Phosphatidylethanol in Maternal or Neonatal Blood to Detect Alcohol Exposure during Pregnancy: A Systematic Review
Abstract
1. Introduction
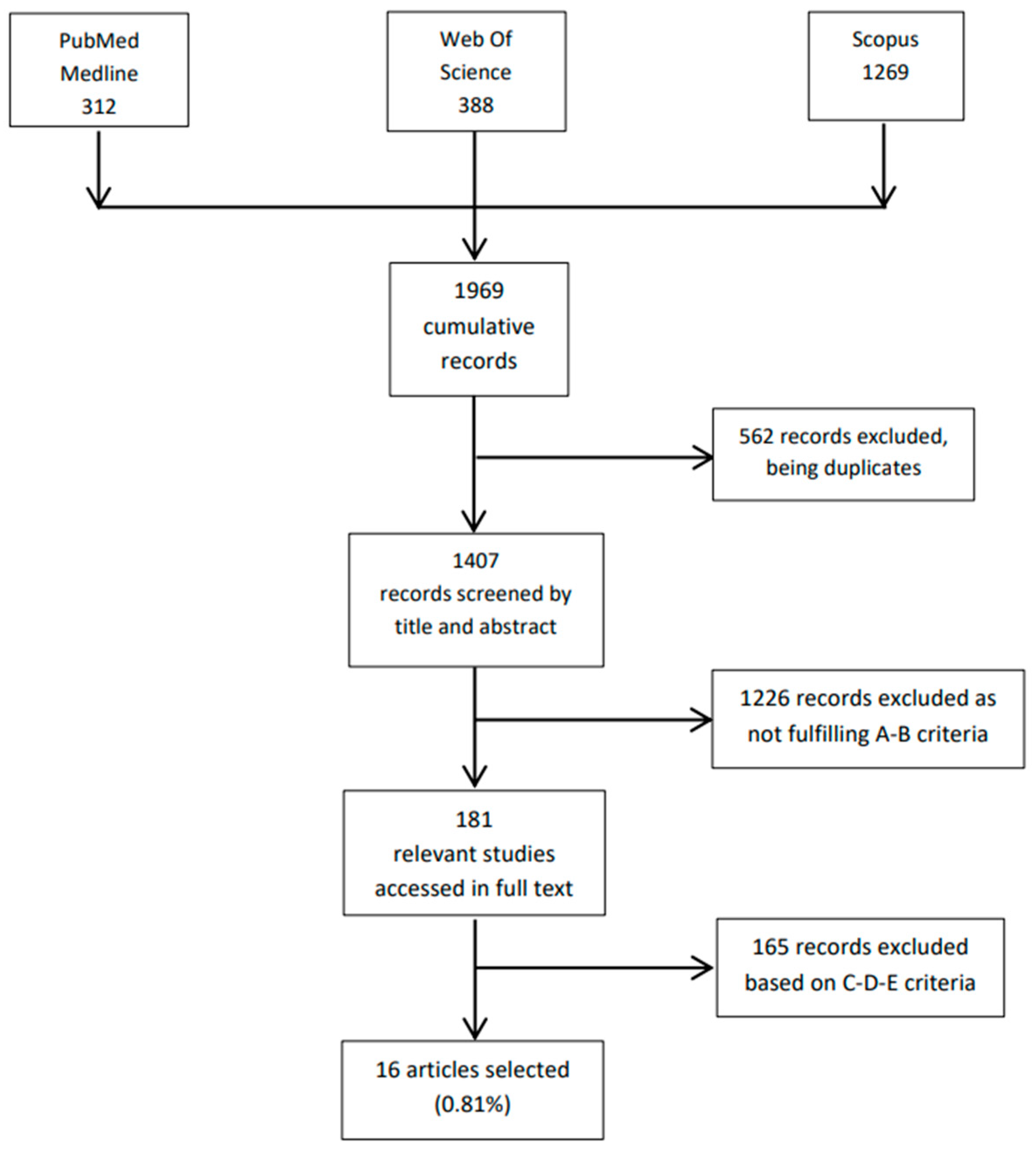
2. Materials and Methods
- Titles and abstracts available in the English language.
