhalek, M.A.; et al. Upregulation of leukemia-induced non-coding activator RNA (LUNAR1) predicts poor outcome in pediatric T-acute lymphoblastic leukemia. Immunobiology 2021, 226, 152149. [Google Scholar] [CrossRef]
- Ouimet, M.; Drouin, S.; Lajoie, M.; Caron, M.; St-Onge, P.; Gioia, R.; Richer, C.; Sinnett, D. A childhood acute lymphoblastic leukemia-specific lncRNA implicated in prednisolone resistance, cell proliferation, and migration. Oncotarget 2017, 8, 7477–7488. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Luo, J.; Ma, Y.; Yang, Z. Long Noncoding RNA Maternally Expressed Gene 3 Is Downregulated, and Its Insufficiency Correlates with Poor-Risk Stratification, Worse Treatment Response, as Well as Unfavorable Survival Data in Patients with Acute Myeloid Leukemia. Technol. Cancer Res. Treat. 2020, 19, 1533033820945815. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Gao, H.; Xia, H.; Li, S.; Li, N.; Duan, Y.; Ren, Y.; Zhang, H.; Liu, J.; Gao, W. Prognostic significance of long non coding maternally expressed gene 3 in pediatric acute myeloid leukemia. Medicine 2021, 100, e26959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, P.; Liu, Q.; Liu, Z. LncRNA MEG3 Regulates Imatinib Resistance in Chronic Myeloid Leukemia via Suppressing MicroRNA-21. Biomol. Ther. 2017, 25, 490–496. [Google Scholar] [CrossRef]
- Lyu, Y.; Lou, J.; Yang, Y.; Feng, J.; Hao, Y.; Huang, S.; Yin, L.; Xu, J.; Huang, D.; Ma, B.; et al. Dysfunction of the WT1-MEG3 signaling promotes AML leukemogenesis via p53-dependent and -independent pathways. Leukemia 2017, 31, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, L.; Liu, X.; Nie, Z.; Luo, J. Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomed. Pharm. 2018, 104, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M.; et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629. [Google Scholar] [CrossRef]
- Qin, J.; Bao, H.; Li, H. Correlation of long non-coding RNA taurine-upregulated gene 1 with disease conditions and prognosis, as well as its effect on cell activities in acute myeloid leukemia. Cancer Biomark. 2018, 23, 569–577. [Google Scholar] [CrossRef]
- Salehi, M.; Sharifi, M. Induction of apoptosis and necrosis in human acute erythroleukemia cells by inhibition of long non-coding RNA PVT1. Mol. Biol. Res. Commun. 2018, 7, 89–96. [Google Scholar] [CrossRef]
- El-Khazragy, N.; Elayat, W.; Matbouly, S.; Seliman, S.; Sami, A.; Safwat, G.; Diab, A. The prognostic significance of the long non-coding RNAs “CCAT1, PVT1” in t(8;21) associated Acute Myeloid Leukemia. Gene 2019, 707, 172–177. [Google Scholar] [CrossRef]
- Salehi, M.; Sharifi, M.; Bagheri, M. Knockdown of Long Noncoding RNA Plasmacytoma Variant Translocation 1 with Antisense Locked Nucleic Acid GapmeRs Exerts Tumor-Suppressive Functions in Human Acute Erythroleukemia Cells Through Downregulation of C-MYC Expression. Cancer Biother. Radiopharm. 2019, 34, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Ruppert, A.S.; Fobare, S.; Chen, T.L.; Liu, C.; Lehman, A.; Blachly, J.S.; Zhang, X.; Lucas, D.M.; Grever, M.R.; et al. The long noncoding RNA, treRNA, decreases DNA damage and is associated with poor response to chemotherapy in chronic lymphocytic leukemia. Oncotarget 2017, 8, 25942–25954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Zhao, F.; Xu, R.; Jiang, J.; Zhang, C.; Liu, H.; Huang, H. Long non-coding RNA taurine-upregulated gene 1 correlates with poor prognosis, induces cell proliferation, and represses cell apoptosis via targeting aurora kinase A in adult acute myeloid leukemia. Ann. Hematol. 