Abstract
Background: Although polysomnography (PSG) is a gold standard tool for diagnosing sleep apnea (SA), it can reduce the patient’s sleep quality by the placement of several disturbing sensors and can only be interpreted by a highly trained sleep technician or scientist. In recent years, electrocardiogram (ECG)-derived respiration (EDR) and heart rate variability (HRV) have been used to automatically diagnose SA and reduce the drawbacks of PSG. Up to now, most of the proposed approaches focus on machine-learning (ML) algorithms and feature engineering, which require prior expert knowledge and experience. The present study proposes an SA detection algorithm to differentiate a normal and apnea event using a deep-learning (DL) framework based on 1D and 2D deep CNN with empirical mode decomposition (EMD) of a preprocessed ECG signal. The EMD is ideally suited to extract essential components which are characteristic of the underlying biological or physiological processes. In addition, the simple and compact architecture of 1D deep CNN, which only performs 1D convolutions, and pretrained 2D deep CNNs, are suitable for real-time and low-cost hardware implementation. Method: This study was validated using 7 h to nearly 10 h overnight ECG recordings from 33 subjects with an average apnea-hypopnea index (AHI) of 30.23/h originated from PhysioNet Apnea-ECG database (PAED). In preprocessing, the raw ECG signal was normalized and filtered using the FIR band pass filter. The preprocessed ECG signal was then decomposed using the empirical mode decomposition (EMD) technique to generate several features. Several important generated features were selected using neighborhood component analysis (NCA). Finally, deep learning algorithm based on 1D and 2D deep CNN were used to perform the classification of normal and apnea event. The synthetic minority oversampling technique (SMOTE) was also applied to evaluate the influence of the imbalanced data problem. Results: The segment-level classification performance had 93.8% accuracy with 94.9% sensitivity and 92.7% specificity based on 5-fold cross-validation (5fold-CV), meanwhile, the subject-level classification performance had 83.5% accuracy with 75.9% sensitivity and 88.7% specificity based on leave-one-subject-out cross-validation (LOSO-CV). Conclusion: A novel and robust SA detection algorithm based on the ECG decomposed signal using EMD and deep CNN was successfully developed in this study.
1. Introduction
Sleep apnea (SA) is a common sleep disorder in which pauses in breathing or periods of shallow breathing during sleep occur more often than normal, which often remains undiagnosed and untreated [1]. Each pause possibly lasts between 10 and 20 s and happens from 5 to over 100 times per hour during overnight sleep. SA can present with or without symptoms and is accompanied by major neurocognitive and cardiovascular sequelae. SA is characterized by increased collapsibility of the upper airway during sleep, resulting in significantly reduced (hypopnea) or absent (apnea) airflow at the nose and/or mouth, which is usually accompanied by oxyhemoglobin desaturation and is typically terminated by a brief micro-arousal [2]. Repeated apnea episodes result in a prolonged decrease in oxyhemoglobin saturation and sleep fragmentation, with less slow-wave and rapid eye movement (REM) sleep.
SA has a high prevalence, especially among middle-aged and elderly people. According to the American Academy of Sleep Apnea’s diagnostic criteria, approximately 936 million adults aged 30 to 69 years have mild to severe SA syndrome and 425 million adults aged 30 to 69 years have moderate to severe SA (AASM) [3,4]. Further research into the relationship between the incidence of SA and age found that 88% of men aged 65 to 69 years had five or more events per hour, while that incidence increased to 90% in men aged 70 to 85 years. In addition, SA has been linked to an increased risk of other diseases such as hypertension, heart failure, a heart attack, and a cardiovascular event [5].
Among the causes, there is the use of different diagnostic tools. For AASM, the gold standard for a SA diagnosis remains comprehensive polysomnography [6], although alternative diagnosis methods have been studied. In recent decades, different SA detection methods have been proposed using a single-lead physiological signal to satisfy the non-intrusive demand and hardware conditions of wearable mobile equipment, such as pulse oximetry (SpO2), nasal airflow, thoracic (THO) and abdominal (ABD) movement electroencephalography (EEG), and electrocardiography (ECG). Based on SpO2 signal, Alvarez et al. built a home polysomnography-based SA screening system using machine learning [7], and Mendonca et al. designed a wireless home monitoring device for automatic diagnosis OSA patient [8]. Based on nasal airflow, Haidar et al. proposed an apnea-hypopnea events recognition system using convolutional neural network (CNN) [9] and Yue et al. developed a multi-resolution residual network for the diagnosis and classification of OSA [10]. Based on THO and ABD movement signals, Lin et al. identified various types of apneas using wearable piezoelectric bands [11]. Based on EEG signal, Zhao et al. developed an algorithm to automatically differentiate SA events using entropy-based EEG sub-band feature extraction and machine learning-based classifiers [12]. Bhattacharjee et al. aimed to detect SA based on Rician modeling of feature variation and in multi-band EEG [13,14], and Taran et al. introduced an apnea event detection algorithm using Hermite basis function adaptive decomposition for EEG [15].
ECG waveform analysis and heart rate variability (HRV) have recently been used and developed as an alternative method of detecting sleep disordered breathing, including apnea and hypopnea events [16,17]. The ECG signal is particularly interesting to study because it enables the physiological demonstration of SA occurrence and is easy to record with wearable devices. When an apnea event occurs, blood oxygen levels drop, and the cardiovascular system is prompted to keep the body’s oxygen supply adequate. As a result, abnormal heart activity or high heart rate variability may indicate the presence of SA. The respiratory effort produces an alteration in the ECG electrode’s position, which severally influences the ECG signal’s amplitude [18]. Meanwhile, the HRV assesses the time interval variation between successive heartbeats, known as the R-R interval (RRI). The variation in the RRI is a symptom of apnea events, hence can provide the physiological basis to detect SA. The possibility of diagnosing SA by single-lead ECG was presented in 1984 by Guilleminault et al. [19], followed by an apnea ECG PhysioNet database publication after competition called the Computers in Cardiology 2000 Challenge [16], which provided minute-by-minute ECG data with apnea labeling. In recent years, more studies using single-lead ECG to detect apnea episodes have been proposed and validated using the PhysioNet database. The majority of them concentrated on machine learning (ML) and feature engineering, which necessitates the use of a specific feature extraction method to extract ECG features. Otherwise, the deep learning approach has been developed in recent years. The SA detection literature review based on single-lead ECG using ML and DL frameworks is summarized in Table 1.

