Radiomics Nomogram Based on High-b-Value Diffusion-Weighted Imaging for Distinguishing the Grade of Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
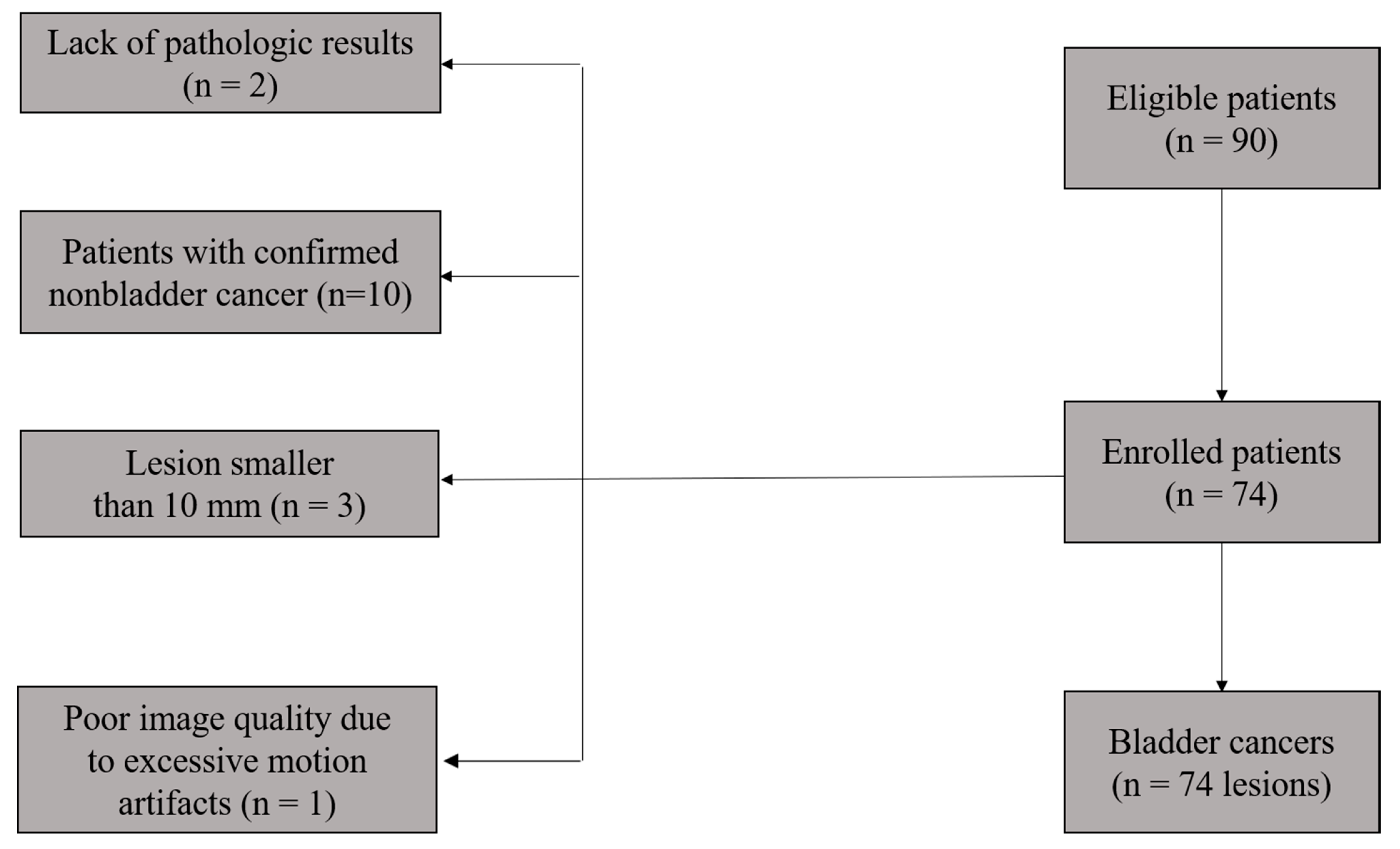
2.1. Patient Characteristics
2.2. Image Acquisition
2.3. Image Segmentation, Preprocessing, and Feature Extraction
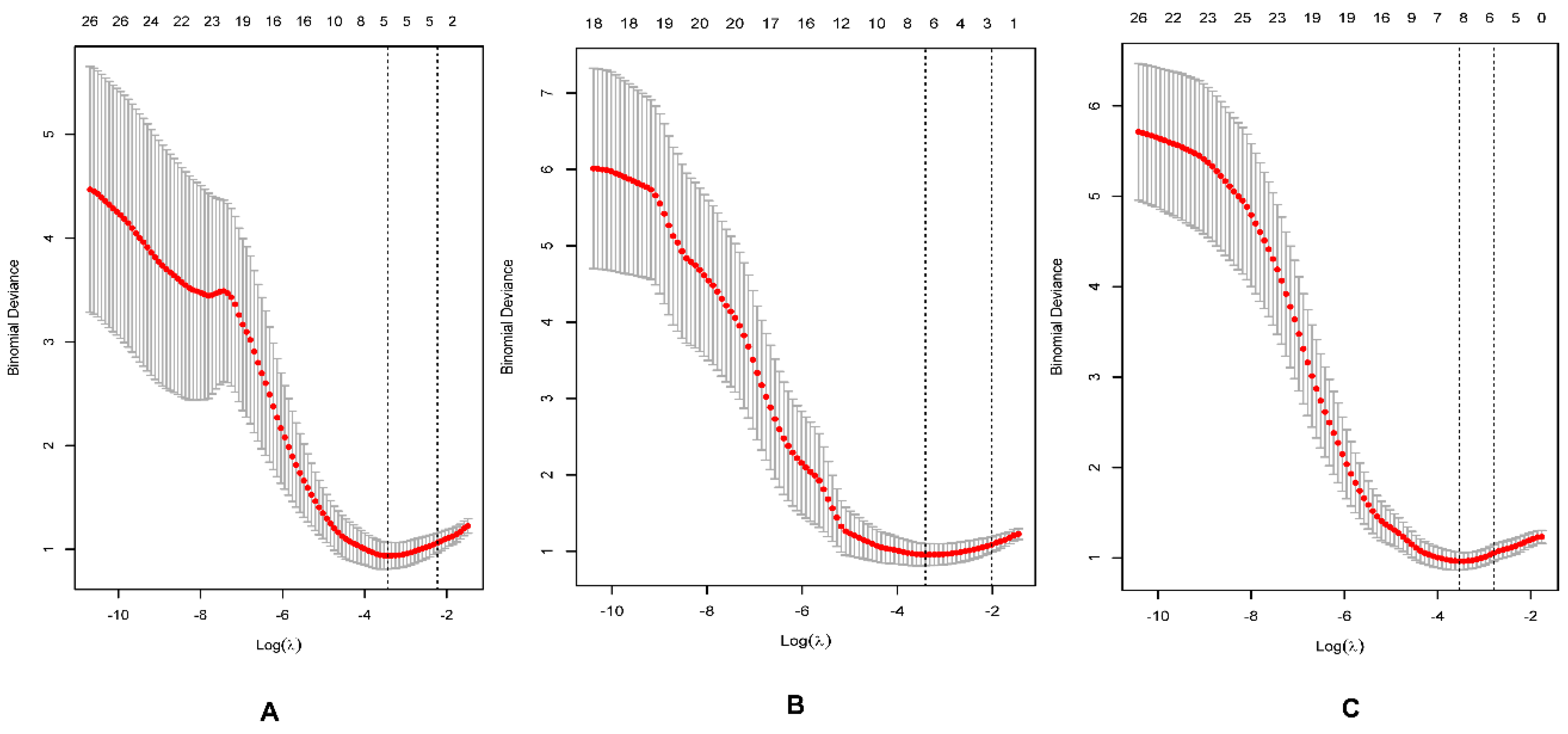
2.4. Feature Selection and Model Building
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Feature Selection
3.3. Performance of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Comperat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Roupret, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; McKenney, J.K. Urinary Bladder Pathology: World Health Organization Classification and American Joint Committee on Cancer Staging Update. Arch. Pathol. Lab. Med. 2019, 143, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Chang, Y.H.; Chen, K.K.; Yu, H.J.; Sun, C.H.; Ho, D.M. Prognostic significance of the 2004 WHO/ISUP classification for prediction of recurrence, progression, and cancer-specific mortality of non-muscle-invasive urothelial tumors of the urinary bladder: A clinicopathologic study of 1,515 cases. Am. J. Clin. Pathol 2010, 133, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Alfred, W.J.; Lebret, T.; Comperat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernandez, V.; Espinos, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Donat, S.M. Quality control in transurethral resection of bladder tumours. BJU Int. 2008, 102, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Ark, J.T.; Keegan, K.A.; Barocas, D.A.; Morgan, T.M.; Resnick, M.J.; You, C.; Cookson, M.S.; Penson, D.F.; Davis, R.; Clark, P.E.; et al. Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int. 2014, 113, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Y.; Dan, G.; Zhong, Z.; Karaman, M.M.; Li, Z.; Hu, D.; Zhou, X.J. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur. Radiol. 2022, 32, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, X.J. Diffusion MRI of cancer: From low to high b-values. J. Magn. Reson. Imaging 2019, 49, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Koga, F.; Kajino, K.; Yoshita, S.; Ishii, C.; Tanaka, H.; Saito, K.; Masuda, H.; Fujii, Y.; Yamada, T.; et al. Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J. Magn. Reason. Imaging 2014, 39, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Koga, F.; Yoshida, S.; Masuda, H.; Ishii, C.; Tanaka, H.; Komai, Y.; Yokoyama, M.; Saito, K.; Fujii, Y.; et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: Potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur. Radiol. 