Abstract
Microaxial left ventricular assist devices (LVAD) are increasingly used to support patients with cardiogenic shock; however, outcome results are limited to single-center studies, registry data and select reviews. We conducted a systematic review and meta-analysis, searching three databases for relevant studies reporting on microaxial LVAD use in adults with cardiogenic shock. We conducted a random-effects meta-analysis (DerSimonian and Laird) based on short-term mortality (primary outcome), long-term mortality and device complications (secondary outcomes). We assessed the risk of bias and certainty of evidence using the Joanna Briggs Institute and the GRADE approaches, respectively. A total of 63 observational studies (3896 patients), 6 propensity-score matched (PSM) studies and 2 randomized controlled trials (RCTs) were included (384 patients). The pooled short-term mortality from observational studies was 46.5% (95%-CI: 42.7–50.3%); this was 48.9% (95%-CI: 43.8–54.1%) amongst PSM studies and RCTs. The pooled mortality at 90 days, 6 months and 1 year was 41.8%, 51.1% and 54.3%, respectively. Hemolysis and access-site bleeding were the most common complications, each with a pooled incidence of around 20%. The reported mortality rate of microaxial LVADs was not significantly lower than extracorporeal membrane oxygenation (ECMO) or intra-aortic balloon pumps (IABP). Current evidence does not suggest any mortality benefit when compared to ECMO or IABP.
1. Introduction
The incidence of cardiogenic shock (CS) has increased in recent years, yet long-term mortality has not substantially improved in the last 20 years [1]. It is associated with significant multi-organ failure and in-hospital mortality reaching in excess of 60% [2,3]. Amongst survivors, up to 20% are re-admitted within 30 days [1]. Acute myocardial infarction is the most common cause of CS and accounts for 10% of patients with CS [1,4]. A spectrum of disease exists in cardiogenic shock—the Society of Cardiovascular Angiography and Interventions (SCAI) classifies CS from Stages A (at-risk) to E (in extremis). Within Stage C (classic) CS, patients typically present with hypoperfusion requiring either inotropes or temporary circulatory support devices [5]. Various temporary circulatory support devices are available for these patients—this ranges from counterpulsation devices such as the intra-aortic balloon pump (IABP), percutaneously inserted left ventricular assist devices (pLVADs, including microaxial and centrifugal), paracorporeal VADs and extracorporeal membrane oxygenation [6,7].
Despite the wide range of options for temporary circulatory support, outcomes remain variable [7,8,9,10,11,12,13]. Microaxial LVADs placed retrogradely across the aortic or pulmonary valves [7,14] are increasingly being used to support patients with cardiogenic shock of various etiologies [14,15,16,17,18], or as a “bridge to decision” in end-stage heart failure [19]. They reduce the ventricular afterload and ventricular end-diastolic pressure, and increase the mean arterial pressure [20] and cardiac output [21]. Smaller percutaneous devices have been authorized for use for up to 4 days, [22] whereas larger surgically inserted devices are authorized for use for up to 14 days [23].
In addition to the multiple device options, existing studies and reviews investigating its use report favorable survival outcomes and safety outcomes in patients with CS [9,24]. However, the outcomes of microaxial LVADs based on the various types and different etiologies of CS have not been elucidated in detail [24]. In addition, potential predictors of mortality have yet to be explored. We conducted this systematic review and meta-analysis to investigate the short- and long-term mortality outcomes and device-related complications of microaxial LVADs in all etiologies of CS, and to explore the potential risk factors associated with mortality.
2. Methodology
2.1. Search Strategy and Selection Criteria
This review was registered on PROSPERO (CRD42020202807) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Table S1) [25]. We searched MEDLINE, Embase and Scopus databases from 1 January 2003 to 13 July 2022 using the keywords ‘Impella’ and ‘cardiogenic shock’ (Table S2). We included studies published in English, reporting on ≥10 non-overlapping adult patients (>18 years) receiving microaxial LVADs for CS. In cases of overlapping patient data, we included the larger study. We excluded studies reporting on animals and where device was inserted prophylactically or electively during percutaneous coronary intervention. We also excluded those studies where outcomes were not stratified by device option in CS, and national or international registry databases that could contribute to duplication of patient data.
2.2. Data Extraction
Data collection included study design (author and study name, year of publication, country, setting, number of patients), patient demographics (age, gender, comorbidities), pre-LVAD clinical characteristics (body mass index, left ventricular ejection fraction [LVEF], comorbidities), etiology of CS (acute myocardial infarction cardiogenic shock [AMICS] or non-myocardial infarction cardiogenic shock [NMICS]), device characteristics (mode of insertion, cannulation access, concomitant extracorporeal membrane oxygenation [ECMO] use, duration of support) and outcomes of interest (in-hospital mortality, 30 days, 90 days, 6 months, 1 year and device-related complications).
2.3. Risk of Bias and Certainty Assessment
Risk of bias in individual studies was assessed using the appropriate Joanna Briggs Institute (JBI) checklists. Egger’s test was used to assess the possibility of publication bias. As inter-study heterogeneity can be misleadingly large when assessed using I2 statistics for observational studies [26], we used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach to rate the certainty of evidence [27,28]. The screening of articles, data collection and risk of bias assessment were conducted independently by two reviewers (TSR and NWL), and any conflicts were resolved by a third reviewer (KR).
2.4. Outcomes
The primary outcome was short-term mortality, defined as 30-day mortality or in-hospital mortality, whichever was longer. Secondary outcomes include long-term mortality at 90 days, 6 months and 1 year, and device-related complications (device malfunction, access-site bleeding, hemolysis, limb ischemia and stroke). Tables S3 and S4 summarize the definitions of CS, device-related complications and severity of LVEF [29].
2.5. Statistical Analysis
For continuous variables, we pooled the means and standard deviations (SDs) in accordance with Wan et al. [30]. Categorical data are reported as pooled proportions with 95% confidence intervals (CIs), whereas continuous outcomes are reported as pooled means with 95% CIs. All analyses were conducted in R4.0.1. Random effects meta-analyses (DerSimonian and Laird) were conducted using the Freeman–Tukey double arcsine transformation, and 95% CIs were computed using the Clopper–Pearson method [31,32,33]. We pooled the results of the propensity-score matched (PSM) studies and RCTs together as previous studies have shown that the estimates obtained from PSM studies are similar and as robust as RCTs [34,35,36]. Sensitivity analysis was conducted by excluding studies with higher risks of bias (defined as <7). Planned subgroup analyses were conducted with continuity correction of 0.5 to allow for inclusion of studies with zero events, and included the geographical region (Europe, North America or Asia), etiology of CS (AMICS or NMICS), the mode of insertion (percutaneous (which comprises Impella 2.5 and CP) or surgical (which comprises Impella 5.0 and Impella 5.5)), cannulation access for insertion (axillary or femoral), duration of support (more or less than 4 days), concomitant use of ECMO, IABP prior to microaxial LVAD use and pre-LVAD LVEF (above or below 20%). Summary-level meta-regression was conducted if there was a minimum of 6 data points in order to explore sources of heterogeneity and to identify potential prognostically relevant study-level covariates [37].
2.6. Role of the Funding Source
This study had no funding source.
3. Results
From 4173 articles, we reviewed 206 full-text articles. In total, we included 71 studies (63 observational, 8 PSM/RCTs) detailing 4280 adult patients that reported on the use of microaxial LVADs in CS (Figure 1) [16,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106]. The findings of the one-armed observational studies and the findings of the PSM/RCTs are reported separately. Of the observational studies, 34 were reported by centers from Europe, 25 from North America, 3 from Asia and 1 from South America, whereas all of the RCTs and PSMs were reported by centers from Europe. Percutaneously inserted devices were more commonly used than surgically inserted devices.