Table 1.
Literature review of SA detection method based on single-lead ECG signal.
The novelty and significance of this study can mainly be described as follows:
- (1)
- The proposed method used EMD features generated from preprocessed overnight ECG signals to identify various symptoms of SA episodes. Due to external stimulation, biomedical signals are, in general, non-linear and non-stationary, thus EMD is ideally suited to extract essential components which are characteristic of the underlying biological or physiological processes.
- (2)
- The proposed deep learning framework can outperform SA classification state-of-the-art methods with potential for real-time and low-cost hardware implementation. The deep CNN models have a simple and compact architecture; moreover, they reduce the need for feature extraction.
- (3)
- The proposed method applied the synthetic minority oversampling technique (SMOTE) to overcome the imbalance problem. The comparison performance with other re-sampling methods is also discussed.
The remaining focus of this paper is organized as follows. Section 2 describes the databases that were used as well as the proposed SA detection system that is based on EMD and deep CNN models. Section 3 contains the results of the experiments. Section 4 describes the study findings discussions. Finally, Section 5 brings the study to a close.
2. Materials and Methods
2.1. Overnight Apnea ECG Database
The Apnea ECG PhysioNet Database (AEPD) [40] is an open public database and was utilized to validate and enable the proposed algorithm performance to be evaluated and compared with the existing literature. The AEPD ECG recordings were sampled at 100 Hz, and the duration of each participant’s sleep recording ranged between 7 and 10 h. [16]. The database contained 35 participants’ recordings with SA annotations on a minute-by-minute basis; each minute of ECG recording was annotated as either normal breathing or disordered breathing with apnea episode.
AEPD had three participant categories: A: apnea (Table 2), B: borderline (Table 3), and C: healthy control (Table 4), each with 20, 5, and 10 subjects. Group A participants were discovered to be related to people with OSA and had total apnea durations of more than 100 min for each recording. This group’s participants ranged in age from 38 to 63, and their AHI ranged from 21 to 83. Group B participants were borderline, with total apnea episode durations ranging from 10–96 min. This group’s age ranged from 42 to 53, and the AHI ranged from 0 to 25. Group C participants were healthy controls with no SA or very low levels of disease and total apnea durations ranging from 0 to 3 min. Group B recording b05 and Group C recording c05 were eliminated because b05 contained a grinding noise and c05 was identical to c06. Consequently, there were only 33 recordings included in this study, with totally 9160 normal events and 6019 apnea events (imbalanced dataset), after eliminating some contaminated events caused by the patient’s movement, poor patch contact, electrical interference, measurement noise, or other disturbances. Table 2, Table 3 and Table 4 provide the details on each participant’s characteristics and the sample size information used in this study.

Table 2.
Apnea ECG PhysioNet Database (APED) apnea participants description.

Table 3.
Apnea ECG PhysioNet Database (APED) borderline participants description.

Table 4.
Apnea ECG PhysioNet Database (APED) healthy control participants description.
2.2. The Overall Deep Learning Framework for Automatic Sleep Apnea Classification Based on Empirical Mode Decomposition Derived from Single-Lead Electrocardiogram
Figure 1 shows the overall proposed sleep apnea detection system flowchart based on the deep learning framework and the ECG signal decomposition. This study proposes an automatic SA classification algorithm based on the deep learning framework and EMD derived from a single-lead ECG signal of apnea and normal breathing. Overnight single-lead ECG signals from 33 participants were the input with each participant’s 7~8 h recordings, and then partitioned into subsequent 60-s ECG windows. The participants of the mixed group category comprised AEPD group A, B, and C. The raw ECG signal was preprocessed and included normalization, windowing, and band-pass filtering. The EMD algorithm was used to obtain decomposition features to discriminate SA and normal breathing episodes. Feature selection based on the neighborhood component analysis (NCA) was then applied to reduce the number of EMD features (IMFs). In total, 4 IMFs were used in this study, specifically , , as the result of the addition IMF1 and 2 and as the result of the addition IMF1, 2, and 3. Thereafter, the synthetic minority oversampling technique (SMOTE) was applied to deal with the data imbalance problem. Finally, a 1D deep CNN model was established to obtain the classification results using cross-validation.

