Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. MSOT
2.2. Study Population
2.3. Experimental
2.4. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gopcevic, K.R.; Gkaliagkousi, E.; Nemcsik, J.; Acet, Ö.; Bernal-Lopez, M.R.; Bruno, R.M.; Climie, R.E.; Fountoulakis, N.; Fraenkel, E.; Fraenkel, A.; et al. Pathophysiology of Circulating Biomarkers and Relationship With Vascular Aging: A Review of the Literature From VascAgeNet Group on Circulating Biomarkers, European Cooperation in Science and Technology Action 18216. Front. Physiol. 2021, 12, 789690. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Roustit, M. Human Skin Microcirculation. Compr. Physiol. 2020, 10, 1105–1154. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Elgrably, B.; Saar, G.; Vandoorne, K. Multi-Scale Imaging of Vascular Pathologies in Cardiovascular Disease. Front. Med. 2022, 8, 754369. [Google Scholar] [CrossRef]
- Li, D.; Humayun, L.; Vienneau, E.; Vu, T.; Yao, J. Seeing through the Skin: Photoacoustic Tomography of Skin Vasculature and Beyond. JID Innov. 2021, 1, 100039. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.V. Photoacoustic imaging and characterization of the microvasculature. J. Biomed. Opt. 2010, 15, 011101. [Google Scholar] [CrossRef]
- Wang, L.V. Prospects of photoacoustic tomography. Med. Phys. 2008, 35, 5758–5767. [Google Scholar] [CrossRef]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Lal, C.; Leahy, M.J. An Updated Review of Methods and Advancements in Microvascular Blood Flow Imaging. Microcirculation 2016, 23, 345–363. [Google Scholar] [CrossRef]
- Taruttis, A.; Herzog, E.; Razansky, D.; Ntziachristos, V. Real-time imaging of cardiovascular dynamics and circulating gold nanorods with multispectral optoacoustic tomography. Opt. Express 2010, 18, 19592–19602. [Google Scholar] [CrossRef]
- Wang, H.; Shan, Z.; Li, W.; Chu, M.; Yang, J.; Yi, D.; Zhan, J.; Yuan, Z.Y.; Raikwar, S.; Wang, S.; et al. Guidelines for assessing mouse endothelial function via ultrasound imaging: A report from the International Society of Cardiovascular Translational Research. J. Cardiovasc. Transl. Res. 2015, 8, 89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zafar, H.; Breathnach, A.; Subhash, H.M.; Leahy, M.J. Linear-array-based photoacoustic imaging of human microcirculation with a range of high frequency transducer probes. J. Biomed. Opt. 2015, 20, 51021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Rank, E.; Meiburger, K.M.; Sinz, C.; Hodul, A.; Zhang, E.; Hoover, E.; Minneman, M.; Ensher, J.; Beard, P.C.; et al. Non-invasive multimodal optical coherence and photoacoustic tomography for human skin imaging. Sci. Rep. 2017, 7, 17975. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, S.; Wang, Y.; Gu, Y.; Xing, D. Noninvasive and high-resolving photoacoustic dermoscopy of human skin. Biomed. Opt. Express 2016, 7, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.M.; Climie, R.; Gallo, A. Aortic pulsatility drives microvascular organ damage in essential hypertension: New evidence from choroidal thickness assessment. J. Clin. Hypertens. 2021, 23, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Granja, T.; de Andrade, S.F.; Rodrigues, L.M. Multispectral Optoacoustic Tomography for Functional Imaging in Vascular Research. J. Vis. Exp. 2022, 184, e63883. [Google Scholar] [CrossRef]
- Rosenberry, R.; Nelson, M.D. Reactive hyperemia: A review of methods, mechanisms, and considerations. American journal of physiology. Regul. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Takahashi, N.; Shimada, T.; Sugamori, T.; Sakane, T.; Umeno, T.; Hirano, Y.; Oyake, N.; Murakami, Y. Short duration of reactive hyperemia in the forearm of subjects with multiple cardiovascular risk factors. Circ. J. 2006, 70, 115–123. [Google Scholar] [CrossRef]
- Huang, A.L.; Silver, A.E.; Shvenke, E.; Schopfer, D.W.; Jahangir, E.; Titas, M.A.; Shpilman, A.; Menzoian, J.O.; Watkins, M.T.; Raffetto, J.D.; et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arter. Thromb. Vasc Biol. 2007, 27, 2113–2119. [Google Scholar] [CrossRef]
- Paine, N.J.; Hinderliter, A.L.; Blumenthal, J.A.; Adams, K.F., Jr.; Sueta, C.A.; Chang, P.P.; O’Connor, C.M.; Sherwood, A. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am. Heart J. 2016, 178, 108–114. [Google Scholar] [CrossRef]
- Granja, T.; Andrade, S.; Rodrigues, L. Optoaccoustic Tomography—good news for microcirculatory research Biomedical and Biopharmaceutical Research. PAT 2021, 18, 200–212. [Google Scholar] [CrossRef]
- iThera Scientific Instruction Manual—MSOT Invasion. User’s Guide 2021. Available online: https://www.usf.edu/research-innovation/comparative-medicine/documents/training/msot-invision-series-user-manual-3-user-guide.pdf (accessed on 10 October 2022).