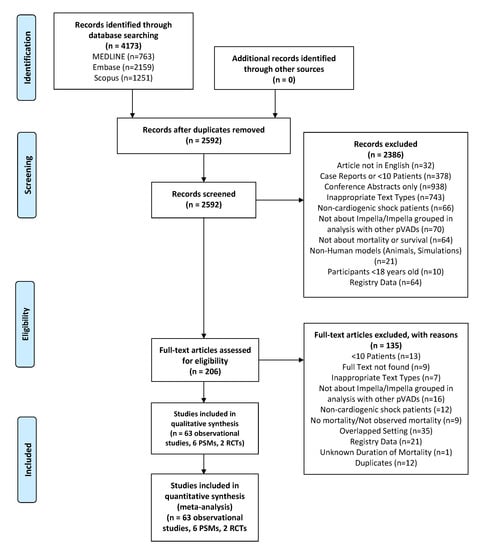
Figure 1.
Flow diagram of selection of articles based on PRISMA statement. Abbreviations: PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses; pVAD = percutaneous ventricular assist device; RCT = randomized controlled trial.
3.1. Demographics of Included Studies
Table 1 presents the baseline demographics of the observational studies. Among the 63 studies, 14 studies reported on patients with AMICS, 4 studies reported on patients with NMICS, and 38 studies reported on both patients with NMICS and patients with AMICS. The etiology of cardiogenic shock was not reported in 8 studies. Patients were predominantly male (75.0%, 95%-CI: 70.9% to 78.8%), and were supported for an average of 6.2 days (95%-CI: 4.7 to 7.7). The pooled intensive care unit (ICU) stay was 13.7 days (95%-CI: 10.2 to 17.2), and the pooled hospital length of stay was 20.6 days (95%-CI: 13.0 to 28.2).

Table 1.
Demographics of included studies.
Table 1 refers to pooled demographics of 384 patients across the PSM studies and RCTs. The pooled age was 62.2 years, and the majority (82.2%) were male. The pooled duration of microaxial LVAD support was 2.9 days, and the pooled ICU stay was 8.6 days. The pooled hospital stay was 15.3 days.
3.2. Primary Meta-Analysis
Amongst the observational studies (63 studies, 3896 patients), the pooled short-term mortality was 46.5% (95%-CI: 42.7% to 50.3%, Figure 2). As all studies had a JBI score of ≥7, sensitivity analysis excluding studies with higher risks of bias was not possible. We excluded studies with a JBI score of <10 as an exploratory analysis, and this yielded similar pooled estimates for the short-term mortality (44.7%, 95%-CI: 40.2% to 49.2%).
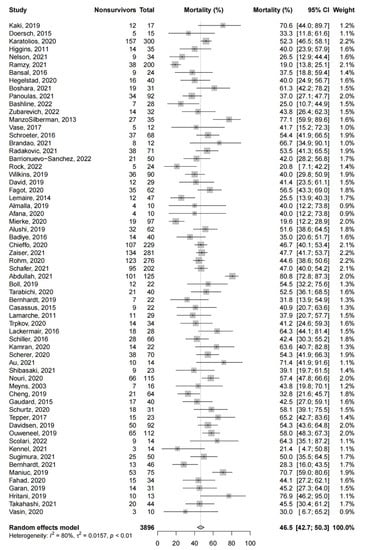
Figure 2.
Short-term mortality in observational studies. Forest plot summarizing the short-term mortality of patients receiving microaxial LVAD for cardiogenic shock amongst observational studies. Abbreviations: CI = confidence interval.
There were no significant differences in short-term mortality with respect to the etiology of CS, mode of insertion and concomitant use of ECMO. Patients who presented with AMICS (52.1%, 95%-CI: 46.8% to 57.3%, 14 studies) had a comparatively higher short-term mortality than patients who presented with NMICS (42.0%, 95%-CI: 33.5 to 50.8%, 5 studies, p = 0.085). Mortality was significantly higher among patients receiving concomitant ECMO (51.5%, 95%-CI: 47.1% to 55.9%, 8 studies) than patients receiving microaxial LVADs only (44.6%, 95%-CI: 39.6% to 49.6%, 40 studies, p = 0.043).
No significant differences were found in short-term mortality when considering the geographical location (North America, South America, Europe or Asia), patient demographic factors (pre-LVAD LVEF (≤20% or >20%)) or device factors (duration of microaxial LVAD support (≤4 days or >4 days) and cannulation site (axillary or femoral)). Table S5 summarizes the results of the subgroup analysis.
Univariate meta-regression found significant associations between mortality and previous cerebrovascular accidents (regression coefficient (B): 0.29, 95%-CI: 0.15 to 0.56, p = 0.038) and hyperlipidemia (B: 0.68, 95%-CI: 0.02 to 0.32, p = 0.030), and an inverse association with the duration of the device support (B: −0.015, 95%-CI: −0.015, 95%-CI: −0.022 to −0.009, p < 0.0001). However, there was no significant association between mortality and patient demographics, including age, other comorbidities (hypertension, diabetes mellitus, previous acute myocardial infarction, heart failure, smoking) and pre-LVAD LVEF. Table S6 summarizes the meta-regression analysis.
Among the PSM studies and RCTs, the pooled short-term mortality (Figure 3) was 48.9% (95%-CI: 43.8% to 54.1%) From one study that compared microaxial LVADs to IABP alone, microaxial LVADs did not significantly reduce the risk of mortality (RR: 0.94, 95%-CI: 0.58–1.53, p = 0.81). Five studies provided a comparison between microaxial LVADs and other devices; we report these findings qualitatively. From two studies comparing microaxial LVADs with IABP, one study found approximately 46% of patients expired in both cohorts [87], and similar findings were reported in the other (hazard ratio for mortality: 0.96, p = 0.92 [77]. One study found that microaxial LVADs (49.4%) were associated with a trend to lower mortality compared to ECMO (61.4%, p = 0.16) [64], which was echoed by another PSM study (55% vs. 67.5%, p = 0.36) [105], while another study found that concurrent microaxial LVAD with ECMO (47%) significantly reduced mortality compared to ECMO alone (80%, p < 0.001) [79]. Finally, microaxial LVAD was shown to improve mortality (20%) compared to patients without any mechanical circulatory support (47%, p = 0.0024) [88].
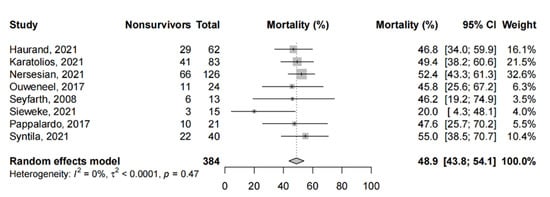
Figure 3.
Short-term mortality in propensity-score matched studies and randomized controlled trials. Forest plot summarizing the short-term mortality of patients receiving microaxial LVAD for cardiogenic shock amongst propensity-score matched studies and randomized controlled trials. Abbreviations: CI = confidence interval.
3.3. Secondary Outcomes
3.3.1. Long-Term Mortality
The pooled 90-day, 6-month and 1-year mortality was 41.8% (95%-CI: 34.4% to 49.3%, 5 studies, 448 patients), 51.1% (95%-CI: 45.2% to 57.0%, 9 studies, 676 patients) and 54.3% (95%-CI: 48.9% to 59.7%, 10 studies, 881 patients), respectively, amongst the observational studies (Figure 4). Two PSM studies reported a mortality at the 6-month follow up that ranged between 36.6% (12/33) and 75% (45/60) [58,74]. One PSM study reported a 1-year mortality rate of 60%.
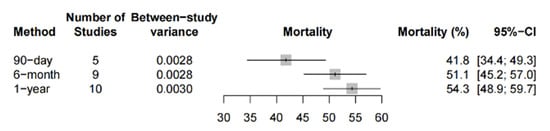
Figure 4.