Figure 1.
The proposed sleep apnea detection system flowchart based on the deep learning framework and EMD of single lead ECG signal.
2.3. ECG Signal Preprocessing and Filtering
The zero-means computation and windowing processing parameters were used in the ECG signal preprocessing. In this method, zero-means subtracts the mean from the ECG signals to remove the baseline wandering effects, and then nocturnal ECG spectra are divided into 60-s intervals, with large noise windows excluded for algorithm development. The AEPD used in this study labeled each 60-s window as having an apnea or normal episode.
2.4. Empirical Mode Decomposition (EMD)
Empirical mode decomposition (EMD) [41] is a time-frequency domain signal analysis that adaptively and locally decomposes any non-stationary time series in a sum of oscillatory waveforms known as Intrinsic Mode Functions (IMF), which represent zero-mean amplitude and frequency modulated components. The main idea of this method is to empirically identify these intrinsic oscillatory modes by their characteristic time scales in the data, and then decompose the data accordingly. The step by step of EMD generation is as follows:
- Correlate all the local maximum and minimum by applying a cubic spline to generate the upper and lower envelope.
- Specify the average of upper and lower envelopes, which is denoted as .
- Calculate the difference between the signal and the average , (potential first IMF).
- Verify if satisfies the two requirements of IMF:
- In the entire data set, the number of extremum and zero-crossings must be equal or differ by no more than one.
- The mean value of the envelope indicated by the local maximum and local minimum is zero at any point.
If meets both requirements, then is the first IMF of the signal. If does not meet the two IMF requirements, the sifting process will be reiterated by treating the as original until it meets the requirements.
- 5.
- After subtracting the signal from the IMF, the sifting process is reiterated to break down the data into IMFs.
Finally, the signal can be expressed as:
where represents the IMFs of the original signal with and is a residue of . The differentiation of ECG decomposed signals, IMF1 to 6, between normal and apnea episodes is clearly described in Figure 2 and Figure 3.
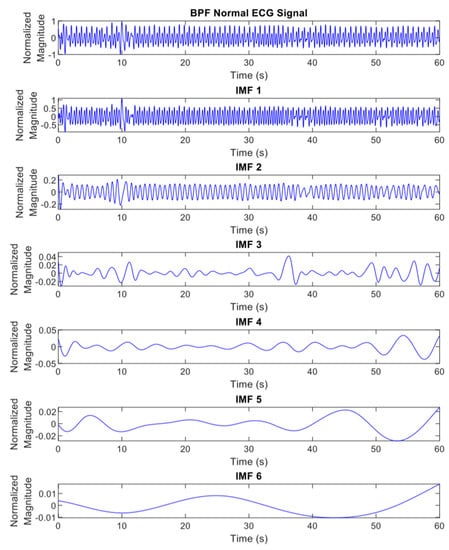
Figure 2.
The EMD results (IMF1 to 6) of preprocessed ECG signals of normal episode.
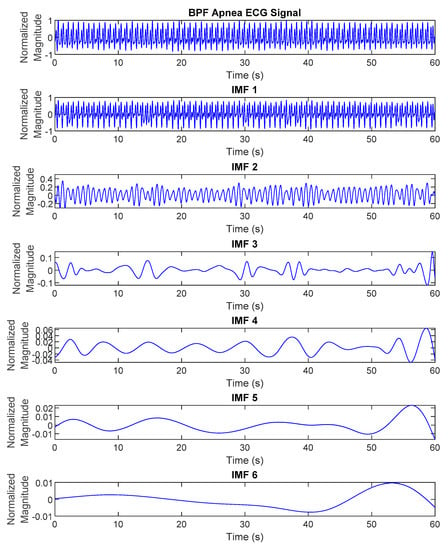
Figure 3.
The EMD results (IMF1 to 6) of preprocessed ECG signals of apnea episode.
2.5. Feature Transformation Using Continuous Wavelet Transform (CWT)
The continuous wavelet transform (CWT) [42] is a technique for extracting time-localized information from a signal by calculating the time convolution of the signal with the analyzing wavelet. Convolution between the signal and a family (set) of wavelets defined for a specific frequency range is required to compute the spectro-temporal representation of a signal using CWT, assigned as:
To extract amplitude and phase, wavelet functions must be complex-valued and well-localized in time and frequency. The complex Morlet wavelet is the most commonly used wavelet for complex spectro-temporal signal characterization. It is made up of a complex oscillation with a fixed frequency (frequency localization) that is constrained by a Gaussian window (time localization). The mother wavelet for the complex Morlet wavelet family is formulated as:
where is the complex oscillatory component with frequency of Hz, is the Gaussian window with a temporal standard deviation of , is applied to confirm that the wavelet energy is equal to one, and the parameter generally specifies the number of cycles at the frequency inside the Gaussian bell. The CWT spectrograms of normal and apnea episode from the EMD preprocessed ECG signal are clearly presented in Figure 4 and Figure 5.
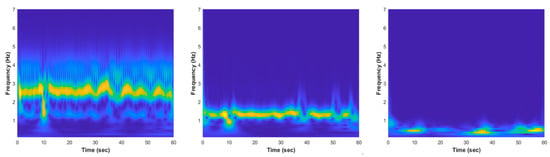
Figure 4.
The EMD CWT spectrogram results of normal episode preprocessed ECG signal, from left to right: , , and .