- PEth used for detecting alcohol consumption during pregnancy.
- PEth quantified in liquid human blood or in dried blood spots through liquid chromatography coupled to mass spectrometry.
- Full text in the English language.
- E.
- Opinion papers, editorials, and narrative reviews without novel data.
3. Results and Discussion
| Authors | Journal and Year | Type of Study | Subjects of the Study | Aim of the Study | Clinical Setting | Inclusion/Exclusion Criteria, Subject Stratification, Types of Cases and Controls | Number of Subjects, Mean Age | Race/Ethnicity | Methods for Estimating Alcohol Use. Reported Alcohol Use | Type of Samples and Timing of Collection | Measured PEth and Mean Concentration | Analytical Method, LOQ or Cut-Off | Main Results and Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bakhireva et al. [1] | Alcoholism: Clinical and Experimental Research 2014 | Prospective cohort/longitudinal study | Mothers and newborns | To examinate validity of maternal PEth and neonatal PEth-DBS for the identification of PAE To assess the sensitivity and specificity of PEth for the detection of PAE | Pregnant women recruited at the University of New Mexico Clinic and followed to early postpartum period | Inclusion criteria:
| PAE group: 28 newborns and 28 women 27 years ± 5.9 Control group: 32 newborns and 32 women 26.3 years ± 4.5 | PAE group:
| TFLB AUDIT | Mothers: Whole blood collected at baseline visit (mean gestational age: PAE group = 20.5 weeks ± 6.3; controls = 20.5 weeks ± 8.1) and at follow-up visit (at delivery) Newborns: Blood DBS collected at birth | 16:0/18:0 | LC-MS/MS LOD = 2 ng/mL LOQ = 8 ng/mL |
|
| Bakhireva et al. [25] | Alcoholism: Clinical and Experimental Research 2017 | Cross-sectional study | Newborns | To estimate the prevalence of PAE in Texas by measuring PEth in infant residual DBS | Neonatal residual DBS stored in the Texas Newborn Screening Repository | Inclusion criteria:
| 1000 residual DBS cards |
| - | DBS cards collected within 48 h of delivery | 16:0/18:1 10.2 ± 37.1 ng/mL | LC-MS/MS LOD = 2.0 ng/mL LOQ = 8.0 ng/mL PAE cut-off = 20 ng/mL |
|
| Baldwin et al. [22] | Alcoholism: Clinical and Experimental Research 2020 | Cross-sectional study | Mothers and newborns | To compare PEth levels in postpartum women and their newborn infants in Montevideo, Uruguay, and Sao Paulo, Brazil. | Pregnant women admitted to the maternity hospitals in Montevideo, Uruguay, and in Sao Paulo, Brazil | Inclusion criteria:
|
|
| Thirty-minutes interview (No data relative to quantity and frequency of alcohol consumption available) Prevalence of alcohol use:
| DBS from whole blood (mothers) collected during pregnancy DBS from heel stick (newborns), both collected within 48 h of delivery | Palmitoyl/oleoyl (16:0/18:0) Uruguay:
| LC-MS/MS LOD = 2 ng/mL LOQ (cut-off) = 8 ng/ml |
|
| Baldwin et al. [21] | International Journal of Alcohol and Drug Research 2015 | Cross-sectional study | Newborns | To analyze the efficacy of screening banked newborn DBS for detection of PEth performing a retrospective assessment of statewide prevalence rates of alcohol consumption in late pregnancy that results in risky prenatal alcohol exposure To investigate the stability of PEth in stored DBS Cards | Stored residual DBS specimens collected for routine metabolic screening from the general population | Inclusion criteria:
| 250 deidentified DBS cards | - | US surveys relying on maternal self-report (BRFSS, NSDUH, PRAMS) | DBS cards collected at birth | 16:0/18:0 | LC-MS/MS LOD = 2 ng/mL LOQ = 8 ng/ml |
|
| Bracero et al. [16] | Reproductive Toxicology 2017 | Prospective cohort/longitudinal study | Mothers | To compare rates of alcohol use between urine ethanol testing and self-reporting (Method 1) and Phosphatidylethanol (PEth) dried blood spot testing and self-reporting (method 2) | Pregnant women attending the prenatal care medical center between July 2013 and March 2014 | Inclusion criteria:
| 314 pregnant women. 24.9 years ± 5.8 |
| ACOG prenatal record questionnaire | DBS from blood specimens, collected during first trimester (mean gestational age: 11.3 ± 7.3 weeks) | Palmitoyl/oleoyl (16:0/18:0) | LC-MS/MS LOD = 2 ng/mL LOQ = 8 ng/ml |
|
| Breunis et al. [18] | BMC Pregnancy and Childbirth 2021 | Prospective cohort/longitudinal study, cross-sectional, single center study | Mothers | To evaluate biochemically assessed prevalence of alcohol consumption during early pregnancy using PEth levels. | Pregnant women who were under the care of the department of the Erasmus MC between September 2016 and October 2017 | Inclusion criteria:
| 684 pregnant women. 31.7 years (SD 4.9) | - | Self-reported consumption | Whole blood collected at gestational week < 15 | 16:0/18:1 (POPEth) 16.0/18.2 (PLPEth) 18.1/18.1 (DOPEth) | LC-MS/MS LOD = 2.0 µg/L (16:0/18:1) 2.0 µg/L (16:0/18:2) 2.0 µg/L (18:1/18:1) LOQ = 6.0 µg/L (16:0/18:1) 6.0 µg/L (16:0/18:2) 3.0 µg/L (18:1/18:1) |
|
| Comasco et al. [7] | Alcoholism: Clinical and Experimental Research 2012 | Prospective cohort/longitudinal study | Mothers | To evaluate methods to assess maternal drinking during pregnancy—To investigate possible influences of PAE | Women attending the maternity clinic at Uppsala University Hospital between October 2007 and May 2009 | Inclusion criteria:
| 77 random blood samples from 2264 pregnant women30.4 years (17–49) | - | AUDIT (16–18 weeks of gestation) for alcohol use before pregnancy AUDIT-C (32 weeks of gestation) for alcohol use during pregnancy | Whole blood collected at 16–18 weeks of gestation | - | LC-MS/MS Cut-off (reporting limit) = 0.1 µmol/L |
|
| Di Battista et al. [26] | Alcoholism: Clinical and Experimental Research 2022 | Retrospective Study | Newborns | To estimate rates of prenatal alcohol exposure (PAE) | Random selection of 2011 residual DBS collected over a 1-week time period. | Inclusion criteria:
| 2006 residual DBS | - | - | - | 16:0/18:1 16:0/18:2 16:0/16:0 18:0/18:2 18:1/18:1 18:0/18:1 16:0/20:4 311 samples tested positive (16:0/18:1 > 20 ng/mL). 24 samples had a value > 284 nM. Main value in Peth—Positive samples 16:0/18:1 = 103 ± 173 nM 16:0/18:2 = 73.5 ± 130 nM 16:0/16:0 = 17.9 ± 18.5 nM 18:0/18:2 = 10.6 ± 19.7 nM 18:1/18:1 = 9.93 ± 17.0 nM 18:0/18:1 = 16.6 ± 24.0 nM 16:0/20:4 = 86.1 ± 120 nM Total Peth= 318 ± 478 nM | LC-MS/MS LOD = 2 nM for all Peths, with the exception of 16:0/18:2 and 16:0/20:4 which had 4 nM. LOQ = 4 nM (16:0/16:0, 18:0/18:2, 16:0/18:1, 18:0/18:1) 8 nM (16:0/18:2, 18:1/18:1, 16:0/20:4) |
|
| Finanger et al. [27] | Alcoholism: Clinical and Experimental Research 2021 | Observational descriptive study | Mothers | Investigate the prevalence of positive PEth values as an indicator of early prenatal alcohol exposure in pregnant women | Rhesus type and antibody screening in pregnant women attending the Clinic between September 2017 and October 2018 | Inclusion criteria:
| 4.533 whole blood samples from 4.067 pregnant women Women with PEth positive sample: 30.3 years ± 5.5 Women with PEth negative sample: 30.2 years ± 4.7 | - | - | Whole blood collected at gestational week 12 and 24 | 16:0/18:1 0.026 µM (0.003-0.287) | UPLC-MSMS LOQ = 0.003 µM | Fifty-eight women had a positive PEth sample collected during pregnancy: first trimester 50; second trimester 3; 5 unknown timing. |
| Kwak et al. [13] | Clinical Toxicology 2012 | Prospective cohort/longitudinal study | Mothers | To evaluate PEth concentrations in pregnant women with positive history of low-to-moderate alcohol ingestion | Pregnant women referred for teratogen-risk counseling because of recent history of alcohol ingestion | Inclusion criteria:
| 13 first-trimester pregnant women Case group: 32.3 years ± 5Control group: 32.4 years ± 4 | - | Self-reported consumption 7.5 (2.5–20) drinks/week | Whole blood collected during firsttrimester of gestation | 16:0/16:0 16:0/18:1 18:1/18:1 PETh 16:0/16:0 = 10.6 nmol/L (1.2–25.3) PETh 16:0/18:1 = 47.8 nmol/L (3.5–177.0) PEth 18:1/18:1 = 3.2 nmol/L (0.2–10.2) | LC-MS/MS LOD = 0.4 nmol/L (16:0/16:0) 0.9 nmol/L (16:0/18:1) 0.4 nmol/L (18:1/18:1) LOQ = 1.5 nmol/L (16:0/16:0) 3.1 nmol/L (16:0/18:1) 1.2 nmol/L (18:1/18:1) | In all cases, PEth 16:0/18:1 levels were above LOQ, whilst PEth 16:0/16:0 and 18:1/18:1 were below LOQ in two and six subjects, respectively. |
| Kwak et al. [20] | Clinical Toxicology 2014 | Prospective cohort/longitudinal study | Mothers | To characterize PEth blood concentrations to differentiate different levels of alcohol exposure in pregnant women | Pregnant women referred to the Clinic for antenatal care | Inclusion criteria:
| 305 first-trimester pregnant women Abstainers 32.7 years ± 3.8 Light drinkers 32.3 years ± 4.0 Moderate drinkers 33.2 years ± 4.4 Heavy drinkers31.9 years ±4.9 | - | Self-reported consumption | Whole blood collected within 3–4 weeks after recruitment (mean gestational age: 6.9–7.8 weeks) | 16:0/16:0 16:0/18:1 18:1/18:1 | LC-MS/MS LOD = 0.4 nmol/L (16:0/16:0) 0.9 nmol/L (16:0/18:1) 0.4 nmol/L (18:1/18:1) LOQ = 1.5 nmol/L (16:0/16:0) 3.1 nmol/L (16:0/18:1) 1.2 nmol/L (18:1/18:1) |
|
| Maxwell et al. [23] | Reproductive Toxicology 2019 | Cross-sectional study | Newborns | To detect PAE assessing biomarkers | DBS collected between January 2013 and February 2015 | Exclusion criteria:
| 162 newborns | Positive PETh testing:
| - | DBS from umbilical cord collected immediately after birth | Palmitoyl/oleoyl (16:0/18:0) | LC-MS/MS LOQ = 8 ng/mL |
|
| Raggio et al. [15] | Journal of Acquired Immune Deficiency Syndromes 2019 | Prospective cohort/longitudinal study | Mothers | To investigate alcohol use and under-reporting of alcohol use in pregnant women HIV-positive in South Africa and Uganda | Women attending the outpatient clinics offering HIV care in Uganda and South Africa | Inclusion criteria:
| 163 pregnant women 255 non-pregnant women 29 years (24–35) | - | AUDIT-C | DBS from venous blood draws | - | LC-MS/MS LOQ = 8 ng/mL |
|
3.1. Main Aims of the Included Studies
3.2. Reported Alcohol Intake
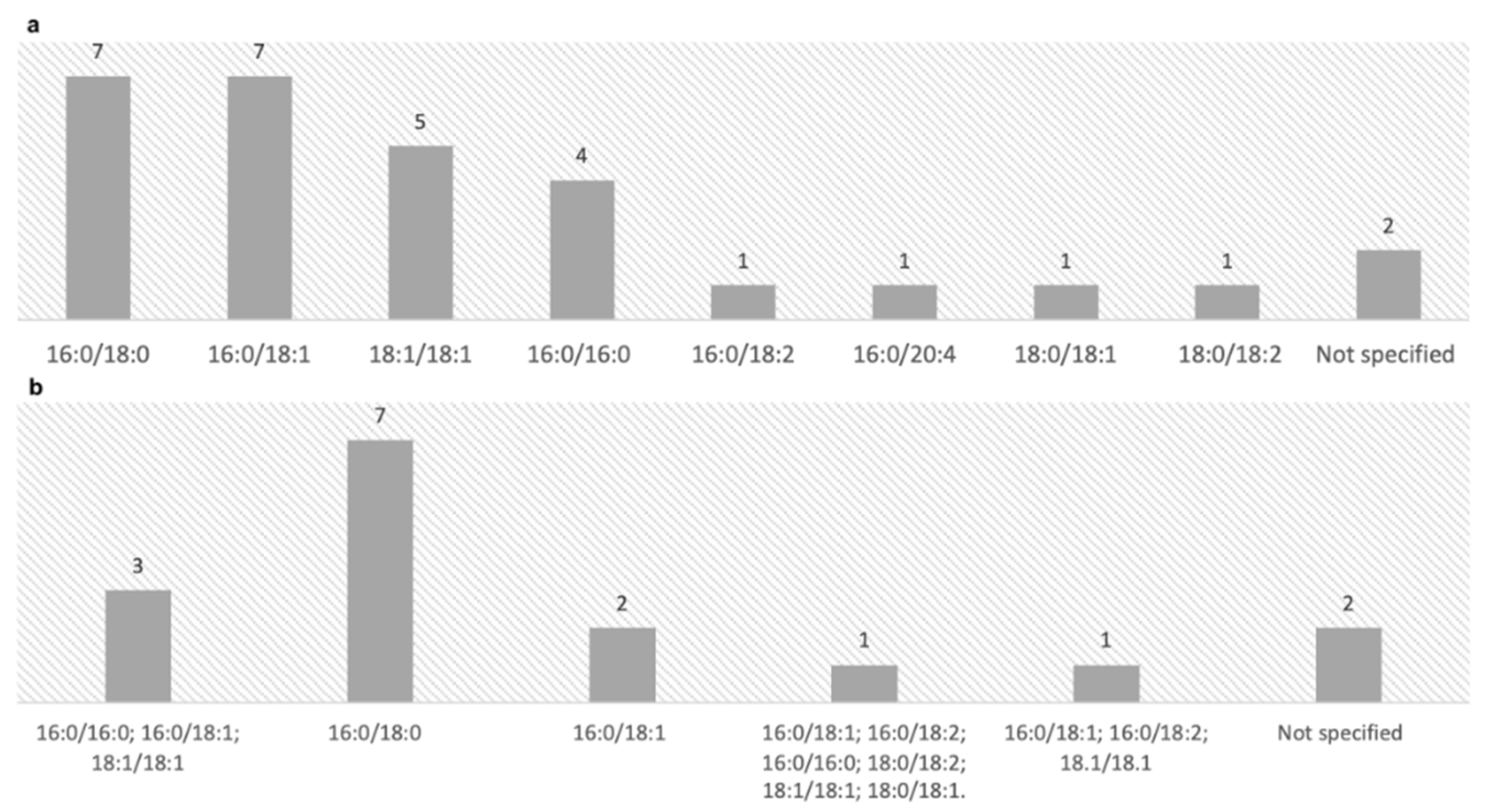
3.3. Isoforms of PEth
3.4. Units of Measurement
3.5. Interpretative Cut-Offs and Sensitivity/Specificity of PEth
3.6. Biological Matrices Involved and Categorization of the Studies
3.6.1. PEth in Maternal Blood
- in one record before the 15th week of gestational age [18];
- in one record between the 16th–18th week of gestational age [7];
- in one record two blood withdrawals were performed [27]; the first at the 12th week of gestational age and the second one at the 24th week of gestational age;
- in one record before the 34th week of gestational age [15].