Long-term mortality in observational studies. Forest plot summarizing the 90-day, 6-month and 1-year mortality of patients receiving microaxial LVAD for cardiogenic shock based on observational studies. Abbreviations: CI = confidence interval.
3.3.2. Complications
Table 2 shows the top five device-related complications (hemolysis, access-site bleeding device malfunction, limb ischemia, stroke) reported amongst 50 observational studies (3101 patients). Hemolysis (24.9%, 95%-CI: 14.9% to 36.4%, 1708 patients, 23 studies), access-site bleeding (25.8%, 95%-CI: 14.7% to 38.5%, 1679 patients, 23 studies) and device malfunction (6.0%, 95%-CI: 3.1% to 9.3%, 690 patients, 17 studies) were the three most common complications in this patient cohort. From the PSM studies and RCTs, the pooled incidence of stroke (three studies) was 0.4% (95%-CI: 0.0% to 2.4%), whereas hemolysis occurred in 40.8% (95%-CI: 4.4% to 84.4%, four studies) of patients. Bleeding was reported among four studies (6.4%, 95%-CI: 3.3% to 10.4%), and three studies reported on device malfunction (3.2%, 95%-CI: 0.0% to 26.9%). Finally, 6.8% (95-CI: 0.0% to 21.6%) of patients (six studies) suffered from limb ischemia.

Table 2.
Complications of Observational Studies.
3.4. Risk of Bias and Certainty of Evidence Assessment
Using appropriate JBI checklists, all studies were of high quality (score of ≥7, Table S7). We assessed the certainty of evidence for all primary and secondary outcome measures using the GRADE approach (Table S8). For both observational studies and RCTs, the certainty of evidence was high according to the GRADE evaluation for our primary outcome of short-term mortality and that of long-term mortality, whereas the complications were deemed to be of moderate-to-high certainty. Egger’s test found that Pegger was 0.96, indicating that publication bias is unlikely.
4. Discussion
This review comprising 71 studies and 4280 patients demonstrated that microaxial LVAD in CS was associated with mortality rates approaching 50%. Patients were predominantly middle-aged males. The 90-day, 6-month and 1-year mortality (observational studies) was 41.8%, 51.1% and 54.3%, respectively. Short-term mortality was relatively higher in patients with surgical insertion compared to percutaneous insertion. Comorbitidies including previous cerebrovascular accidents and hyperlipidemia were associated with mortality, whereas longer durations of device support were associated with survival.
Our study provides further insights into the characteristics of microaxial LVAD devices that may affect mortality. We found that patients receiving surgically inserted devices had a relatively higher mortality rate than percutaneously inserted devices. This is likely to be because multifactorial-percutaneously inserted devices generate a maximum of 2.5 to 4.0 L/min of blood flow, [107] whereas surgically inserted devices generate up to 5.0 and 6.0 L/min [107,108]. Patients with more severe cardiogenic shock may have higher support requirements and intrinsically higher mortality rates due to their clinical presentation. In addition, the surgical insertion of devices might increase the rates of surgical site infection and bleeding. Finally, higher flows generated by surgically inserted devices may lead to higher rates of hemolysis. We also found that the duration of the device support was not associated with a higher mortality. This is contrary to previous studies that have shown that the use of microaxial LVADs for >4 days led to an increased mortality and duration of hospital and coronary care unit stay [109]. Nonetheless, this could be attributed to immortal time bias, which has been described in observational studies [110] and in patients on life-saving devices [111,112], where patients in the treated group have to survive and be event-free until the treatment definition is fulfilled [113].
Mortality rates for CS remain high despite timely goal-directed medical management [7,114,115]. The variable survival rates of CS between the use of mechanical cardiac support devices is evident from the IABP-SHOCK I and II trials that showed that IABP did not significantly improve 30-day survival [10,116], whereas the international Extracorporeal Life Support Organization registry found that 42% of patients receiving venoarterial ECMO survived to discharge [117]. This contrasts with the 51% survival rate of patients receiving microaxial LVADs in the United States [18]. Similarly, in our observational cohort of patients with microaxial LVAD support, we observed short-term survival rates of 53%. However, survival rates of 70% have been reported in advanced cardiac centers with robust protocols comprising the stringent selection criteria team-based management of CS [118,119]. The concomitant use of microaxial LVADs and ECMO is an area of increasing interest to improve outcomes. Microaxial LVADs unload the left ventricle (LV) and may help offset the LV distension secondary to retrograde aortic blood flow in patients on peripheral venoarterial ECMO [120]. Our study found that patients receiving concomitant ECMO had a significantly higher mortality rate than those receiving microaxial LVADs alone (51.5% vs. 44.6%, p = 0.04). However, this can be confounded by the severity of cardiogenic shock, and VA-ECMO may only be initiated in the context of cardiogenic shock refractory to other therapies. As such, it is unclear whether VA-ECMO causes an increase in mortality, or if it is simply initiated in patients with more severe cardiogenic shock.
The long-term mortality reported in our review was higher compared to those reported in major trials on microaxial LVADs [17,121]. The reasons may be multifactorial: both RCTs had fewer patients with a smaller range of etiologies of CS, and robust patient selection criteria and management protocols. On the other hand, patients recruited in the observational studies were heterogenous in selection and management. The higher incidence of complication rates could also have impacted the long-term outcomes. The most frequently reported device-related complication was hemolysis, which was higher than those reported in previous registry reviews [24,122,123]. There was also a discrepancy between RCTs and PSM studies, and observational studies in the incidence of hemolysis (40.8% vs. 23.8%) and access site bleeding (6.4% vs. 25.8%). Possible reasons include a longer pooled duration of device support in observational studies compared to RCTs, varying definitions of hemolysis and the predominant use of percutaneous devices with a smaller pump design. Access-site bleeding was reported in 15 studies with a pooled prevalence of 19.4%, similar to the USpella cohort [122] but lower than the EUROSHOCK cohort [108,123,124]. Notably, our study found that the pooled incidence of limb ischemia was comparable between the observational studies and RCTs (6.3% vs. 8.2%), and was lower compared to ECMO and IABP, whereas the incidence of access-site bleeding was higher compared to ECMO and IABP [37,125,126]. Nonetheless, the incidence of limb ischemia and bleeding may have been affected by multiple factors, such as the use of anticoagulants or presence of peripheral vascular disease, for which, adequate data were not clearly available [7].
The strengths of this review include a comprehensive search strategy and robust inclusion criteria that encompassed all etiologies of CS and types of devices used. It also included a detailed analysis of various patient and intervention factors and their impact on mortality outcomes. Nonetheless, we recognize several limitations. First, there is significant heterogeneity in patient demographics, definitions, variations in patient selection, practices and reporting patterns and the observational nature of the included studies, which we tried to account for by using subgroup and meta-regression analyses. Meta-regression analyses are also inherently constrained by a lack of power, resulting in an increased risk of type II errors. Almost all of the analyses have also been limited to North America and Europe, whereas studies from Asia remain scarce. Hence, the results might not be generalizable to other parts of the world where healthcare systems and workflows are different. Nonetheless, our subgroup analysis on geographical location did not find any significant difference in short-term mortality. Moreover, the GRADE assessment suggested a high certainty in the evidence for the primary outcome and long-term mortality, whereas complications were of moderate to high certainty. With scores of 7 or higher, JBI critical appraisal also deemed all 71 articles as high quality and suitable for inclusion.