Figure 5.
The EMD CWT spectrogram results of apnea episode preprocessed ECG signal, from left to right: , , and .
2.6. Feature Selection Using Neighborhood Component Analysis (NCA)
Feature selection is the process of reducing the number of input variables when developing a predictive model. It is desirable to reduce the number of input variables to both reduce the computational cost of modelling and, in some cases, to improve the performance of the model. Neighborhood component analysis (NCA) is a non-parametric method for selecting features with the goal of maximizing prediction accuracy of classification algorithms [43].
The regularization parameter () was tuned for the best features. NCA discards irrelevant features which reduces the features dimensionality and improves the algorithm performance. The steps involved in NCA are as follows [44]:
- Data is divided into training and testing sets. The training data are then partitioned into 10-folds in which the classifier leaves out one-fold for testing and trained on the other nine folds.
- The regularization parameter, value, is tuned and the NCA model was trained for each using each fold in the training set. This process is repeated for all folds and all values.
- The average loss obtained from each fold for each value was computed and the best value was found, which corresponds to the minimum average loss.
- Finally, the features with feature weight greater than the threshold (T) were extracted.
As the result, the selected EMD features were IMF1, 2, and 3 based on the NCA evaluation of mean, standard deviation, and variance of each feature. The selected IMFs were also combined and generated new features, such as and , to remove most of the noises and artifacts and construct denoised ECG signals. The combination IMFs were defined as follows:
In total, there were 4 IMF features, , , , and , which were processed to the classification stage (Figure 6 and Figure 7 provide details of the IMF features between normal and apnea episodes).
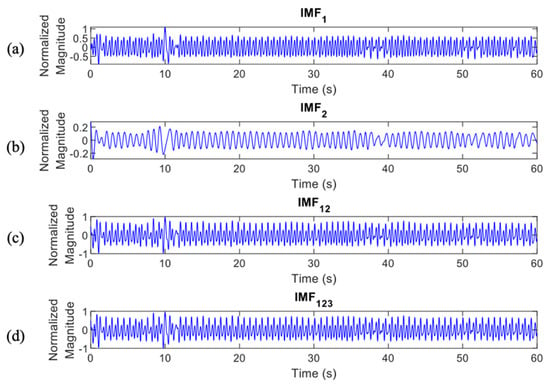
Figure 6.
IMF features of normal episodes which were processed to the classification stage, (a), (b), (c), and (d).
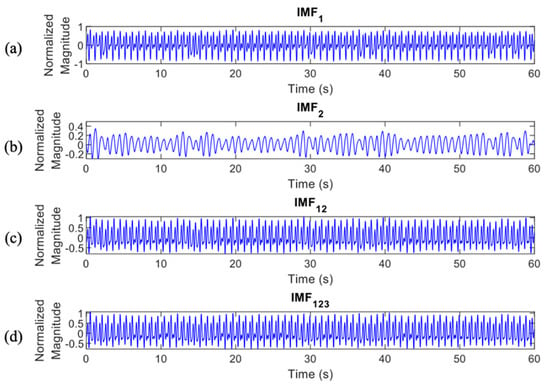
Figure 7.
IMF features of apnea episodes which were processed to the classification stage, (a), (b), (c), and (d).
2.7. Data Augmentation Using Synthetic Minority Oversampling Technique (SMOTE)
The SMOTE algorithm [45] generates artificial examples based on the feature space, rather than data space, similarities between existing minority examples, by the reason of data augmentation. These synthetic examples are generated by connecting a portion or all of the minority class’s K nearest neighbors. Neighbors from the K nearest neighbors are chosen at random depending on the amount of oversampling required.
In more detail, let characterize the class with minority condition. For each data point find the k-nearest neighbor, given a specified K. The k-nearest neighbors are defined as the K elements of whose Euclidian distance between itself and have the smallest magnitude in the feature space X. To generate a new data point, choose one of the k-nearest neighbors at random, and then calculate the difference between the chosen data point and its nearest data point. Multiply this difference by a number λ, that ranges from 0 to 1. Finally, add this vector to the selected data point :
where is the selected instance from minority class, is one of the k-nearest neighbors of and is random generated number.
2.8. One-Dimensional Deep Convolutional Neural Network (1D Deep CNN)
Deep-learning (DL) is the most recent achievement of the machine-learning (ML) era, displaying near-human [46], and now super-human abilities in a variety of applications such as voice-to-text translations, object detection and recognition, anomaly detection, emotion recognition from audio or video recordings, and so on.
The proposed 1D deep CNN architecture, adapted from a study by Chang et al. [25], comprises input, feature extraction, classification, and output stages, as shown in Figure 8 and Table 5.
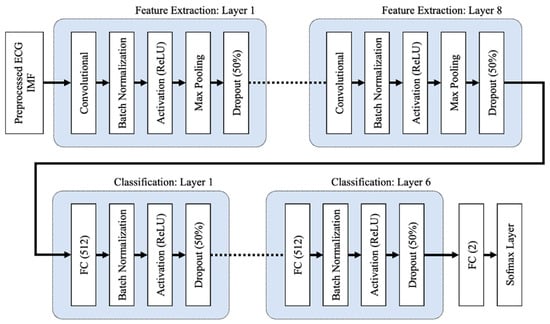
Figure 8.
The 1D deep CNN architecture, comprises of input, feature extraction, and classification stages.