2011, 21, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Ghar, M.E.; El-Assmy, A.; Refaie, H.F.; El-Diasty, T. Bladder cancer: Diagnosis with diffusion-weighted MR imaging in patients with gross hematuria. Radiology 2009, 251, 415–421. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Hu, X.; Li, Z.; Feng, C.; Hu, D.; Kamel, I.R. Application of R2* and Apparent Diffusion Coefficient in Estimating Tumor Grade and T Category of Bladder Cancer. AJR Am. J. Roentgenol. 2020, 214, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Avcu, S.; Koseoglu, M.N.; Ceylan, K.; Bulut, M.D.; Unal, O. The value of diffusion-weighted MRI in the diagnosis of malignant and benign urinary bladder lesions. Br. J. Radiol. 2011, 84, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Chen, J.H. Pitfalls and Limitations of Diffusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Urinary Bladder Cancer. Transl. Oncol. 2015, 8, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Choi, S.H.; Kim, Y.J.; Kim, K.G.; Sohn, C.H.; Kim, J.H.; Yun, T.J.; Chang, K.H. Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging--correlation with tumor grade. Radiology 2011, 261, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Huang, S.; Guo, J.; Zhuang, X.; Han, H. Use of a high b-value for diffusion weighted imaging of peritumoral regions to differentiate high-grade gliomas and solitary metastases. J. Magn. Reason. Imaging 2015, 42, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Choi, S.H.; Ryoo, I.; Kim, S.C.; Yeom, J.A.; Shin, H.; Jung, S.C.; Lee, A.L.; Yoon, T.J.; Kim, T.M.; et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: Comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 2013, 269, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, X.; Zhang, X.; Liu, Y.; Ouyang, L.; Du, P.; Li, S.; Tian, Q.; Ling, J.; Guo, Y.; et al. Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: A double-center study. Eur. Radiol. 2020, 30, 4816–4827. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Zhang, X.; Tian, Q.; Wu, Y.; Zhang, G.; Meng, J.; Yang, Z.; Lu, H. Preoperative prediction of muscular invasiveness of bladder cancer with radiomic features on conventional MRI and its high-order derivative maps. Abdom. Radiol (NY). 2017, 42, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Tian, Q.; Wang, H.; Cui, L.B.; Li, S.; Tang, X.; Li, B.; Dolz, J.; Ayed, I.B.; et al. Quantitative Identification of Nonmuscle-Invasive and Muscle-Invasive Bladder Carcinomas: A Multiparametric MRI Radiomics Analysis. J. Magn. Reason. Imaging 2019, 49, 1489–1498. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Tian, Q.; Li, B.; Wu, Y.; Yang, Z.; Liang, Z.; Liu, Y.; Cui, G.; Lu, H. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J. Magn. Reason. Imaging 2017, 46, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Du, P.; Zhang, F.; Li, S.; Zhang, Z.; Yuan, J.; Liang, Z.; Zhang, X.; Guo, Y.; et al. A predictive nomogram for individualized recurrence stratification of bladder cancer using multiparametric MRI and clinical risk factors. J. Magn. Reason. Imaging 2019, 50, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, D.; Meng, J.; Zhou, J.; Chen, Z.; Huang, S.; Lu, B.; Qiu, Y.; Baker, M.E.; Ye, Z.; et al. Development and Validation of a Novel Computed-Tomography Enterography Radiomic Approach for Characterization of Intestinal Fibrosis in Crohn's Disease. Gastroenterology 2021, 160, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Liang, C.H.; He, L.; Tian, J.; Liang, C.S.; Chen, X.; Ma, Z.L.; Liu, Z.Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Razik, A.; Das, C.J.; Sharma, R.; Malla, S.; Sharma, S.; Seth, A.; Srivastava, D.N. Utility of first order MRI-Texture analysis parameters in the prediction of histologic grade and muscle invasion in urinary bladder cancer: A preliminary study. Br. J. Radiol. 2021, 94, 20201114. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.T.; Xu, S.; Wood, B.J.; Turkbey, B.; Choyke, P.L.; Pinto, P.A.; Wang, S.; Summers, R.M. Automated prostate cancer detection using T2-weighted and high-b-value diffusion-weighted magnetic resonance imaging. Med. Phys. 2015, 42, 2368–2378. [Google Scholar] [CrossRef]
- Borkowski, K.; Krzyżak, A.T. The generalized Stejskal-Tanner equation for non-uniform magnetic field gradients. J. Magn. Reason. 2018, 296, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Krzyzak, A.T.; Klodowski, K. The b matrix calculation using the anisotropic phantoms for DWI and DTI experiments. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 418–421. [Google Scholar]
- Borkowski, K.; Klodowski, K.; Figiel, H.; Krzyzak, A.T. A theoretical validation of the B-matrix spatial distribution approach to diffusion tensor imaging. Magn Reason. Imaging 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]


| Variables | Characteristics |
|---|---|
| Age (years) * | 61 ± 10 (37–79) |
| Gender | |
| Male | 63 (85) |
| Female | 11 (15) |
| No. of lesions | |
| Unifocal | 56 (76) |
| Multifocal | 18 (24) |
| Primary or recurrent tumors | |
| Primary | 72 (97) |
| Recurrent | 2 (3) |
| Tumor size (cm) * | 3.1 ± 1.6 (1.0–10.1) |
| Pathologic stage | |
| T1 | 41 (55) |
| T2 | 20 (27) |
| T3 | 3 (4) |
| T4 | 10 (16) |
| Histologic grade | |