5. Conclusions
This review summarizes the mortality outcomes and complications of microaxial LVADs in patients with CS. Short-term mortality was 46.5% whereas 6-month and 1-year mortalities were 51% and 54%, respectively. Complications such as hemolysis and access site bleeding were high as reported in the observational studies. Nonetheless, the use of temporary circulatory support in cardiogenic shock remains inherently challenging as patients are usually critically ill with multi-organ pathologies, and patient care is heterogenous. In addition, the current evidence base is limited in concluding whether or not microaxial LVADs confer a survival benefit in patients with CS. Further RCTs are warranted to better assess the effectiveness and role of microaxial LVADs in CS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12101629/s1, Table S1: Preferred Reporting Items for Systematic reviews and Meta-analyses checklist; Table S2: Search strategies for databases; Table S3: Definitions of complications reported while patients were receiving microaxial LVAD support.; Table S4: American Society of Echocardiography Guidelines on severity of cardiogenic shock based on two-dimensional echocardiogram-derived left ventricular ejection fraction.; Table S5: Results of subgroup analysis; Table S6: Results of meta-regression analysis; Table S7: Joanna Briggs Institute Critical Appraisal Checklist.; Table S8: Grading of Recommendations, Assessment, Development, and Evaluations. Refs. [29,42,48,76,77,92] are cited in Supplementary Materials.
Author Contributions
Conceptualization: K.R.; methodology and data curation: S.R.T., C.J.W.L., W.L.N. and R.R.L.; software and formal analysis: R.R.L., C.S.T. and K.R.; visualization: S.R.T.; C.J.W.L., W.L.N. and R.R.L.; writing—original draft preparation: S.R.T., C.J.W.L., W.L.N. and R.R.L.; writing—review and editing: S.R.T., C.J.W.L., W.L.N., R.R.L., C.S.T., S.L.L., R.C., W.L., K.S., S.M., G.M. and K.R.; supervision: K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Kollengode Ramanathan serves as a co-chair of the Scientific Oversight Committee of the Extracorporeal Life support Organization (ELSO), and reports honoraria for educational lectures from Baxter Ltd., and Fresenius Ltd. All other authors declare no competing interest.
References
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic shock. J. Am. Heart. Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, C.; Rueda, F.; Oliveras, T.; Serra, J.; Labata, C.; Ferrer, M.; Bayes-Genis, A. P779 Cardiogenic shock in STEMI patients: Prevalence, management and acute phase mortality over the last three decades. Eur. Heart J. 2018, 39, ehy564.P779. [Google Scholar]
- Goldberg, R.J.; Spencer, F.A.; Gore, J.M.; Lessard, D.; Yarzebski, J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: A population-based perspective. Circulation 2009, 119, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- De Chambrun, M.P.; Donker, D.W.; Combes, A. What’s new in cardiogenic shock? Intensive Care Med. 2020, 46, 1016–1019. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Fernando, S.M.; Price, S.; Mathew, R.; Slutsky, A.S.; Combes, A.; Brodie, D. Mechanical circulatory support in the treatment of cardiogenic shock. Curr. Opin. Crit. Care 2022, 28, 434–441. [Google Scholar] [CrossRef]
- Combes, A.; Price, S.; Slutsky, A.S.; Brodie, D. Temporary circulatory support for cardiogenic shock. Lancet 2020, 396, 199–212. [Google Scholar] [CrossRef]
- Shaefi, S.; O’Gara, B.; Kociol, R.; Joynt, K.; Mueller, A.; Nizamuddin, J.; Mahmood, E.; Talmor, D.; Shahul, S. Effect of Cardiogenic Shock Hospital Volume on Mortality in Patients With Cardiogenic Shock. J. Am. Heart Assoc. 2015, 4, e001462. [Google Scholar] [CrossRef]
- Iannaccone, M.; Albani, S.; Giannini, F.; Colangelo, S.; Boccuzzi, G.G.; Garbo, R.; Brilakis, E.S.; D’Ascenzo, F.; de Ferrari, G.M.; Colombo, A. Short term outcomes of Impella in cardiogenic shock: A review and meta-analysis of observational studies. Int. J. Cardiol. 2020, 324, 44–51. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Kapur, N.K.; Jumean, M.F. Defining the Role for Percutaneous Mechanical Circulatory Support Devices for Medically Refractory Heart Failure. Curr. Heart Fail. Rep. 2013, 10, 177–184. [Google Scholar] [CrossRef]
- Smith, L.; Peters, A.; Mazimba, S.; Ragosta, M.; Taylor, A.M. Outcomes of patients with cardiogenic shock treated with TandemHeart® percutaneous ventricular assist device: Importance of support indication and definitive therapies as determinants of prognosis. Catheter. Cardiovasc. Interv. 2018, 92, 1173–1181. [Google Scholar] [CrossRef]
- Lemor, A.; Dehkordi, S.H.H.; Basir, M.B.; Villablanca, P.A.; Jain, T.; Koenig, G.C.; Alaswad, K.; Moses, J.W.; Kapur, N.K.; O’Neill, W. Impella Versus Extracorporeal Membrane Oxygenation for Acute Myocardial Infarction Cardiogenic Shock. Cardiovasc. Revascularization Med. 2020, 21, 1465–1471. [Google Scholar] [CrossRef]
- Cheung, A.W.; White, C.; Davis, M.K.; Freed, D.H. Short-term mechanical circulatory support for recovery from acute right ventricular failure: Clinical outcomes. J. Heart Lung Transplant. 2014, 33, 794–799. [Google Scholar] [CrossRef]
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560. [Google Scholar] [CrossRef]
- Bansal, A.; Bhama, J.K.; Patel, R.; Desai, S.; Mandras, S.A.; Patel, H.; Collins, T.; Reilly, J.P.; Ventura, H.O.; Parrino, P.E. Using the Minimally Invasive Impella 5.0 via the Right Subclavian Artery Cutdown for Acute on Chronic Decompensated Heart Failure as a Bridge to Decision. Ochsner J. 2016, 16, 210–216. [Google Scholar]
- Griffith, B.P.; Anderson, M.B.; Samuels, L.E.; Pae Jr, W.E.; Naka, Y.; Frazier, O.H. The RECOVER I: A multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J. Thorac. Cardiovasc. Surg. 2013, 145, 548–554. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Grines, C.; Schreiber, T.; Moses, J.; Maini, B.; Dixon, S.R.; Ohman, E.M. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am. Heart J. 2018, 202, 33–38. [Google Scholar] [CrossRef]
- Lima, B.; Kale, P.; Gonzalez-Stawinski, G.V.; Kuiper, J.J.; Carey, S.; Hall, S.A. Effectiveness and Safety of the Impella 5.0 as a Bridge to Cardiac Transplantation or Durable Left Ventricular Assist Device. Am. J. Cardiol. 2016, 117, 1622–1628. [Google Scholar] [CrossRef]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of mechanical circulatory support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef]
- Burzotta, F.; Russo, G.; Previ, L.; Bruno, P.; Aurigemma, C.; Trani, C. Impella: Pumps overview and access site management. Minerva Cardioangiol. 2018, 66, 606–611. [Google Scholar] [CrossRef]