Table 5.
The detail layer’s description summary of the proposed 1D deep CNN model.
To initialize the weights, the CNN and FC layers both use the He normal initialization method. The weights are initialized with the previous layer of neurons in mind, which helps the cost function reach the global minimum faster and more efficiently. The batch normalization layers, which are added after the CNN and FC layers in both the feature extraction and classification layers, normalize the data before it enters the ReLU activation layer, which improves the neural network’s speed, performance, and stability. By selecting the maximum activation about a neuron in a feature map, the max pooling layers in the feature extraction layers reduce network complexity and the possibility of overfitting. When the pooling size is set to 2, the size of each feature map is cut in half. Dropout layers with a dropout rate of 0.5 are used to reduce overfitting by randomly omitting 50% of the nodes during the proposed CNN model’s training process. Overfitting results in a high training accuracy but a low testing accuracy. The proposed 1D deep CNN model was trained to minimize cross entropy using the Adam optimizer, which is a stochastic gradient descent extension that computes individual adaptive learning rates for different parameters based on estimates of the gradient’s first and second moments.
2.9. Two-Dimensional Deep Convolutional Neural Network (2D Deep CNN)
Transfer learning is the enhancement of learning in a new task by the transfer of knowledge from a previously learned network. Fine-tuning with transfer learning is typically considerably faster and easier than training a network with randomly initialized weights from beginning. There were three kinds of pretrained CNN models that were employed for transfer learning in this study, i.e., AlexNet, GoogLeNet, and ResNet-50. Table 6 explains the main differences among the pretrained CNN models, AlexNet, GoogLeNet, and ResNet-50.

Table 6.
The main differences among pretrained 2D Deep CNN models that were used in this study.
AlexNet [47] architecture consists of 8 layers, including 5 convolution layers (with 2 Convolution 2D layers, 3 Grouped Convolution 2D layers, 5 Rectified Linear Unit (ReLU) layers, 2 Cross-Channel Normalization layers, 3 Max-Pooling 2D layers) and 3 fully connected layers (with 3 Fully Connected layer, 2 ReLU layers, 2 Dropout layers for regularization, a Softmax layer using a normalized exponential function). The network’s originality is the effective implementation of the ReLU activation function, as well as the usage of the Dropout mechanism and data augmentation technique to prevent overfitting. A Cross-Channel Normalization layer is used in the network to increase model generalization. Furthermore, maximum overlap pooling is employed to eliminate the blurring effect generated by average pooling.
GoogLeNet [48] is a pretrained CNN that has 22 layers with 9 inception layers. In a convolutional vision network, an inception layer determines the optimal local sparse structure, which can be approximated and covered by readily available dense components. The network includes an initial structure to expand the network’s width and depth while eliminating the fully connected layer and replacing it with average pooling to prevent the gradient disappearance.
ResNet-50 [49] denotes a residual network (ResNet) built with 50 layers. The central concept of a ResNet is the presentation of an “identity shortcut connection” that bypasses one or more layers. A shortcut (or skip) connection is used to solve the problem of vanishing or exploding gradients by re-routing the input and adding to the previous layer’s concept. A layer learns the concepts of the previous layer and merges with inputs from that previous layer during learning.
2.10. Cross-Validation
Cross-validation is a statistical method for evaluating and comparing learning algorithms that divides data into two groups: a training set (for model training) and a testing set (for model testing or validation) [50]. The training and testing sets must cross over in successive rounds so that each data point can be validated. Cross-validation is used for two main reasons: First, the performance of the learned model from available data can be investigated using a single algorithm. In other words, it is used to assess an algorithm’s generalizability. The second goal is to compare the performance of two or more different algorithms to find the best algorithm for the available data or, alternatively, to compare the performance of two or more parameterized model variants. Leave-one-subject-out cross-validation (LOSO-CV) and k-fold cross-validation (kfold-CV) were performed at segment-level and subject-level validation, respectively.
In the kfold-CV (Figure 9a), the first step is to a create division of the data samples randomly into k subgroups. Afterward, each subgroup can be chosen as the testing set with the remaining (k-1) subgroups as the training set. In this manner, kfold-CV repeats training and testing k times, and the final accuracy is the average of the k accuracy values for each iteration. Meanwhile, in the LOSO-CV, the records of only one subject are excluded for each training process. In other words, consider N the number of people in the dataset, training is finished on N-1 subjects and validation is finished on a single subject. This process is repeated N times (Figure 9b).
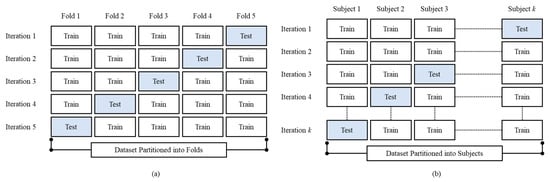
Figure 9.
The proposed method cross-validation illustration, (a) kfold-CV (k = 5) and (b) LOSO-CV.
3. Experimental Results
This study was conducted using MATLAB 2021b software (developed by MathWorks, Natick, MA, USA) on several computers with 24 GB installed RAM, Intel® CoreTM i5-8400 CPU ≅ 2.80 GHz, and NVIDIA GeForce GTX 1060 6 GB installed graphic card. The commonly used performance evaluation parameters for the apnea detection system are defined as follows:
where TP indicates the number of positive events predicted correctly (apnea events predicted as apnea events), FP indicates the number of actual negative events (normal events) predicted as positive events (apnea events), TN indicates the number of negative events predicted correctly (normal events predicted as normal events), FN indicates the number of actual positive events (apnea events) predicted as negative events (normal events). The receiver operating characteristic (ROC) then plotted and computed the area under the ROC curve (AUC) to globally specify the apnea detection performance [51].
Youden’s index is commonly used to assess the effectiveness of an overall diagnostic test when deciding between two or more diagnostic tests [52]. In other words, this index was used to choose the best classification result from a variety of input systems, such as dataset setting and feature. Youden’s index is a function of sensitivity and specificity and ranges from 0 to 1, with a value close to 1 indicating that the diagnostic test is meaningful and a value close to 0 indicating that the diagnostic test is useless. The Youden’s index () is calculated as the sum of the measurement correctly diagnosed for the diseased group (sensitivity and healthy group (specificity).
3.1. Per Segment Classification
Per segment classification was performed using 5-fold cross-validation (5fold-CV). The detailed classification results are given in Table 7 and Figure 10, Figure 11, Figure 12 and Figure 13. The best per segment classification performance of mixed group SA imbalanced dataset was from of ResNet-50 with 92.5% accuracy, 91.4% sensitivity, 93.1% specificity, and 0.9758 AUC value. The best per segment classification performance of mixed group SA balanced dataset based on SMOTE was from with 93.8% accuracy, 94.9% sensitivity, 92.7% specificity, and 0.9833 of AUC value.