3.6.2. PEth in Neonatal Blood
3.6.3. PEth in Both Maternal and Neonatal Blood
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bakhireva, L.N.; Leeman, L.; Savich, R.D.; Cano, S.; Gutierrez, H.; Savage, D.D.; Rayburn, W.F. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2014, 38, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Jańczewska, I.; Wierzba, J.; Cichoń-Kotek, M.; Jańczewska, A. Fetal alcohol spectrum disorders—Diagnostic difficulties in the neonatal period and new diagnostic approaches. Dev. Period Med. 2019, 23, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Bakhireva, L.N.; Savage, D.D. Focus on biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol. Res. Health 2011, 34, 56–63. [Google Scholar] [PubMed]
- Bager, H.; Christensen, L.P.; Husby, S.; Bjerregaard, L. Biomarkers for the Detection of Prenatal Alcohol Exposure: A Review. Alcohol. Clin. Exp. Res. 2017, 41, 251–261. [Google Scholar] [CrossRef]
- Goodlett, C.R.; Peterson, S.D.; Lundahl, K.R.; Pearlman, A.D. Binge-like alcohol exposure of neonatal rats via intragastric intubation induces both Purkinje cell loss and cortical astrogliosis. Alcohol. Clin. Exp. Res. 1997, 21, 1010–1017. [Google Scholar] [CrossRef]
- May, P.A.; Blankenship, J.; Marais, A.S.; Gossage, J.P.; Kalberg, W.O.; Joubert, B.; Cloete, M.; Barnard, R.; De Vries, M.; Hasken, J.; et al. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol. Depend. 2013, 133, 502–512. [Google Scholar] [CrossRef]
- Comasco, E.; Hallberg, G.; Helander, A.; Oreland, L.; Sundelin-Wahlsten, V. Alcohol consumption among pregnant women in a Swedish sample and its effects on the newborn outcomes. Alcohol. Clin. Exp. Res. 2012, 36, 1779–1786. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P. Maternal risk factors for fetal alcohol spectrum disorders: Not as simple as it might seem. Alcohol. Res. Health 2011, 34, 15–26. [Google Scholar]
- Bakhireva, L.N. Growing potential and remaining uncertainties in assessing prenatal alcohol exposure in dry blood spots. Pediatr. Res. 2020, 88, 159–160. [Google Scholar] [CrossRef]
- Joya, X.; Friguls, B.; Ortigosa, S.; Papaseit, E.; Martínez, S.E.; Manich, A.; Garcia-Algar, O.; Pacifici, R.; Vall, O.; Pichini, S. Determination of maternal-fetal biomarkers of prenatal exposure to ethanol: A review. J. Pharm. Biomed. Anal. 2012, 69, 209–222. [Google Scholar] [CrossRef]
- Cook, J.D. Biochemical markers of alcohol use in pregnant women. Clin. Biochem. 2003, 36, 9–19. [Google Scholar] [CrossRef]
- Hansson, P.; Caron, M.; Johnson, G.; Gustavsson, L.; Alling, C. Blood phosphatidylethanol as a marker of alcohol abuse: Levels in alcoholic males during withdrawal. Alcohol. Clin. Exp. Res. 1997, 21, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S.; Han, J.Y.; Ahn, H.K.; Kim, M.H.; Ryu, H.M.; Kim, M.Y.; Chung, H.J.; Cho, D.H.; Shin, C.Y.; Velazquez-Armenta, E.Y.; et al. Blood levels of phosphatidylethanol in pregnant women reporting positive alcohol ingestion, measured by an improved LC-MS/MS analytical method. Blood levels of phosphatidylethanol in pregnant women reporting positive alcohol ingestion, measured by an improved LC-MS/MS analytical method. Clin. Toxicol. 2012, 50 , 886–891. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Savich, R.D.; Raisch, D.W.; Cano, S.; Annett, R.D.; Leeman, L.; Garg, M.; Goff, C.; Savage, D.D. The feasibility and cost of neonatal screening for prenatal alcohol exposure by measuring phosphatidylethanol in dried blood spots. Alcohol. Clin. Exp. Res. 2013, 37, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Raggio, G.A.; Psaros, C.; Fatch, R.; Goodman, G.; Matthews, L.T.; Magidson, J.F.; Amanyire, G.; Cross, A.; Asiimwe, S.; Hahn, J.A.; et al. High Rates of Biomarker-Confirmed Alcohol Use Among Pregnant Women Living with HIV in South Africa and Uganda. J. Acquir. Immune Defic. Syndr. 2019, 82, 443–451. [Google Scholar] [CrossRef]