2018, 97, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Kang, Q.; Zhu, X.; Chen, Q.; Wang, X.; Chen, Y.; Ouyang, J.; Zhang, L.; Tan, H.; Chen, R.; et al. A long noncoding RNA critically regulates Bcr-Abl-mediated cellular transformation by acting as a competitive endogenous RNA. Oncogene 2015, 34, 1768–1779. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Xu, G. Silencing of long noncoding RNA TUG1 inhibits viability and promotes apoptosis of acute myeloid leukemia cells by targeting microRNA-221-3p/KIT axis. Clin. Hemorheol. Microcirc. 2020, 76, 425–437. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, X. Knockdown of LncRNA-UCA1 suppresses chemoresistance of pediatric AML by inhibiting glycolysis through the microRNA-125a/hexokinase 2 pathway. J. Cell Biochem. 2018, 119, 6296–6308. [Google Scholar] [CrossRef]
- Liang, Y.; Li, E.; Zhang, H.; Zhang, L.; Tang, Y.; Wanyan, Y. Silencing of lncRNA UCA1 curbs proliferation and accelerates apoptosis by repressing SIRT1 signals by targeting miR-204 in pediatric AML. J. Biochem. Mol. Toxicol 2020, 34, e22435. [Google Scholar] [CrossRef]
- Sun, M.D.; Zheng, Y.Q.; Wang, L.P.; Zhao, H.T.; Yang, S. Long noncoding RNA UCA1 promotes cell proliferation, migration and invasion of human leukemia cells via sponging miR-126. Eur. Rev. Med. Pharm. Sci. 2018, 22, 2233–2245. [Google Scholar] [CrossRef]
- Trimarchi, T.; Bilal, E.; Ntziachristos, P.; Fabbri, G.; Dalla-Favera, R.; Tsirigos, A.; Aifantis, I. Genome-wide Mapping and Characterization of Notch-Regulated Long Noncoding RNAs in Acute Leukemia. Cell 2014, 158, 593–606. [Google Scholar] [CrossRef]
- Ronchetti, D.; Manzoni, M.; Agnelli, L.; Vinci, C.; Fabris, S.; Cutrona, G.; Matis, S.; Colombo, M.; Galletti, S.; Taiana, E.; et al. lncRNA profiling in early-stage chronic lymphocytic leukemia identifies transcriptional fingerprints with relevance in clinical outcome. Blood Cancer J. 2016, 6, e468. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Xie, J.; Han, H.; Dong, Q.; Wang, W. Long Non-Coding RNA ZNF667-AS1 Knockdown Curbs Liver Metastasis in Acute Myeloid Leukemia by Regulating the microRNA-206/AKAP13 Axis. Cancer Manag. Res. 2020, 12, 13285–13300. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, H.; Qin, X. MicroRNA-206 serves as a tumor suppressor in pediatric acute myeloid leukemia by targeting Cyclin D1. Pathol. Res. Pr. 2019, 215, 152554. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Taheri, M. Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed. Pharm. 2019, 118, 109129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Huang, Y.; Su, R.; Yu, Y.Y. Silencing long non-coding RNA HOTAIR exerts anti-oncogenic effect on human acute myeloid leukemia via demethylation of HOXA5 by inhibiting Dnmt3b. Cancer Cell Int. 2019, 19, 114. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, C.; Chen, S.; Cai, X.; Shi, Y.; Lin, B.; Chen, Y. Overexpression of long non-coding RNA HOTAIR predicts a poor prognosis in patients with acute myeloid leukemia. Oncol. Lett. 2015, 10, 2410–2414. [Google Scholar] [CrossRef]