- Yang, F.F.; Cao, L.; Wen, H.; Fu, T.T.; Ma, S.Q.; Liu, C.Y.; Quan, L.H.; Liao, Y.H. Multispectral optoacoustic tomography (MSOT) for imaging the particle size-dependent intratumoral distribution of polymeric micelles. Int. J. Nanomed. 2018, 13, 8549–8560. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, S.; Minson, C.T. Human cutaneous reactive hyperaemia: Role of BKCa channels and sensory nerves. J. Physiol. 2007, 585 Pt 1, 295–303. [Google Scholar] [CrossRef]
- Alomari, M.A.; Solomito, A.; Reyes, R.; Khalil, S.M.; Wood, R.H.; Welsch, M.A. Measurements of vascular function using strain-gauge plethysmography: Technical considerations, standardization, and physiological findings. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H99–H107. [Google Scholar] [CrossRef]
- Bahadir, Z.; Tisdell, E.; Arce Esquivel, A.A.; Dobrosielski, D.A.; Welsch, M.A. Influence of venous emptying on the reactive hyperemic blood flow response. Dyn. Med. 2007, 6, 3. [Google Scholar] [CrossRef]
- Dennis, J.J.; Wiggins, C.C.; Smith, J.R.; Isautier, J.M.; Johnson, B.D.; Joyner, M.J.; Cross, T.J. Measurement of muscle blood flow and O2 uptake via near-infrared spectroscopy using a novel occlusion protocol. Sci. Rep. 2021, 11, 918. [Google Scholar] [CrossRef]
- McLay, K.M.; Nederveen, J.P.; Pogliaghi, S.; Paterson, D.H.; Murias, J.M. Repeatability of vascular responsiveness measures derived from nearinfrared spectroscopy. Physiol. Rep. 2016, 4, e12772. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Chang, W.; Chi, Z.; Shang, Q.; Wu, M.; Pan, T.; Huang, L.; Jiang, H. Photoacoustic imaging of hemodynamic changes in forearm skeletal muscle during cuff occlusion. Biomed. Opt. Express 2020, 11, 4560–4570. [Google Scholar] [CrossRef]
- Didier, K.D.; Hammer, S.M.; Alexander, A.M.; Caldwell, J.T.; Sutterfield, S.L.; Smith, J.R.; Ade, C.J.; Barstow, T.J. Microvascular blood flow during vascular occlusion tests assessed by diffuse correlation spectroscopy. Exp. Physiol. 2020, 105, 201–210. [Google Scholar] [CrossRef]
- Young, G.M.; Krastins, D.; Chang, D.; Lam, J.; Quah, J.; Stanton, T.; Russell, F.; Greaves, K.; Kriel, Y.; Askew, C.D.; et al. Influence of cuff-occlusion duration on contrast-enhanced ultrasound assessments of calf muscle microvascular blood flow responsiveness in older adults. Exp. Physiol. 2020, 105, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Young, G.M.; Krastins, D.; Chang, D.; Lam, J.; Quah, J.; Stanton, T.; Russell, F.; Greaves, K.; Kriel, Y.; Askew, C.D. The Association Between Contrast-Enhanced Ultrasound and Near-Infrared Spectroscopy-Derived Measures of Calf Muscle Microvascular Responsiveness in Older Adults Heart. Lung Circ. 2021, 30, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: A systematic review and metaanalysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Braverman, I.M. The Cutaneous Microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef]
- Karlas, A.; Kallmayer, M.; Fasoula, N.-A.; Liapis, E.; Bariotakis, M.; Krönke, M.; Anastasopoulou, M.; Reber, J.; Eckstein, H.H.; Ntziachristos, V. Multispectral optoacoustic tomography of muscle perfusion and oxygenation under arterial and venous occlusion: A human pilot study. J. Biophotonics 2020, 13, e201960169. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.A.; Silva, H.N. Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. J. Appl. Physiol. 2020, 128, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Silva, H.; Ferreira, H.; Renault, M.A.; Gadeau, A.P. Observations on the perfusion recovery of regenerative angiogenesis in an ischemic limb model under hyperoxia. Physiol. Rep. 2018, 6, e13736. [Google Scholar] [CrossRef]
- Florindo, M.; Nuno, S.; Andrade, S.; Rocha, C.; Rodrigues, L.M. The Acute Modification of the Upper-Limb Perfusion in Vivo Evokes a Prompt Adaptive Hemodynamic Response to Re-Establish Cardiovascular Homeostasis. Physiology21 Annual Conference Abstract Book 2021. Available online: https://static.physoc.org/app/uploads/2021/06/10115155/Physiology-2021-Abstract-Book.pdf (accessed on 10 October 2022).