- Bracero, L.A.; Maxwell, S.; Nyanin, A.; Seybold, D.J.; White, A.; Broce, M. Improving screening for alcohol consumption during pregnancy with phosphatidylethanol. Reprod. Toxicol. 2017, 74, 104–107. [Google Scholar] [CrossRef]
- Stevens, S.; Anstice, N.; Cooper, A.; Goodman, L.; Rogers, J.; Wouldes, T.A. Multiple Tools Are Needed for the Detection of Prenatal Alcohol Exposure: Findings from a Community Antenatal Setting. Alcohol. Clin. Exp. Res. 2020, 44, 1001–1011. [Google Scholar] [CrossRef]
- Breunis, L.J.; Wassenaar, S.; Sibbles, B.J.; Aaldriks, A.A.; Bijma, H.H.; Steegers, E.; Koch, B. Objective assessment of alcohol consumption in early pregnancy using phosphatidylethanol: A cross-sectional study. BMC Pregnancy Childbirth 2021, 21, 342. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Kwak, H.S.; Han, J.Y.; Choi, J.S.; Ahn, H.K.; Ryu, H.M.; Chung, H.J.; Cho, D.H.; Shin, C.Y.; Velazquez-Armenta, E.Y.; Nava-Ocampo, A.A. Characterization of phosphatidylethanol blood concentrations for screening alcohol consumption in early pregnancy. Clin. Toxicol. 2014, 52, 25–31. [Google Scholar] [CrossRef]
- Baldwin, A.E.; Jones, J.; Jones, M.; Plate, C.; Lewis, D. Retrospective assessment of prenatal alcohol exposure by detection of phosphatidylethanol in stored dried blood spot cards: An objective method for determining prevalence rates of alcohol consumption during pregnancy. Int. J. Alcohol. Drug Res. 2015, 4, 131–137. [Google Scholar] [CrossRef]
- Baldwin, A.E.; Hayes, N.; Ostrander, E.; Magri, R.; Sass, N.; Dos Anjos Mesquita, M.; Martínez, M.; Juliani, M.C.; Cabral, P.; Fleming, M. Phosphatidylethanol Levels in Postpartum Women and Their Newborns in Uruguay and Brazil. Alcohol. Clin. Exp. Res. 2020, 44, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.; Thompson, S.; Zakko, F.; Bracero, L.A. Screening for prenatal alcohol exposure and corresponding short-term neonatal outcomes. Reprod. Toxicol. 2019, 85, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Lilly, C.; Hamilton, C.; Baldwin, A.; Breyel, J.; Tolliver, A.; Mullins, C.; John, C.; Maxwell, S. Prevalence of alcohol use in late pregnancy. Pediatr. Res. 2020, 88, 312–319. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Sharkis, J.; Shrestha, S.; Miranda-Sohrabji, T.J.; Williams, S.; Miranda, R.C. Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol. Clin. Exp. Res. 2017, 41, 1004–1011. [Google Scholar] [CrossRef]
- DiBattista, A.; Ogrel, S.; MacKenzie, A.E.; Chakraborty, P. Quantitation of phosphatidylethanols in dried blood spots to determine rates of prenatal alcohol exposure in Ontario. Alcohol. Clin. Exp. Res. 2022, 46, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Finanger, T.; Spigset, O.; Gråwe, R.W.; Andreassen, T.N.; Løkken, T.N.; Aamo, T.O.; Bratt, G.E.; Tømmervik, K.; Langaas, V.S.; Finserås, K.; et al. Phosphatidylethanol as Blood Biomarker of Alcohol Consumption in Early Pregnancy: An Observational Study in 4067 Pregnant Women. Alcohol. Clin. Exp. Res. 2021, 45, 886–892. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kwak, H.S.; Han, J.Y.; Choi, J.S.; Ahn, H.K.; Oh, Y.J.; Velázquez-Armenta, E.Y.; Nava-Ocampo, A.A. Could a first-trimester blood phosphatidylethanol concentration ≥4 nM be useful to identify women with moderate-to-heavy prenatal alcohol exposure who are at high risk of adverse pregnancy outcomes? Med. Hypotheses 2015, 85, 965–968. [Google Scholar] [CrossRef]