- Hill, J.; Banning, A.; Burzotta, F.; Chieffo, A.; Schieffer, B.; Schafer, A.; Mstelmaszuk-Zadykowicz, N.; Sun, S.; Spelman, T.; Doshi, S.; et al. A systematic literature review and meta-analysis of impella devices used in cardiogenic shock and high risk percutaneous coronary interventions. Interv. Cardiol. 2019, 11, 163. [Google Scholar] [CrossRef]
- Abiomed, I. Approves Impella 5.0 and Impella LD Extended Duration of Use to 14 Days for Cardiogenic Shock Derived from AMI or Cardiomyopathy; FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Vargas, K.G.; Jäger, B.; Kaufmann, C.C.; Biagioli, A.; Watremez, S.; Gatto, F.; Özbek, C.; Razouk, A.; Geppert, A.; Huber, K. Impella in cardiogenic shock following acute myocardial infarction: A systematic review and meta-analysis. Wien. Klin. Wochenschr. 2020, 132, 716–725. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Iorio, A.; Spencer, F.A.; Falavigna, M.; Alba, A.; Lang, E.; Burnand, B.; McGinn, T.; Hayden, J.; Williams, K.; Shea, B.; et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 2015, 350, h870. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Clopper, C.J.; Pearson, E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Miller, J.J. The inverse of the Freeman–Tukey double arcsine transformation. Am. Stat. 1978, 32, 138. [Google Scholar]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2013, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Hartz, A.J. A comparison of observational studies and randomized, controlled trials. N. Engl. J. Med. 2000, 342, 1878–1886. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Haidich, A.B.; Pappa, M.; Pantazis, N.; Kokori, S.I.; Tektonidou, M.G.; Lau, J. Comparison of evidence of treatment effects in randomized and nonrandomized studies. Jama 2001, 286, 821–830. [Google Scholar] [CrossRef]
- Jia, D.; Yang, I.X.; Ling, R.R.; Syn, N.; Poon, W.H.; Murughan, K.; Ramanathan, K. Vascular complications of extracorporeal membrane oxygenation: A systematic review and meta-regression analysis. Crit. Care Med. 2020, 48, e1269–e1277. [Google Scholar] [CrossRef]
- Afana, M.; Altawil, M.; Basir, M.; Alqarqaz, M.; Alaswad, K.; Eng, M.; O’Neill, W.W.; Lederman, R.J.; Greenbaum, A.B. Transcaval access for the emergency delivery of 5.0 liters per minute mechanical circulatory support in cardiogenic shock. Catheter. Cardiovasc. Interv. 2020, 97, 555–564. [Google Scholar] [CrossRef]
- Almalla, M.; Kersten, A.; Altiok, E.; Marx, N.; Schröder, J.W. Hemodynamic support with Impella ventricular assist device in patients undergoing TAVI: A single center experience. Catheter. Cardiovasc. Interv. 2019, 95, 357–362. [Google Scholar] [CrossRef]
- Alushi, B.; Douedari, A.; Froehlig, G.; Knie, W.; Wurster, T.H.; Leistner, D.M.; Staehli, B.-E.; Mochmann, H.-C.; Pieske, B.; Landmesser, U.; et al. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart 2019, 6, e000987. [Google Scholar] [CrossRef]
- Au, S.-Y.; Fong, K.-M.; Tsang, C.-F.S.; Chan, K.-C.A.; Wong, C.Y.; Ng, W.-Y.G.; Lee, K.Y.M. Veno-arterial extracorporeal membrane oxygenation with concomitant Impella versus concomitant intra-aortic-balloon-pump for cardiogenic shock. Perfusion 2021, 02676591211033947, online ahead of print. [Google Scholar] [CrossRef]
- Badiye, A.P.; Hernandez, G.A.; Novoa, I.; Chaparro, S.V. Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device. ASAIO J. 2016, 62, 11–14. [Google Scholar] [CrossRef]
- Bernhardt, A.M.; Potapov, E.; Schibilsky, D.; Ruhparwar, A.; Tschöpe, C.; Spillmann, F.; Benk, C.; Schmack, B.; Schmitto, J.D.; Napp, L.C.; et al. First in man evaluation of a novel circulatory support device: Early experience with the Impella 5.5 after CE mark approval in Germany. J. Heart Lung Transplant. 2021, 40, 850–855. [Google Scholar] [CrossRef]
- Bernhardt, A.M.; Zipfel, S.; Reiter, B.; Hakmi, S.; Castro, L.; Söffker, G.; Kluge, S.; Lubos, E.; Rybczinski, M.; Grahn, H.; et al. Impella 5.0 therapy as a bridge-to-decision option for patients on extracorporeal life support with unclear neurological outcomes. Eur. J. Cardio-Thorac. Surg. 2019, 56, 1031–1036. [Google Scholar] [CrossRef]
- Boll, G.; Fischer, A.; Kapur, N.K.; Salehi, P. Right Axillary Artery Conduit Is a Safe and Reliable Access for Implantation of Impella 5.0 Microaxial Pump. Ann. Vasc. Surg. 2018, 54, 54–59. [Google Scholar] [CrossRef]
- Boshara, A.; Patel, A.; Alasaad, M.; Dutcheshen, K.J.; LaLonde, T.A.; Schreiber, T.L.; Mehta, R.H.; Kaki, A.; Rosman, H.S. Cardiogenic Shock Complicating Acute Myocardial Infarction Treated With Percutaneous Coronary Intervention Supported by Impella: Implications of Advanced Age and Refractory Shock on Outcomes. Crit. Care Explor. 2021, 3, e0447. [Google Scholar] [CrossRef]
- Brandão, M.; Caeiro, D.; Pires-Morais, G.; Almeida, J.G.; Teixeira, P.G.; Silva, M.P.; Braga, P. Impella support for cardiogenic shock and high-risk percutaneous coronary intervention: A single-center experience. Rev. Port. Cardiol. 2021, 40, 853–861. [Google Scholar] [CrossRef]
- Casassus, F.; Corre, J.; Leroux, L.; Chevalereau, P.; Fresselinat, A.; Seguy, B.; Calderon, J.; Coste, P.; Ouattara, A.; Roques, X.; et al. The Use of Impella 2.5 in Severe Refractory Cardiogenic Shock Complicating an Acute Myocardial Infarction. J. Interv. Cardiol. 2015, 28, 41–50. [Google Scholar] [CrossRef]
- Cheng, R.; Tank, R.; Ramzy, D.; Azarbal, B.; Chung, J.; Esmailian, F.; Kobashigawa, J.A.; Moriguchi, J.D.; Arabia, F.A. Clinical Outcomes of Impella Microaxial Devices Used to Salvage Cardiogenic Shock as a Bridge to Durable Circulatory Support or Cardiac Transplantation. ASAIO J. 2019, 65, 642–648. [Google Scholar] [CrossRef]
- Chieffo, A.; Ancona, M.B.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in ITaly: The IMP-IT registry. EuroIntervention 2020, 15, e1343–e1350. [Google Scholar] [CrossRef]
- David, C.H.; Quessard, A.; Mastroianni, C.; Hekimian, G.; Amour, J.; Leprince, P.; Lebreton, G. Mechanical circulatory support with the Impella 5.0 and the Impella Left Direct pumps for postcardiotomy cardiogenic shock at La Pitié-Salpêtrière Hospital. Eur. J. Cardio-Thorac. Surg. 2020, 57, 183–188. [Google Scholar] [CrossRef]
- Davidsen, C.; Packer, E.J.; Løland, K.H.; Rotevatn, S.; Nygreen, E.L.; Eriksen, E.; Øksnes, A.; Herstad, J.; Haaverstad, R.; Bleie, Ø.; et al. Impella use in acute myocardial infarction complicated by cardiogenic shock and cardiac arrest: Analysis of 10 years registry data. Resuscitation 2019, 140, 178–184. [Google Scholar] [CrossRef]