- Kong, W.; Yin, G.; Zheng, S.; Liu, X.; Zhu, A.; Yu, P.; Zhang, J.; Shan, Y.; Ying, R.; Jin, H. Long noncoding RNA (lncRNA) HOTAIR: Pathogenic roles and therapeutic opportunities in gastric cancer. Genes Dis. 2022, 9, 1269–1280. [Google Scholar] [CrossRef]
- Norouzi, A.; Motaghi, M.; Hassanshahi, G.; Nazari, M. Exploring the expression profile of vitamin D receptor and its related long non-coding RNAs in patients with acute lymphoblastic leukemia. Rev. Assoc. Méd. Bras. 2021, 67, 1113–1117. [Google Scholar] [CrossRef]
- Yang, J.; Qi, M.; Fei, X.; Wang, X.; Wang, K. LncRNA H19: A novel oncogene in multiple cancers. Int. J. Biol. Sci. 2021, 17, 3188–3208. [Google Scholar] [CrossRef]
- Venkatraman, A.; He, X.C.; Thorvaldsen, J.L.; Sugimura, R.; Perry, J.M.; Tao, F.; Zhao, M.; Christenson, M.K.; Sanchez, R.; Yu, J.Y.; et al. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature 2013, 500, 345–349. [Google Scholar] [CrossRef]
- El Hajj, J.; Nguyen, E.; Liu, Q.; Bouyer, C.; Adriaenssens, E.; Hilal, G.; Ségal-Bendirdjian, E. Telomerase regulation by the long non-coding RNA H19 in human acute promyelocytic leukemia cells. Mol. Cancer 2018, 17, 85. [Google Scholar] [CrossRef]
- Gil, J.; Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat. Rev. Mol. Cell Biol. 2006, 7, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gius, D.; Onyango, P.; Muldoon-Jacobs, K.; Karp, J.; Feinberg, A.P.; Cui, H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 2008, 451, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, L.; Zhao, J.; Liu, D.; Li, H.; Hu, M. LncRNA ANRIL/miR-7-5p/TCF4 axis contributes to the progression of T cell acute lymphoblastic leukemia. Cancer Cell Int. 2020, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Bahraini, M.; Faranoush, M.; Mobaraki, S.; Paridar, M.; Amini, A.; Manafi, R.; Safa, M. Overexpression of long non-coding RNA ANRIL in B-acute lymphoblastic leukemia. Iran. J. Pediatr. Hematol. Oncol. 2021, 11, 148–157. [Google Scholar] [CrossRef]
- Onagoruwa, O.T.; Pal, G.; Ochu, C.; Ogunwobi, O.O. Oncogenic Role of PVT1 and Therapeutic Implications. Front. Oncol. 2020, 10, 17. [Google Scholar] [CrossRef]
- Huppi, K.; Pitt, J.J.; Wahlberg, B.M.; Caplen, N.J. The 8q24 gene desert: An oasis of non-coding transcriptional activity. Front. Genet. 2012, 3, 69. [Google Scholar] [CrossRef]
- Zeng, C.; Yu, X.; Lai, J.; Yang, L.; Chen, S.; Li, Y. Overexpression of the long non-coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J. Hematol. Oncol. 2015, 8, 126. [Google Scholar] [CrossRef]
- Ju, J.K.; Han, W.N.; Shi, C.L. Long non-coding RNA (lncRNA) plasmacytoma variant translocation 1 gene (PVT1) modulates the proliferation and apoptosis of acute lymphoblastic leukemia cells by sponging miR-486-5p. Bioengineered 2022, 13, 4587–4597. [Google Scholar] [CrossRef]
- Subhash, S.; Andersson, P.O.; Kosalai, S.T.; Kanduri, C.; Kanduri, M. Global DNA methylation profiling reveals new insights into epigenetically deregulated protein coding and long noncoding RNAs in CLL. Clin. Epigenet. 2016, 8, 106. [Google Scholar] [CrossRef]
- Grieselhuber, N.R.; Klco, J.M.; Verdoni, A.M.; Lamprecht, T.; Sarkaria, S.M.; Wartman, L.D.; Ley, T.J. Notch signaling in acute promyelocytic leukemia. Leukemia 2013, 27, 1548–1557. [Google Scholar] [CrossRef]