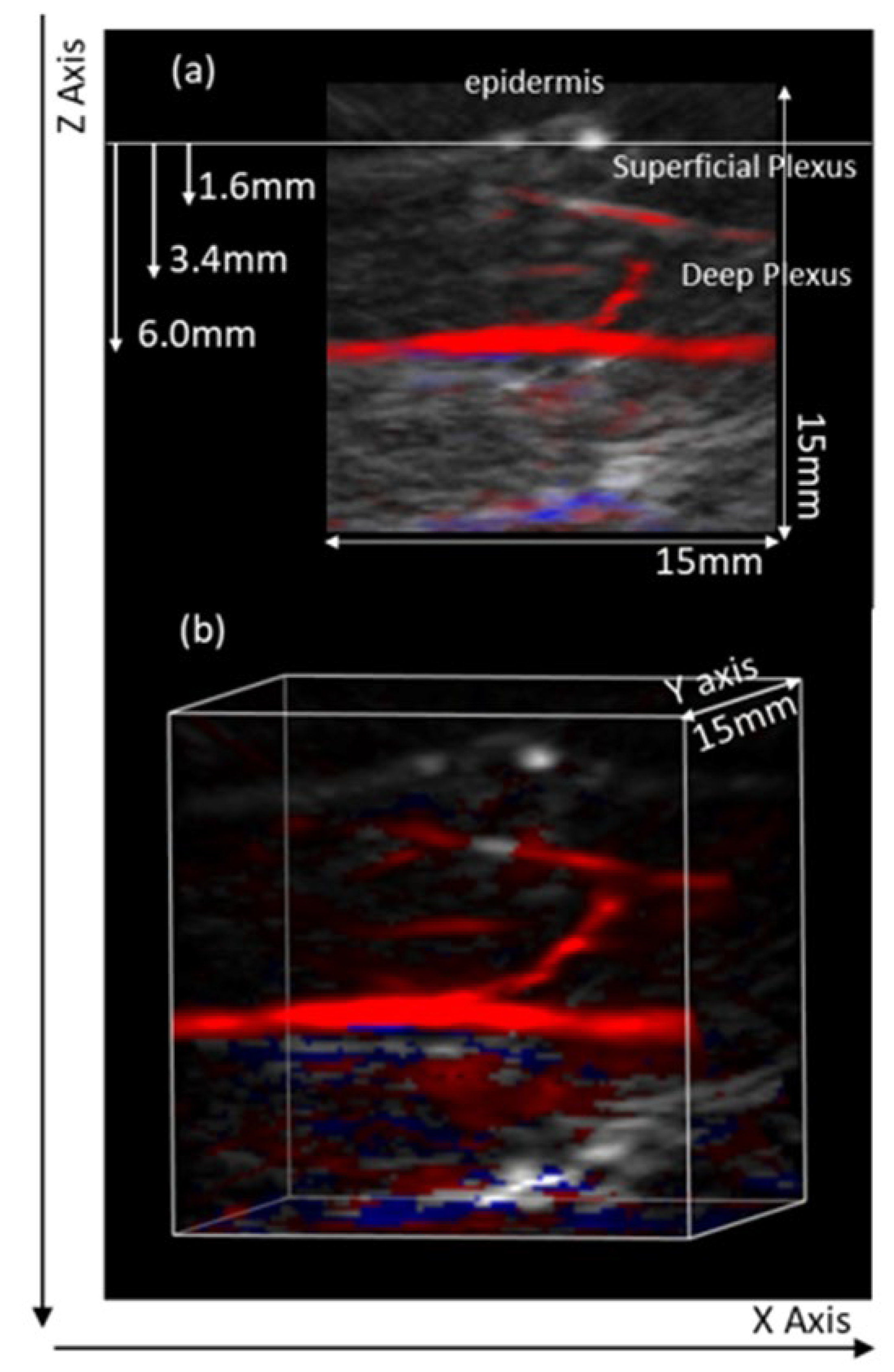

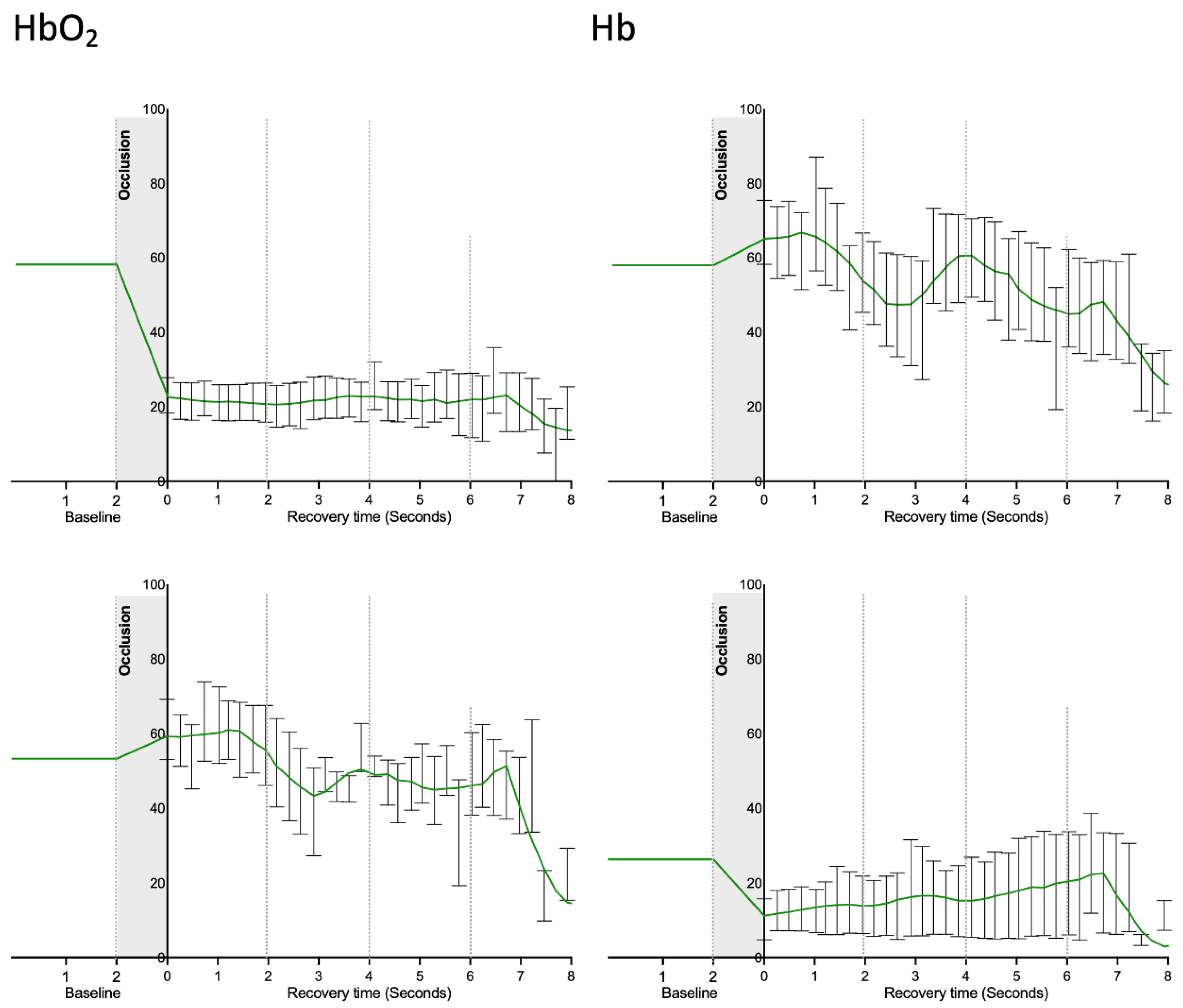

| Participants | Mean ± sd |
|---|---|
| Sex (F female; M male) | F (5); M (5) |
| Smokers | 0 |
| Physical Activity (h/week) | 3.0 ± 1.9 |
| Age, years | 35.8 ± 13.3 |
| Body mass, kg | 68.2 ± 9.8 |
| Height, m | 1.7 ± 0.1 |
| BMI, kg/m2 | 23.7 ± 2.5 |
| MAP, mmHg | 91.4 ± 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro Rodrigues, L.; Granja, T.F.; de Andrade, S.F. Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology. Life 2022, 12, 1628. https://doi.org/10.3390/life12101628
Monteiro Rodrigues L, Granja TF, de Andrade SF. Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology. Life. 2022; 12(10):1628. https://doi.org/10.3390/life12101628
Chicago/Turabian StyleMonteiro Rodrigues, Luis, Tiago F. Granja, and Sergio Faloni de Andrade. 2022. "Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology" Life 12, no. 10: 1628. https://doi.org/10.3390/life12101628
APA StyleMonteiro Rodrigues, L., Granja, T. F., & de Andrade, S. F. (2022). Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology. Life, 12(10), 1628. https://doi.org/10.3390/life12101628