- Doersch, K.; Tong, C.W.; Gongora, E.; Konda, S.; Sareyyupoglu, B. Temporary Left Ventricular Assist Device Through an Axillary Access is a Promising Approach to Improve Outcomes in Refractory Cardiogenic Shock Patients. ASAIO J. 2015, 61, 253–258. [Google Scholar] [CrossRef]
- Fagot, J.; Bouisset, F.; Bonello, L.; Biendel, C.; Lhermusier, T.; Porterie, J.; Roncalli, J.; Galinier, M.; Elbaz, M.; Lairez, O.; et al. Early Evaluation of Patients on Axial Flow Pump Support for Refractory Cardiogenic Shock is Associated with Left Ventricular Recovery. J. Clin. Med. 2020, 9, 4130. [Google Scholar] [CrossRef]
- Fahad, F.; Shaukat, M.H.S.; Yager, N. Incidence and Outcomes of Acute Kidney Injury Requiring Renal Replacement Therapy in Patients on Percutaneous Mechanical Circulatory Support with Impella-CP for Cardiogenic Shock. Cureus 2020, 12, e6591. [Google Scholar] [CrossRef]
- Garan, A.R.; Takeda, K.; Salna, M.; Vandenberge, J.; Doshi, D.; Karmpaliotis, D.; Kirtane, A.J.; Takayama, H.; Kurlansky, P. Prospective Comparison of a Percutaneous Ventricular Assist Device and Venoarterial Extracorporeal Membrane Oxygenation for Patients With Cardiogenic Shock Following Acute Myocardial Infarction. J. Am. Heart Assoc. 2019, 8, e012171. [Google Scholar] [CrossRef]
- Gaudard, P.; Mourad, M.; Eliet, J.; Zeroual, N.; Culas, G.; Rouvière, P.; Albat, B.; Colson, P. Management and outcome of patients supported with Impella 5.0 for refractory cardiogenic shock. Crit. Care 2015, 19, 363. [Google Scholar] [CrossRef]
- Haurand, J.M.; Haberkorn, S.; Haschemi, J.; Oehler, D.; Aubin, H.; Akhyari, P.; Boeken, U.; Kelm, M.; Westenfeld, R.; Horn, P. Outcome of patients with non-ischaemic cardiogenic shock supported by percutaneous left ventricular assist device. ESC Heart Fail. 2021, 8, 3594–3602. [Google Scholar] [CrossRef]
- Helgestad, O.K.L.; Josiassen, J.; Hassager, C.; Jensen, L.O.; Holmvang, L.; Udesen, N.L.J.; Schmidt, H.; Ravn, H.B.; Moller, J.E. Contemporary trends in use of mechanical circulatory support in patients with acute MI and cardiogenic shock. Open Heart 2020, 7, e001214. [Google Scholar] [CrossRef]
- Higgins, J.; Lamarche, Y.; Kaan, A.; Stevens, L.-M.; Cheung, A. Microaxial Devices for Ventricular Failure: A Multicentre, Population-Based Experience. Can. J. Cardiol. 2011, 27, 725–730. [Google Scholar] [CrossRef]
- Hritani, A.W.; Wani, A.S.; Olet, S.; Lauterbach, C.J.; Allaqaband, S.Q.; Bajwa, T.; Jan, M.F. Secular Trend in the Use and Implementation of Impella in High-Risk Percutaneous Coronary Intervention and Cardiogenic Shock: A Real-World Experience. J. Invasive Cardiol. 2019, 31, E265–E270. [Google Scholar] [PubMed]
- Kaki, A.; Blank, N.; Alraies, M.C.; Jani, A.; Shemesh, A.; Kajy, M.; Laktineh, A.; Hasan, R.; Gade, C.L.; Mohamad, T.; et al. Axillary Artery Access for Mechanical Circulatory Support Devices in Patients With Prohibitive Peripheral Arterial Disease Presenting With Cardiogenic Shock. Am. J. Cardiol. 2019, 123, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Kamran, H.; Batra, S.; Venesy, D.M.; Patten, R.D.; Waxman, S.; Pyne, C.; Shah, S.P. Outcomes of Impella CP insertion during cardiac arrest: A single center experience. Resuscitation 2019, 147, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Karatolios, K.; Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Divchev, D.; Syntila, S.; Jerrentrup, A.; Schieffer, B. Comparison of mechanical circulatory support with venoarterial extracorporeal membrane oxygenation or Impella for patients with cardiogenic shock: A propensity-matched analysis. Clin. Res. Cardiol. 2020, 110, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Kennel, P.J.; Lumish, H.; Kaku, Y.; Fried, J.; Kirtane, A.J.; Karmpaliotis, D.; Takayama, H.; Naka, Y.; Sayer, G.; Uriel, N.; et al. A case series analysis on the clinical experience of Impella 5.5® at a large tertiary care centre. ESC Heart Fail. 2021, 8, 3720–3725. [Google Scholar] [CrossRef]
- Lackermair, K.; Sattler, S.; Huber, B.; Grabmaier, U.; Weckbach, L.; Bauer, A.; Theiss, H.; Hausleiter, J.; Mehilli, J.; Massberg, S.; et al. Retrospective analysis of circulatory support with the Impella CP® device in patients with therapy refractory cardiogenic shock. Int. J. Cardiol. 2016, 219, 200–203. [Google Scholar] [CrossRef]
- Lamarche, Y.; Cheung, A.; Ignaszewski, A.; Higgins, J.; Kaan, A.; Griesdale, D.; Moss, R. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J. Thorac. Cardiovasc. Surg. 2010, 142, 60–65. [Google Scholar] [CrossRef]
- Lemaire, A.; Anderson, M.B.; Lee, L.Y.; Scholz, P.; Prendergast, T.; Goodman, A.; Lozano, A.M.; Spotnitz, A.; Batsides, G. The Impella Device for Acute Mechanical Circulatory Support in Patients in Cardiogenic Shock. Ann. Thorac. Surg. 2014, 97, 133–138. [Google Scholar] [CrossRef]
- Maniuc, O.; Salinger, T.; Anders, F.; Müntze, J.; Liu, D.; Hu, K.; Ertl, G.; Frantz, S.; Nordbeck, P. Impella CP use in patients with non-ischaemic cardiogenic shock. ESC Heart Fail. 2019, 6, 863–866. [Google Scholar] [CrossRef]
- Manzo-Silberman, S.; Fichet, J.; Mathonnet, A.; Varenne, O.; Ricome, S.; Chaib, A.; Zuber, B.; Spaulding, C.; Cariou, A. Percutaneous left ventricular assistance in post cardiac arrest shock: Comparison of intra aortic blood pump and IMPELLA Recover LP2.5. Resuscitation 2013, 84, 609–615. [Google Scholar] [CrossRef]
- Meyns, B.; Dens, J.; Sergeant, P.; Herijgers, P.; Daenen, W.; Flameng, W. Initial experiences with the Impella device in patients with cardiogenic shock. Thorac. Cardiovasc. Surg. 2003, 51, 312–317. [Google Scholar]
- Mierke, J.; Loehn, T.; Ende, G.; Jahn, S.; Quick, S.; Speiser, U.; Jellinghaus, S.; Pfluecke, C.; Linke, A.; Ibrahim, K. Percutaneous Left Ventricular Assist Device Leads to Heart Rhythm Stabilisation in Cardiogenic Shock: Results from the Dresden Impella Registry. Heart Lung Circ. 2020, 30, 577–584. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sundararajan, S.; Klein, E.; Joyce, L.D.; Durham, L.A.; Joyce, D.L.; Mohammed, A.A. Sustained Use of the Impella 5.0 Heart Pump Enables Bridge to Clinical Decisions in 34 Patients. Tex. Heart Inst. J. 2021, 48, e207260. [Google Scholar] [CrossRef]
- Nersesian, G.; Potapov, E.V.; Nelki, V.; Stein, J.; Starck, C.; Falk, V.; Schoenrath, F.; Krackhardt, F.; Tschöpe, C.; Spillmann, F. Propensity score-based analysis of 30-day survival in cardiogenic shock patients supported with different microaxial left ventricular assist devices. J. Card. Surg. 2021, 36, 4141–4152. [Google Scholar] [CrossRef]