Table 7.
Mixed group SA per segment classification performance results using 5fold-CV.
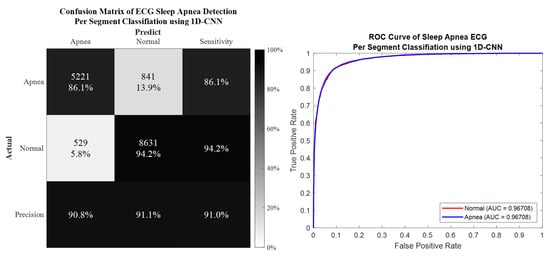
Figure 10.
The confusion matrix and ROC curve of the best 1D deep CNN 5fold-CV performance of imbalanced dataset.
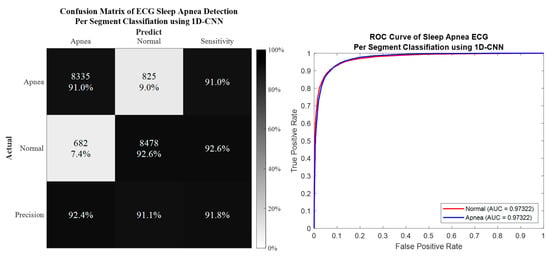
Figure 11.
The confusion matrix and ROC curve of the best 1D deep CNN 5fold-CV performance of SMOTE dataset.
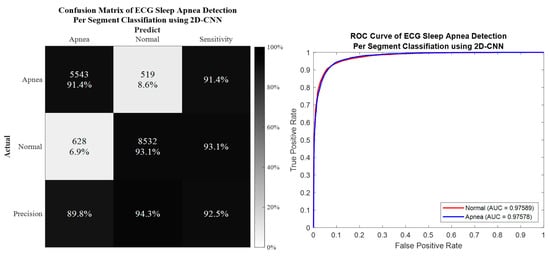
Figure 12.
The confusion matrix and ROC curve of the best 2D deep CNN (ResNet-50) 5fold-CV of imbalanced dataset.
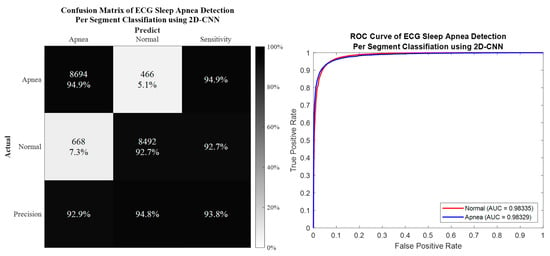
Figure 13.
The confusion matrix and ROC curve of the best 2D deep CNN (ResNet-50) 5fold-CV of SMOTE dataset.
3.2. Per Subject Classification
A more applicable approach is so-called per-subject classification, which is required to test a new unseen participant into the trained model. Per-subject classification was performed using leave-one-subject-out cross-validation (LOSO-CV). The detailed classification results are given in Table 8, Table 9, Table 10 and Table 11. The best per subject classification performance of mixed group SA was from ResNet-50 with 83.5% accuracy, 75.9% sensitivity, and 88.7% specificity.

Table 8.
Mixed group SA per subject classification based on 1D deep CNN performance results using LOSO-CV.

Table 9.
Mixed group SA per subject classification based on 2D deep CNN AlexNet performance results using LOSO-CV (best performance from ).

Table 10.
Mixed group SA per subject classification based on 2D deep CNN GoogLeNet performance results using LOSO-CV (best performance from ).