- Congrains-Castillo, A.; Niemann, F.S.; Santos Duarte, A.S.; Olalla-Saad, S.T. LEF1-AS1, long non-coding RNA, inhibits proliferation in myeloid malignancy. J. Cell Mol. Med. 2019, 23, 3021–3025. [Google Scholar] [CrossRef] [PubMed]
- Vrba, L.; Garbe, J.C.; Stampfer, M.R.; Futscher, B.W. A lincRNA connected to cell mortality and epigenetically-silenced in most common human cancers. Epigenetics 2015, 10, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- El-Khazragy, N.; Esmaiel, M.A.; Mohamed, M.M.; Hassan, N.S. Upregulation of long noncoding RNA Lnc-IRF2-3 and Lnc-ZNF667-AS1 is associated with poor survival in B-chronic lymphocytic leukemia. Int. J. Lab. Hematol. 2020, 42, 284–291. [Google Scholar] [CrossRef]
- Li, J.; Zi, Y.; Wang, W.; Li, Y. [ARTICLE WITHDRAWN] Long Noncoding RNA MEG3 Inhibits Cell Proliferation and Metastasis in Chronic Myeloid Leukemia via Targeting miR-184. Oncol. Res. 2018, 26, 297–305. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yang, L.; Liu, X.J.; Wang, X.Z.; Pan, Y.X.; Luo, J.M. The Long Noncoding RNA MEG3 and its Target miR-147 Regulate JAK/STAT Pathway in Advanced Chronic Myeloid Leukemia. EBioMedicine 2018, 34, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kou, D.; Liu, B.; Huang, Y.; Li, S.; Qi, Y.; Guo, Y.; Huang, T.; Qi, X.; Jia, L. LncRNA MEG3 contributes to drug resistance in acute myeloid leukemia by positively regulating ALG9 through sponging miR-155. Int. J. Lab. Hematol. 2020, 42, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lian, Z.; Padden, C.; Gerstein, M.B.; Rozowsky, J.; Snyder, M.; Gingeras, T.R.; Kapranov, P.; Weissman, S.M.; Newburger, P.E. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 2009, 113, 2526–2534. [Google Scholar] [CrossRef]
- Díaz-Beyá, M.; Brunet, S.; Nomdedéu, J.; Pratcorona, M.; Cordeiro, A.; Gallardo, D.; Escoda, L.; Tormo, M.; Heras, I.; Ribera, J.M.; et al. The lincRNA HOTAIRM1, located in the HOXA genomic region, is expressed in acute myeloid leukemia, impacts prognosis in patients in the intermediate-risk cytogenetic category, and is associated with a distinctive microRNA signature. Oncotarget 2015, 6, 31613–31627. [Google Scholar] [CrossRef]
- Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef]
- Chapuis, N.; Tamburini, J.; Cornillet-Lefebvre, P.; Gillot, L.; Bardet, V.; Willems, L.; Park, S.; Green, A.S.; Ifrah, N.; Dreyfus, F.; et al. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: Therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica 2010, 95, 415–423. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Sun, Y.; Yu, D.; Wen, X.; Wang, H.; Cui, J.; Wang, G.; Hoffman, A.R.; Hu, J.F. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014, 42, 9588–9601. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, J.D.; Zhang, T.J.; Ma, J.C.; Xiao, G.F.; Chen, Q.; Deng, Z.Q.; Lin, J.; Qian, J.; Yao, D.M. Overexpression of lncRNA PANDAR predicts adverse prognosis in acute myeloid leukemia. Cancer Manag. Res. 2018, 10, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Song, W.; Wang, J. TUG1 confers Adriamycin resistance in acute myeloid leukemia by epigenetically suppressing miR-34a expression via EZH2. Biomed. Pharm. 2019, 109, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Chen, W.; Li, X. Urothelial cancer associated 1: A long noncoding RNA with a crucial role in cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1407–1419. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, N.; Watabe, K.; Lu, Z.; Wu, F.; Xu, M.; Mo, Y.Y. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis. 2014, 5, e1008. [Google Scholar] [CrossRef]