- Nouri, S.N.; Malick, W.; Masoumi, A.; Fried, J.A.; Topkara, V.K.; Brener, M.I.; Ahmad, Y.; Prasad, M.; Rabbani, L.E.; Takeda, K.; et al. Impella percutaneous left ventricular assist device as mechanical circulatory support for cardiogenic shock: A retrospective analysis from a tertiary academic medical center. Catheter. Cardiovasc. Interv. 2020, 99, 37–47. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; de Brabander, J.; Karami, M.; Sjauw, K.D.; Engström, A.E.; Vis, M.M.; Henriques, J.P. Real-life use of left ventricular circulatory support with Impella in cardiogenic shock after acute myocardial infarction: 12 years AMC experience. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 338–349. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 69, 278–287. [Google Scholar] [CrossRef]
- Panoulas, V.; Monteagudo-Vela, M. Predictors of Short-term Survival in Cardiogenic Shock Patients Requiring Left Ventricular Support Using the Impella CP or 5.0. CJC Open 2021, 3, 1002–1009. [Google Scholar] [CrossRef]
- Pappalardo, F.; Schulte, C.; Pieri, M.; Schrage, B.; Contri, R.; Soeffker, G.; Greco, T.; Lembo, R.; Müllerleile, K.; Colombo, A.; et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur. J. Heart Fail. 2016, 19, 404–412. [Google Scholar] [CrossRef]
- Radakovic, D.; Zittermann, A.; Knezevic, A.; Razumov, A.; Opacic, D.; Wienrautner, N.; Flottmann, C.; Rojas, S.V.; Fox, H.; Schramm, R.; et al. Left ventricular unloading during extracorporeal life support for myocardial infarction with cardiogenic shock: Surgical venting versus Impella device. Interact. Cardiovasc. Thorac. Surg. 2021, 34, 137–144. [Google Scholar] [CrossRef]
- Rohm, C.L.; Gadidov, B.; Ray, H.E.; Mannino, S.F.; Prasad, R. Vasopressors and Inotropes as Predictors of Mortality in Acute Severe Cardiogenic Shock Treated With the Impella Device. Cardiovasc. Revascularization Med. 2020, 31, 71–75. [Google Scholar] [CrossRef]
- Schäfer, A.; Westenfeld, R.; Sieweke, J.-T.; Zietzer, A.; Wiora, J.; Masiero, G.; Martinez, C.S.; Tarantini, G.; Werner, N. Complete Revascularisation in Impella-Supported Infarct-Related Cardiogenic Shock Patients Is Associated With Improved Mortality. Front. Cardiovasc. Med. 2021, 8, 678748. [Google Scholar] [CrossRef]
- Scherer, C.; Lüsebrink, E.; Kupka, D.; Stocker, T.J.; Stark, K.; Stremmel, C.; Orban, M.; Petzold, T.; Germayer, A.; Mauthe, K.; et al. Long-Term Clinical Outcome of Cardiogenic Shock Patients Undergoing Impella CP Treatment vs. Standard of Care. J. Clin. Med. 2020, 9, 3803. [Google Scholar] [CrossRef]
- Schiller, P.; Vikholm, P.; Hellgren, L. The Impella® Recover mechanical assist device in acute cardiogenic shock: A single-centre experience of 66 patients. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 452–458. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Herdis, K.; Wachter, A.; Annalen Bleckmann, M.D.; Hasenfu, G.; Wolfgang Schillinger, M.D. Use of the Impella device for acute coronary syndrome complicated by cardiogenic shock–experience from a single heart center with analysis of long-term mortality. J. Invasive Cardiol. 2016, 28, 467–472. [Google Scholar]
- Schurtz, G.; Rousse, N.; Saura, O.; Balmette, V.; Vincent, F.; Lamblin, N.; Porouchani, S.; Verdier, B.; Puymirat, E.; Robin, E.; et al. IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock. J. Clin. Med. 2021, 10, 759. [Google Scholar] [CrossRef]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef]
- Sieweke, J.-T.; Akin, M.; Beheshty, J.-A.; Flierl, U.; Bauersachs, J.; Schäfer, A. Unloading in Refractory Cardiogenic Shock After Out-Of-Hospital Cardiac Arrest Due to Acute Myocardial Infarction—A Propensity Score-Matched Analysis. Front. Cardiovasc. Med. 2021, 8, 704312. [Google Scholar] [CrossRef]
- Sugimura, Y.; Katahira, S.; Immohr, M.B.; Sipahi, N.F.; Mehdiani, A.; Assmann, A.; Rellecke, P.; Tudorache, I.; Westenfeld, R.; Boeken, U.; et al. Initial experience covering 50 consecutive cases of large Impella implantation at a single heart centre. ESC Heart Fail. 2021, 8, 5168–5177. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kubo, S.; Ikuta, A.; Osakada, K.; Takamatsu, M.; Taguchi, Y.; Ohya, M.; Shimada, T.; Miura, K.; Tada, T.; et al. Incidence, predictors, and clinical outcomes of mechanical circulatory support-related complications in patients with cardiogenic shock. J. Cardiol. 2021, 79, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, S.; Ikegami, H.; Russo, M.J.; Lee, L.Y.; Lemaire, A. The role of the axillary Impella 5.0 device on patients with acute cardiogenic shock. J. Cardiothorac. Surg. 2020, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Masood, M.F.; Garcia, M.B.; Pisani, M.; Ewald, G.A.; Lasala, J.M.; Bach, R.G.; Singh, J.; Balsara, K.R.; Itoh, A. Left Ventricular Unloading by Impella Device Versus Surgical Vent During Extracorporeal Life Support. Ann. Thorac. Surg. 2017, 104, 861–867. [Google Scholar] [CrossRef]
- Trpkov, C.; Gibson, J.D.; Miller, R.J.; Grant, A.D.; Schnell, G.; Har, B.J.; Clarke, B. Percutaneous Left Ventricular Assist Device in Cardiogenic Shock: A Five-Year Single Canadian Center Initial Experience. CJC Open 2020, 2, 370–378. [Google Scholar] [CrossRef]
- Vase, H.; Christensen, S.; Christiansen, A.; Therkelsen, C.J.; Christiansen, E.H.; Eiskjaer, H.; Poulsen, S.H. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation 2017, 112, 70–74. [Google Scholar] [CrossRef]
- Vasin, S.; Philipp, A.; Floerchinger, B.; Rastogi, P.; Lunz, D.; Mueller, T.; Schmid, C.; Camboni, D. Increasing use of the Impella®-pump in severe cardiogenic shock: A word of caution. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 711–714. [Google Scholar] [CrossRef]
- Wilkins, C.E.; Herrera, T.L.; Nagahiro, M.K.; Weathers, L.B.; Girotra, S.V.; Sandhu, F. Outcomes of Hemodynamic Support With Impella for Acute Myocardial Infarction Complicated by Cardiogenic Shock at a Rural Community Hospital Without On-Site Surgical Back-up. J. Invasive Cardiol. 2019, 31, E23–E29. [Google Scholar]
- Zaiser, A.S.; Fahrni, G.; Hollinger, A.; Knobel, D.T.; Bovey, Y.; Zellweger, N.M.; Siegemund, M. Adverse Events of Percutaneous Microaxial Left Ventricular Assist Devices—A Retrospective, Single-Centre Cohort Study. J. Clin. Med. 2021, 10, 3710. [Google Scholar] [CrossRef]
- Abdullah, K.Q.A.; Roedler, J.V.; Dahl, J.V.; Szendey, I.; Haake, H.; Eckardt, L.; Topf, A.; Ohnewein, B.; Jirak, P.; Motloch, L.J.; et al. Impella use in real-world cardiogenic shock patients: Sobering outcomes. PLoS ONE 2021, 16, e0247667. [Google Scholar] [CrossRef]
- Barrionuevo-Sánchez, M.I.; Ariza-Solé, A.; Ortiz-Berbel, D.; González-Costello, J.; Gómez-Hospital, J.A.; Lorente, V.; Comin-Colet, J. Usefulness of Impella support in different clinical settings in cardiogenic shock. J. Geriatr. Cardiol. JGC 2022, 19, 115. [Google Scholar]