- Viel, G.; Boscolo-Berto, R.; Cecchetto, G.; Fais, P.; Nalesso, A.; Ferrara, S.D. Phosphatidylethanol in blood as a marker of chronic alcohol use: A systematic review and meta-analysis. Int. J. Mol. Sci. 2012, 13, 14788–14812. [Google Scholar] [CrossRef]
- Varga, A.; Hansson, P.; Lundqvist, C.; Alling, C. Phosphatidylethanol in blood as a marker of ethanol consumption in healthy volunteers: Comparison with other markers. Alcohol. Clin. Exp. Res. 1998, 22, 1832–1837. [Google Scholar] [CrossRef]
- Afshar, M.; Burnham, E.L.; Joyce, C.; Clark, B.J.; Yong, M.; Gaydos, J.; Cooper, R.S.; Smith, G.S.; Kovacs, E.J.; Lowery, E.M. Cut-point levels of phosphatidylethanol to identify alcohol misuse in a mixed cohort including critically ill patients. Alcohol. Clin. Exp. Res. 2017, 41, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, C.; McCall, K.E.; Cooper, G.; Favretto, D.; Vaiano, F.; Bertol, E.; Mactier, H. Determining the pattern and prevalence of alcohol consumption in pregnancy by measuring biomarkers in meconium. Arch. Dis. Child. Fetal Neonatal 2018, 103, F216–F220. [Google Scholar] [CrossRef]
- Bakdash, A.; Burger, P.; Goecke, T.W.; Fasching, P.A.; Reulbach, U.; Bleich, S.; Hastedt, M.; Rothe, M.; Beckmann, M.W.; Pragst, F.; et al. Quantification of fatty acid ethyl esters (FAEE) and ethyl glucuronide (EtG) in meconium from newborns for detection of alcohol abuse in a maternal health evaluation study. Anal. Bioanal. Chem. 2010, 396, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Bakhireva, L.N.; Kane, M.A.; Bearer, C.F.; Bautista, A.; Jones, J.W.; Garrison, L.; Begay, M.G.; Ozechowski, T.; Lewis, J. Prenatal alcohol exposure prevalence as measured by direct ethanol metabolites in meconium in a Native American tribe of the southwest. Birth Defects Res. 2019, 111, 53–61. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschetto, L.; Perilli, M.; Cinquetti, A.; Giraudo, C.; Gardi, M.; Cecchetto, G.; Viel, G. Phosphatidylethanol in Maternal or Neonatal Blood to Detect Alcohol Exposure during Pregnancy: A Systematic Review. Life 2022, 12, 1528. https://doi.org/10.3390/life12101528
Franceschetto L, Perilli M, Cinquetti A, Giraudo C, Gardi M, Cecchetto G, Viel G. Phosphatidylethanol in Maternal or Neonatal Blood to Detect Alcohol Exposure during Pregnancy: A Systematic Review. Life. 2022; 12(10):1528. https://doi.org/10.3390/life12101528
Chicago/Turabian StyleFranceschetto, Lisa, Matteo Perilli, Alessandro Cinquetti, Chiara Giraudo, Mario Gardi, Giovanni Cecchetto, and Guido Viel. 2022. "Phosphatidylethanol in Maternal or Neonatal Blood to Detect Alcohol Exposure during Pregnancy: A Systematic Review" Life 12, no. 10: 1528. https://doi.org/10.3390/life12101528
APA StyleFranceschetto, L., Perilli, M., Cinquetti, A., Giraudo, C., Gardi, M., Cecchetto, G., & Viel, G. (2022). Phosphatidylethanol in Maternal or Neonatal Blood to Detect Alcohol Exposure during Pregnancy: A Systematic Review. Life, 12(10), 1528. https://doi.org/10.3390/life12101528