- Wander, S.A.; Zhao, D.; Slingerland, J.M. p27: A barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin. Cancer Res. 2011, 17, 12–18. [Google Scholar] [CrossRef]
- Hughes, J.M.; Legnini, I.; Salvatori, B.; Masciarelli, S.; Marchioni, M.; Fazi, F.; Morlando, M.; Bozzoni, I.; Fatica, A. C/EBPα-p30 protein induces expression of the oncogenic long non-coding RNA UCA1 in acute myeloid leukemia. Oncotarget 2015, 6, 18534–18544. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, X.F.; Wang, M.; Zhang, P.P.; Zhang, F.; Zhang, J.J. Long non-coding RNA UCA1 promotes autophagy by targeting miR-96-5p in acute myeloid leukaemia. Clin. Exp. Pharm. Physiol. 2020, 47, 877–885. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Chen, X. Long non-coding RNA UCA1 modulates cell proliferation and apoptosis by regulating miR-296-3p/Myc axis in acute myeloid leukemia. Cell Cycle 2020, 19, 1454–1465. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiao, C.; Lin, Y.; Chen, M.; Zhang, J.; Wang, J.; Zhang, Z. lncRNA UCA1 Contributes to Imatinib Resistance by Acting as a ceRNA Against miR-16 in Chronic Myeloid Leukemia Cells. DNA Cell Biol. 2017, 36, 18–25. [Google Scholar] [CrossRef]
- Fang, K.; Han, B.W.; Chen, Z.H.; Lin, K.Y.; Zeng, C.W.; Li, X.J.; Li, J.H.; Luo, X.Q.; Chen, Y.Q. A distinct set of long non-coding RNAs in childhood MLL-rearranged acute lymphoblastic leukemia: Biology and epigenetic target. Hum. Mol. Genet. 2014, 23, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Chen, T.Q.; Zeng, Z.C.; Pan, Q.; Huang, W.; Han, C.; Fang, K.; Sun, L.Y.; Yang, Q.Q.; Wang, D.; et al. The lncRNA LAMP5-AS1 drives leukemia cell stemness by directly modulating DOT1L methyltransferase activity in MLL leukemia. J. Hematol. Oncol. 2020, 13, 78. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, S.; Li, J.; Zhang, H.; Qian, C.; Wang, H.; Liu, J.; Zhao, Y. TCF7L2 activated HOXA-AS2 decreased the glucocorticoid sensitivity in acute lymphoblastic leukemia through regulating HOXA3/EGFR/Ras/Raf/MEK/ERK pathway. Biomed. Pharm. 2019, 109, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Tsuiji, H.; Yoshimoto, R.; Hasegawa, Y.; Furuno, M.; Yoshida, M.; Nakagawa, S. Competition between a noncoding exon and introns: Gomafu contains tandem UACUAAC repeats and associates with splicing factor-1. Genes Cells 2011, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Sattari, A.; Siddiqui, H.; Moshiri, F.; Ngankeu, A.; Nakamura, T.; Kipps, T.; Croce, C. Upregulation of long noncoding RNA MIAT in aggressive form of chronic lymphocytic leukemias. Oncotarget 2014, 7, 54174–54182. [Google Scholar] [CrossRef]
- Winkler, L.; Jimenez, M.; Zimmer, J.T.; Williams, A.; Simon, M.D.; Dimitrova, N. Functional elements of the cis-regulatory lincRNA-p21. Cell Rep. 2022, 39, 110687. [Google Scholar] [CrossRef]