| Low | 22 (30) |
| High | 52 (70) |
| Lymph node metastasis | |
| Yes | 13 (18) |
| No | 61 (82) |
| Treatment methods | |
| TURBT | 43 (58) |
| Radical cystectomy | 27 (36) |
| Partial cystectomy | 4 (5) |
| Rad-Score | Variables | Coefficients |
|---|---|---|
| ADC1000 | Intercept | 1.199576464 |
| CONVENTIONAL_#std | −0.582534519 | |
| CONVENTIONAL_#Q2 | −0.615993908 | |
| SHAPE_Sphericity (only for 3D ROI (nz > 1) | −0.77836837 | |
| GLRLM_RLNU | 0.340808516 | |
| NGLDM_Busyness | 0.029560922 | |
| ADC1700 | Intercept | 1.255336302 |
| CONVENTIONAL_#std | −0.54250182 | |
| SHAPE_Sphericity (only for 3D ROI (nz > 1) | −1.110704238 | |
| SHAPE_Compacity only for 3D ROI (nz > 1) | 0.36442195 | |
| GLRLM_HGRE | −0.538256985 | |
| NGLDM_Busyness | 0.050853414 | |
| GLZLM_HGZE | −0.10091227 | |
| GLZLM_GLNU | 0.004710521 | |
| ADC3000 | Intercept | 1.234603905 |
| HISTO_Skewness | 0.306892167 | |
| SHAPE_Sphericity (only for 3D ROI (nz > 1) | −0.918088191 | |
| SHAPE_Compacity only for 3D ROI (nz > 1) | 0.869977739 | |
| GLCM_Correlation | −0.62276962 | |
| GLRLM_LGRE | 0.139654102 | |
| GLRLM_SRLGE | −0.098482715 | |
| NGLDM_Contrast | −0.076828157 |
| AUC (95%CI) | Sensitivity | Specitivity | |
|---|---|---|---|
| Training cohort (n = 58) | |||
| ADC1000 | 0.901 (0.825–0.977) | 0.683 | 1.000 |
| ADC1700 | 0.920 (0.849–0.990) | 0.854 | 0.882 |
| ADC3000 | 0.901 (0.817–0.985) | 0.805 | 0.882 |
| Test cohort (n = 16) | |||
| ADC1000 | 0.582 (0.226–0.937) | 0.548 | 0.800 |
| ADC1700 | 0.745 (0.475–1.000) | 0.909 | 0.600 |
| ADC3000 | 0.745 (0.451–1.000) | 0.727 | 0.800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Zhou, Z.; Huang, Q.; Meng, X.; Li, Z.; Wang, Y. Radiomics Nomogram Based on High-b-Value Diffusion-Weighted Imaging for Distinguishing the Grade of Bladder Cancer. Life 2022, 12, 1510. https://doi.org/10.3390/life12101510
Feng C, Zhou Z, Huang Q, Meng X, Li Z, Wang Y. Radiomics Nomogram Based on High-b-Value Diffusion-Weighted Imaging for Distinguishing the Grade of Bladder Cancer. Life. 2022; 12(10):1510. https://doi.org/10.3390/life12101510
Chicago/Turabian StyleFeng, Cui, Ziling Zhou, Qiuhan Huang, Xiaoyan Meng, Zhen Li, and Yanchun Wang. 2022. "Radiomics Nomogram Based on High-b-Value Diffusion-Weighted Imaging for Distinguishing the Grade of Bladder Cancer" Life 12, no. 10: 1510. https://doi.org/10.3390/life12101510
APA StyleFeng, C., Zhou, Z., Huang, Q., Meng, X., Li, Z., & Wang, Y. (2022). Radiomics Nomogram Based on High-b-Value Diffusion-Weighted Imaging for Distinguishing the Grade of Bladder Cancer. Life, 12(10), 1510. https://doi.org/10.3390/life12101510