Table 11.
Mixed group SA per subject classification based on 2D deep CNN ResNet-50 performance results using LOSO-CV (best performance from ).
4. Discussion
4.1. Performance Comparison between Proposed Method and Existing Literature
Table 12 compares the proposed SA detection algorithm with several latest best-practiced DL and ML algorithms from the existing literature using AEPD. In recent years, a DL framework in SA classification has been developed. Qin et al. [21] proposed a dual-model deep learning method to perform representation learning and long-term temporal dependance with the adaptive synthetic (ADSYN) sampling method based on the R-R interval to classify a SA event (91.1% accuracy, 88.9% sensitivity, and 92.4% specificity). Yeh et al. [22] developed filter bank decomposition to decompose the ECG signal and a 1D-CNN to extract and classify each sub band decomposed ECG signal (88.6% accuracy, 83.8% sensitivity, and 91.5% specificity). Feng et al. [23] developed a SA detection model based on frequential stacked sparse auto-encoder (FSSAE) and time-dependent cost-sensitive (TDCS) classification (85.1% accuracy, 86.2% sensitivity, and 84.4% specificity). Sheta et al. [24] proposed an automated computer-aided diagnosis system to diagnose apnea events based on ECG using deep learning models (86.3% accuracy and 88.8% sensitivity). Chang et al. [25] developed a 1D deep CNN model using single-lead ECG recordings to construct a sleep apnea detection system (87.9% accuracy, 92% sensitivity, 81.1% specificity). Singh et al. [30] proposed a CNN-based deep learning approach using the time-frequency scalogram transformation of an ECG signal (86.22% accuracy, 90% sensitivity, and 83.8% specificity). Li et al. [53] proposed a SA detection system using a deep neural network-based and Hidden Markov model (HMM) based on single-lead ECG (84.7% accuracy, 88.9 sensitivity, and 82.1 specificity).

Table 12.
The comparison of SA classification performance with existing literature.
The ML framework development for SA classification is very dependable with feature engineering. Lin et al. [20] developed a SA detection method using the ML framework and bag-of-features derived from an ECG spectrogram (91.4% accuracy, 89.8% sensitivity, and 92.4 specificity). Bozkurt et al. [27] aimed to develop new machine learning-based respiratory scoring in the SA diagnostic process with feature engineering of HRV and ECG signal (85.1% accuracy, 85% sensitivity, 86% specificity). Surrel et al. [34] used the R-R intervals and R-S amplitude series with apnea scoring energy feature generation and SVM (85.70% accuracy, 81.40% sensitivity, and 88.40% specificity). Hassan et al. [35] addressed the automated sleep apnea detection system using a data-adaptive signal decomposition tunable-Q factor wavelet transform (TQWT) for single-lead ECG and a random under sampling boosting (RUSBoost) classifier (88.9% accuracy, 87.6% sensitivity, and 91.5% specificity). The proposed method achieved significantly better performance in accuracy, sensitivity, specificity, and AUC compared to all other considered methods.
4.2. Dealing with Imbalanced Dataset Problem
The imbalanced dataset problem exists when an uneven distribution of elements across the decision classes occurs: one class of data (typically the diseased class) is smaller than the other classes. In the study of SA classification, most events are normal and only a minority of apnea events occur, since not only participants with apnea were included but also the borderline and healthy control participants. This problem causes a condition where the trained classifier’s performance for normal events data, expressed as the specificity, is much higher than the sensitivity. The sensitivity metric represents the ability of the classifier to recognize patients (apnea events class) and if a classifier is only accurate for normal people, it is not valuable. To overcome this problem, certain synthetic data balancing methods need to be applied to balance the dataset (see Table 13 for some existing research in dealing with imbalanced data of SA classification).

Table 13.
Literature survey of sleep apnea detection with data augmentation approach for dealing with imbalanced dataset problem.
The main objective of balancing classes is to either increase the frequency of the minority class or decrease the frequency of the majority class by re-sampling techniques. Random over sampling (ROS) aims to increase the number of instances in the minority class by randomly replicating them to present a higher representation of the minority class in the sample. Random under sampling (RUS) balances class distribution by randomly eliminating majority class examples. Table 6 presents the mixed group SA classification performance comparison using AEPD for several balancing methods, including ROS, RUS, and SMOTE (the proposed method).
As shown in Table 14, all methods could improve the SA classification performance, especially the sensitivity, which was much closer to the specificity. SMOTE and ROS could achieve superior performance and RUS had a slightly lower performance for all IMF features. Even though the best classification performance was also achieved by the ROS, SMOTE is still favored. This method can mitigate the problem of overfitting caused by ROS as synthetic examples are generated rather than replication of instances.