- Bao, X.; Wu, H.; Zhu, X.; Guo, X.; Hutchins, A.P.; Luo, Z.; Song, H.; Chen, Y.; Lai, K.; Yin, M.; et al. The p53-induced lincRNA-p21 derails somatic cell reprogramming by sustaining H3K9me3 and CpG methylation at pluripotency gene promoters. Cell Res. 2015, 25, 80–92. [Google Scholar] [CrossRef]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef]
- Kröber, A.; Seiler, T.; Benner, A.; Bullinger, L.; Brückle, E.; Lichter, P.; Döhner, H.; Stilgenbauer, S. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood 2002, 100, 1410–1416. [Google Scholar] [CrossRef]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M.; et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef]
- Ouillette, P.; Erba, H.; Kujawski, L.; Kaminski, M.; Shedden, K.; Malek, S.N. Integrated genomic profiling of chronic lymphocytic leukemia identifies subtypes of deletion 13q14. Cancer Res. 2008, 68, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Dal Bo, M.; Rossi, F.M.; Rossi, D.; Deambrogi, C.; Bertoni, F.; Del Giudice, I.; Palumbo, G.; Nanni, M.; Rinaldi, A.; Kwee, I.; et al. 13q14 deletion size and number of deleted cells both influence prognosis in chronic lymphocytic leukemia. Genes Chromosom. Cancer 2011, 50, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Garding, A.; Bhattacharya, N.; Claus, R.; Ruppel, M.; Tschuch, C.; Filarsky, K.; Idler, I.; Zucknick, M.; Caudron-Herger, M.; Oakes, C.; et al. Epigenetic upregulation of lncRNAs at 13q14.3 in leukemia is linked to the In Cis downregulation of a gene cluster that targets NF-kB. PLoS Genet. 2013, 9, e1003373. [Google Scholar] [CrossRef] [PubMed]
- Palamarchuk, A.; Efanov, A.; Nazaryan, N.; Santanam, U.; Alder, H.; Rassenti, L.; Kipps, T.; Croce, C.M.; Pekarsky, Y. 13q14 deletions in CLL involve cooperating tumor suppressors. Blood 2010, 115, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, K.; Li, A.; Yan, J.; Setoyama, T.; Johannes, G.J.; Orom, U.A.; Tchou, J.; Liu, Q.; Zhang, L.; Speicher, D.W.; et al. Identification of a long non-coding RNA-associated RNP complex regulating metastasis at the translational step. EMBO J. 2013, 32, 2672–2684. [Google Scholar] [CrossRef]
| Name of lncRNA | Expression in Leukemia Type | Function | Target | Effect | Prognostic Impact | References |
|---|---|---|---|---|---|---|
| AC092652.2-202 | CLL-up-regulated | Oncogene | Increased expression is associated with adverse prognosis | [90] | ||
| ANRIL | AML-up-regulated | Oncogene | PRC1, PRC2 | repression of INK4b-ARF-INK4a tumor-suppressor locus, increased proliferation | Increased expression is associated with adverse prognosis | [91,92] |
| ADIPOR1 | regulates glucose metabolism | [93] | ||||
| ALL-up-regulated | Oncogene | miR-7-5p | Accelerates proliferation and inhibits apoptosis | Increased expression is associated with adverse prognosis | [94] | |
| BALR-2 | ALL-up-regulated | Oncogene Drug resistance | Poor overall survival | [95] | ||
| BALR-6 | ALL with MLL rearrangements-up-regulated | Oncogene | SP1 and CREB1 | Promotes proliferation and cell survival | [96] | |
| BGL3 | CML-down-regulated | Tumor suppressor | miR-17, miR-20a, miR-20b, miR-93, miR-106a, miR-106b | Alters the function of PTEN tumor suppressor | [97] | |
| BM74240 | CLL-down-regulated | Tumor suppressor | Increased expression is a marker of good prognosis | [98] | ||
| CCDC26 | AML-up-regulated | Oncogene | c-KIT | Induces cell proliferation | Increased expression is associated with adverse prognosis | [99,100,101] |