- Bashline, M.J.; Rhinehart, Z.; Kola, O.; Fowler, J.; Kaczorowski, D.; Hickey, G. Impella 5.0 is associated with a reduction in vasoactive support and improves hemodynamics in cardiogenic shock: A single-center experience. Int. J. Artif. Organs 2022, 45, 462–469. [Google Scholar] [CrossRef]
- Ramzy, D.; Anderson, M.; Batsides, G.; Ono, M.; Silvestry, S.; D’Alessandro, D.A.; Funamoto, M.; Zias, E.A.; Lemaire, A.; Soltese, E. Early Outcomes of the First 200 US Patients Treated with Impella 5.5: A Novel Temporary Left Ventricular Assist Device. Innovations 2021, 16, 365–372. [Google Scholar] [CrossRef]
- Rock, J.R.; Kos, C.A.; Lemaire, A.; Ikegami, H.; Russo, M.J.; Moin, D.; Iyer, D. Single center first year experience and outcomes with Impella 5.5 left ventricular assist device. J. Cardiothorac. Surg. 2022, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.L.; Trott, G.; Schneider, D.; Goldraich, L.A.; Tonietto, T.F.; Moura, L.Z.; Bertoldi, E.G.; Rover, M.M.; Wolf, J.M.; de Souza, D.; et al. Cardiogenic shock treated with temporary mechanical circulatory support in Brazil: The effect of learning curve. Int. J. Artif. Organs 2022, 45, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, I.; Masawa, T.; Abe, S.; Ogawa, H.; Takei, Y.; Tezuka, M.; Seki, M.; Kato, T.; Watanabe, R.; Koshiji, N.; et al. Benefit of veno-arterial extracorporeal membrane oxygenation combined with Impella (ECpella) therapy in acute coronary syndrome with cardiogenic shock. J. Cardiol. 2022, 80, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Syntila, S.; Chatzis, G.; Markus, B.; Ahrens, H.; Waechter, C.; Luesebrink, U.; Divchev, D.; Schuett, H.; Tsalouchidou, P.-E.; Jerrentrup, A.; et al. Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis. J. Clin. Med. 2021, 10, 3583. [Google Scholar] [CrossRef] [PubMed]
- Zubarevich, A.; Rad, A.A.; Szczechowicz, M.; Luedike, P.; Koch, A.; Pizanis, N.; Kamler, M.; Ruhparwar, A.; Weymann, A.; Schmack, B. Early Experience with the Impella Pump: Single Center Registry. Artif. Organs 2022, 46, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Trani, C.; Doshi, S.N.; Townend, J.; van Geuns, R.J.; Hunziker, P.; Schieffer, B.; Karatolios, K.; Møller, J.E.; Ribichini, F.L.; et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int. J. Cardiol. 2015, 201, 684–691. [Google Scholar] [CrossRef]
- Ramzy, D.; Soltesz, E.; Anderson, M. New Surgical Circulatory Support System Outcomes. ASAIO J. 2020, 66, 746–752. [Google Scholar] [CrossRef]
- Rohm, C.L.; Gadidov, B.; Leitson, M.; Ray, H.E.; Prasad, R. Predictors of Mortality and Outcomes of Acute Severe Cardiogenic Shock Treated with the Impella Device. Am. J. Cardiol. 2019, 124, 499–504. [Google Scholar] [CrossRef]
- Shintani, A.K.; Girard, T.; Eden, S.K.; Arbogast, P.G.; Moons, K.G.M.; Ely, E.W. Immortal time bias in critical care research: Application of time-varying Cox regression for observational cohort studies*. Crit. Care Med. 2009, 37, 2939–2945. [Google Scholar] [CrossRef]
- Poon, W.H.; Ramanathan, K.; Ling, R.R.; Yang, I.X.; Tan, C.S.; Schmidt, M.; Shekar, K. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2021, 25, 292. [Google Scholar] [CrossRef]
- Ramanathan, K.; Shekar, K.; Ling, R.R.; Barbaro, R.P.; Wong, S.N.; Tan, C.S.; Brodie, D. Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit. Care 2021, 25, 211. [Google Scholar] [CrossRef]
- Lévesque, L.E.; A Hanley, J.; Kezouh, A.; Suissa, S. Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ 2010, 340, b5087. [Google Scholar] [CrossRef]
- Batsides, G.; Massaro, J.; Cheung, A.; Soltesz, E.; Ramzy, D.; Anderson, M.B. Outcomes of Impella 5.0 in cardiogenic shock: A systematic review and meta-analysis. Innovations 2018, 13, 254–260. [Google Scholar] [CrossRef]
- Glazier, J.J.; Kaki, A. Improving survival in cardiogenic shock: Is Impella the answer? Am. J. Med. 2018, 131, e403–e404. [Google Scholar] [CrossRef]
- Prondzinsky, R.; Lemm, H.; Swyter, M.; Wegener, N.; Unverzagt, S.; Carter, J.M.; Russ, M.; Schlitt, A.; Buerke, U.; Christoph, A.; et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome*. Crit. Care Med. 2010, 38, 152–160. [Google Scholar] [CrossRef]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Hodgson, C.; Scheinkestel, C.; Cooper, D.J.; Thiagarajan, R.R.; et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef]
- Basir, M.B.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A. National Cardiogenic Shock Initiative Investigators. Improved outcomes associated with the use of shock protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar]
- Basir, M.B.; Schreiber, T.; Dixon, S.; Alaswad, K.; Patel, K.; Almany, S.; Khandelwal, A.; Hanson, I.; George, A.; Ashbrook, M.; et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter. Cardiovasc. Interv. 2017, 91, 454–461. [Google Scholar] [CrossRef]
- Mourad, M.; Gaudard, P.; De La Arena, P.; Eliet, J.; Zeroual, N.; Rouvière, P.; Roubille, F.; Albat, B.; Colson, P.H. Circulatory Support with Extracorporeal Membrane Oxygenation and/or Impella for Cardiogenic Shock During Myocardial Infarction. ASAIO J. 2018, 64, 708–714. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.S.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Džavík, V.; et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Schreiber, T.; Wohns, D.H.W.; Rihal, C.; Naidu, S.S.; Civitello, A.; Dixon, S.R.; Massaro, J.M.; Maini, B.; Ohman, E.M. The Current Use of Impella 2.5 in Acute Myocardial Infarction Complicated by Cardiogenic Shock: Results from the USpella Registry. J. Interv. Cardiol. 2013, 27, 1–11. [Google Scholar] [CrossRef]
- Lauten, A.; Engström, A.E.; Jung, C.; Empen, K.; Erne, P.; Cook, S.; Windecker, S.; Bergmann, M.W.; Klingenberg, R.; Lüscher, T.F.; et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: Results of the Impella-EUROSHOCK-registry. Circ. Heart Fail. 2013, 6, 23–30. [Google Scholar] [CrossRef]
- Hirst, C.S.; Thayer, K.L.; Harwani, N.; Kapur, N.K. Post-Closure Technique to Reduce Vascular Complications Related to Impella CP. Cardiovasc. Revascularization Med. 2022, 39, 38–42. [Google Scholar] [CrossRef]
- Davidavicius, G.; Godino, C.; Shannon, J.; Takagi, K.; Bertoldi, L.; Mussardo, M.; Chieffo, A.; Arioli, F.; Ielasi, A.; Montorfano, M.; et al. Incidence of Overall Bleeding in Patients Treated With Intra-Aortic Balloon Pump During Percutaneous Coronary Intervention: 12-Year Milan Experience. JACC Cardiovasc. Interv. 2012, 5, 350–357. [Google Scholar] [CrossRef]
- De Jong, M.M.; Lorusso, R.; Al Awami, F.; Matteuci, F.; Parise, O.; Lozekoot, P.; Gelsomino, S. Vascular complications following intra-aortic balloon pump implantation: An updated review. Perfusion 2018, 33, 96–104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).