| CRNDE | CLL-down-regulated | Tumor suppressor | miR-28 | suppresses proliferation, induces apoptosis | [102] | |
| AML-up-regulated | Oncogene | miR-181 miR-136-5p | Promoting proliferation, blocking myeloid differentiation | Increased expression is associated with adverse prognosis | [103,104,105,106] | |
| Drug resistance | MRP1 | [107] | ||||
| DELU2 | CLL-down-regulated | Tumor suppressor | miR-15a, miR-16-1 | suppresses proliferation, induces apoptosis | [108,109] | |
| GAS5 | CLL-down-regulated | Tumor suppressor | miR-222 | Suppresses proliferation | [88] | |
| AML-down-regulated | Tumor suppressor | miR-222 | Low expression associated with inferior outcome in AML-NK | [85,110] | ||
| ALL-up-regulated | Drug resistance | Glucocorticoid receptor | Increases glucocorticoid resistance | Higher risk of short-term relapse | [86,87] | |
| H19 | AML-up-regulated | Oncogene | ID2 miR-19a, miR-19b, miR-29a-3p | proliferation, apoptosis | Increased expression is associated with adverse prognosis | [111,112,113] |
| CML-up-regulated | Oncogene Drug resistance | c-MYC | Increased expression is associated with disease progression and poor prognosis | [97,114] | ||
| ALL-up-regulated | Oncogene | miR-326 | [115] | |||
| HOTAIR | AML-up-regulated | Oncogene | p15INK4b (CDKN2B) miR-193a and c-KIT | gene repression self-renewal | Increased expression is associated with adverse prognosis | [116,117,118] |
| CML-up-regulated | Drug resistance | MRP1 | [119] | |||
| B-ALL-up-regulated | [120] | |||||
| HOTAIRM1 | AML-up-regulated | Oncogene | HOXA1, HOXA4, CD11b, CD18 | chromatin modification, myeloid differentiation | Increased expression is associated with adverse prognosis | [121,122] |
| miR-20a, miR-106b and miR-125b | autophagy | [123] | ||||
| APL-down-regulated | Tumor suppressor | regulates PML-RARa degradation | expression is up-regulated during ATRA-induced granulocytic differentiation promoting cell-cycle progression | [124] | ||
| HOXA-AS2 | ALL-up-regulated | Drug resistance | HOXA3/EGFR/Ras/Raf/MEK/ERK pathway | Decreases glucocorticoid sensitivity | [125] | |
| IRAIN | AML-down-regulated | Tumor suppressor | IGF1R promoter | Suppresses cell proliferation | down-regulated in patients with poor prognosis | [99,126] |
| LAMP-AS1 | MLL rearrangement leukemias-up-regulated | Oncogene | LAMP5 | Reduced 5-year leukemia-free survival | [95] | |
| LEF1-AS1 | CLL-up-regulated | Oncogene | LEF1 | increases survival inhibits apoptosis | [127] | |
| AML-down-regulated | Tumor suppressor | P21Cyp1(CDKN1A) p27Kip1(CDKN1B) | Suppresses proliferation | [128] | ||
| LincRNA-p21 | CLL-down-regulated | Tumor suppressor | p53 hnRNP-K | Induces apoptosis | [129,130] | |
| LUNAR1 | ALL-up-regulated | Oncogene | IGF1R | Increases proliferation | Poor prognosis in T-ALL | [131,132] |
| MALAT1 | CLL-up-regulated | Oncogene | EZH2 | Induces proliferation, suppresses apoptosis | [72] | |
| AML-up-regulated | Oncogene Drug resistance | miR-96 | Increases proliferation | [73,75] | ||
| CML-up-regulated | Oncogene | miR-328 | Increases proliferation | [77] | ||
| ALL-up-regulated | Oncogene | miR-205 | Induces proliferation, suppresses apoptosis | [76] | ||
| MEG3 | AML-down-regulated | Tumor suppressor | p53 | Inhibits tumorigenesis via p53 dependent and independent way | Increased expression is a marker of good prognosis | [133,134,135] |
| Drug resistance | miR-155 | [136] | ||||
| CML-down-regulated | Drug resistance | miR-21 | [137] | |||
| MIAT | CLL-up-regulated | Oncogene | OCT4 | Increased cell growth, suppression of apoptosis | Increased expression is associated with adverse prognosis | [138] |
| NEAT1 | CLL-down-regulated | Tumor suppressor | p53 | Induces apoptosis | [58,59] | |
| AML-down-regulated | Tumor suppressor | miR-23a-3p miR-338-3p PTEN | suppresses proliferation, induces apoptosis | Overexpressed in APL where PML-RARa fusion protein inhibits NEAT1 expression | [61,62,63] | |
| CML-up-regulated | Oncogene | SFPQ-component of paraspeckles | suppresses apoptosis | [62] | ||
| PANDAR | AML-up-regulated | Oncogene | NF-YA transcription factor | Promotes cell survival | Increased expression is associated with adverse prognosis | [139,140] |
| PVT1 | AML-up-regulated | Oncogene | c-MYC | Induces proliferation, suppresses apoptosis | Overexpression in APL and AML with t(8;21) indicates adverse prognosis | [141,142,143] |
| RP11-137H2.4 | ALL-up-regulated | Drug resistance | NRAS/BRAF/NF-κB MAPK cascade members | Increases glucocorticoid resistance | [95] | |
| TRERNA1 | CLL-up-regulated | Oncogene | Increased expression is associated with adverse prognosis | [144] | ||
| TUG1 | AML-up-regulated | Oncogene | AURKA miR-221-3p | increases cell proliferation, suppresses apoptosis | Increased expression is associated with adverse prognosis | [145,146] |
| Drug resistance | miR-34a | [147] | ||||
| UCA1 | AML-up-regulated | Oncogene | p27Kip1 (CDKN1B) | Induces cell proliferation | Overexpressed in AML patients positive for CEPBA mutations | [148] |
| miR-126 | Increases viability, migration and invasion | [149] | ||||
| Drug resistance | miR-125 | [150] | ||||
| CML-up-regulated | Drug resistance | miR-16 | [151] | |||
| ZNF667-AS1 | CLL-up-regulated | Oncogene | Increased expression is associated with adverse prognosis | [152] | ||
| AML-up-regulated | Oncogene | miR-206 | Increased proliferation and invasion of leukemic cells | Increased expression is associated with adverse prognosis | [153,154] | |
| APL-down-regulated | Tumor suppressor | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasic, V.; Karan-Djurasevic, T.; Pavlovic, D.; Zukic, B.; Pavlovic, S.; Tosic, N. Diagnostic and Therapeutic Implications of Long Non-Coding RNAs in Leukemia. Life 2022, 12, 1770. https://doi.org/10.3390/life12111770
Gasic V, Karan-Djurasevic T, Pavlovic D, Zukic B, Pavlovic S, Tosic N. Diagnostic and Therapeutic Implications of Long Non-Coding RNAs in Leukemia. Life. 2022; 12(11):1770. https://doi.org/10.3390/life12111770
Chicago/Turabian StyleGasic, Vladimir, Teodora Karan-Djurasevic, Djordje Pavlovic, Branka Zukic, Sonja Pavlovic, and Natasa Tosic. 2022. "Diagnostic and Therapeutic Implications of Long Non-Coding RNAs in Leukemia" Life 12, no. 11: 1770. https://doi.org/10.3390/life12111770
APA StyleGasic, V., Karan-Djurasevic, T., Pavlovic, D., Zukic, B., Pavlovic, S., & Tosic, N. (2022). Diagnostic and Therapeutic Implications of Long Non-Coding RNAs in Leukemia. Life, 12(11), 1770. https://doi.org/10.3390/life12111770