Table 14.
The comparison SA classification performance among several data augmentation methods (based on the best SA classification model: 2D Deep CNN ResNet-50).
4.3. Intrinsic Mode Decomposition Feature Phenomenon of Apnea ECG Signal
Many studies have described the ECG signal to be highly correlated with respiratory variation [38,56,58,59]. During apnea onset, some key features can particularly be observed on the ECG signal, such as a decline in heart rate and an increase in heart rate followed by the end of the apnea episode. By decomposing the ECG signal using EMD, these key features are enhanced in certain IMFs. EMD deconstructs a signal into its key components (IMFs), which may then be used in evidence-specific dynamics to provide a more complete description of the signal [41,60]. EMD directly extracts energy connected with distinct intrinsic time scales.
The key features phenomenon of the apnea IMF ECG signal is clearly shown in Figure 14. This observation was only performed on three IMFs of the preprocessed apnea ECG (, , and ) since these IMFs were also the selected features input of the proposed method. In the normal event, all IMFs showed almost stable magnitude and frequency (relatively high); this also indicates that the heart rate does not change. Meanwhile, in the apnea event, the key features of heart rate were obviously emphasized. The apnea onset frequency of preprocessed ECG and were not stable (tended to be higher in the end of episode) and lower than the apnea offset. Furthermore, on the and , there were some explicit magnitude and frequency fluctuations.
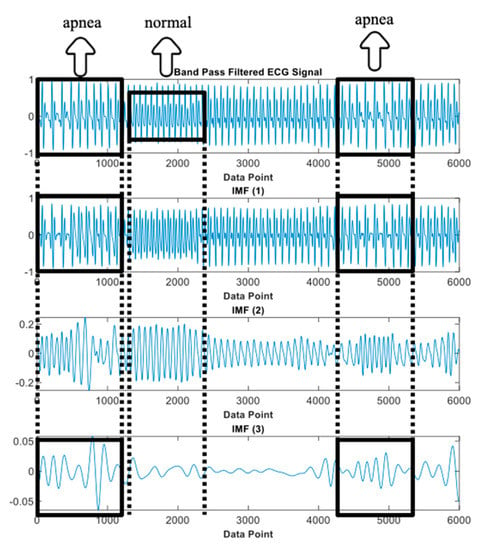
Figure 14.
IMFs of preprocessed ECG observation (IMF1, IMF2, and IMF3).
The key features phenomenon differences between normal and apnea IMF ECG are emphasized and also possible to be observed from the CWT time-frequency spectrogram patterns visualization, as shown in Figure 4 and Figure 5. In the normal ECG spectrogram, the dominant frequency range (yellow color region) was approximately 2–3 Hz, whereas in the apnea ECG spectrogram, the dominant frequency range was around 2–4 Hz with unstable frequency power from 3 to 4 Hz. Additionally, the dominant frequency range of normal ECG spectrogram was 1–2 Hz and apnea ECG spectrogram was 1–3 Hz with lower frequency power in 2–3 Hz (light blue color region). These two spectrogram patterns indicate that the apnea episode had unstable frequency components compared to the normal episode.
4.4. Study Limitations and Future Developments
Despite the fact that the proposed algorithm performed exceptionally well, this study has several limitations. First, the proposed method was validated using a small sample size from AEPD. Second, insufficient physiological data and subject disease history were available for further analysis because AEPD did not provide such information. More data collection from the NCKUH Sleep Center, as well as the combination of multiple databases, may be a solution to these drawbacks. Third, the proposed algorithm could not be applied to patients with cardiovascular disease (CVD) complications because their ECG signal was somewhat irregular and not only affected by SA.
This study’s future developments include using explainable AI (XAI) to automatically identify physiological meanings for OSA features, testing the proposed method’s robustness in a large-scale group of participants from several databases, and developing an algorithm to discriminate SA and CVD using ECG data.
5. Conclusions
This paper proposed a novel algorithm to classify SA and normal breathing episodes using the signal decomposition method. Four different EMD features were considered along with 1D and 2D deep learning classifiers. When the ECG signal of SA and normal breathing episodes was decomposed, high accuracy was obtained. In addition, the algorithm outperformed the current best-practiced approaches in terms of accuracy.
By decomposing the ECG signal using EMD, the key features of normal and apnea episode are enhanced in certain IMFs. Furthermore, transforming the IMFs into the time-frequency spectrogram using CWT, pattern visualization and recognition of the key features made it possible to successfully differentiate the normal and apnea episodes.
Author Contributions
Conceptualization, F.S. and C.-W.L.; methodology, F.S. and C.-W.L.; software, F.S.; validation, F.S.; formal analysis, F.S. and C.-W.L.; investigation, F.S. and C.-W.L.; resources, C.-W.L.; data curation, F.S.; writing—original draft preparation, F.S.; writing—review and editing, C.-W.L.; visualization, F.S. and C.-W.L.; supervision, C.-W.L.; project administration, C.-W.L.; funding acquisition, C.-W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (Taiwan), grant number 108-2628-E-006-003-MY3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Strollo, P.J., Jr.; Rogers, R.M. Obstructive sleep apnea. N. Engl. J. Med. 1996, 334, 99–104. [Google Scholar] [CrossRef]
- Memon, J.; Manganaro, S.N. Obstructive Sleep-disordered Breathing. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Santilli, M.; Manciocchi, E.; D’Addazio, G.; Di Maria, E.; D’Attilio, M.; Femminella, B.; Sinjari, B. Prevalence of obstructive sleep apnea syndrome: A single-center retrospective study. Int. J. Environ. Res. Public Health 2021, 18, 10277. [Google Scholar] [CrossRef] [PubMed]
- Ontario, H.Q. Polysomnography in patients with obstructive sleep apnea: An evidence-based analysis. Ont Health Technol. Assess Ser. 2006, 6, 1–38. [Google Scholar]
- Álvarez, D.; Cerezo-Hernández, A.; Crespo, A.; Gutiérrez-Tobal, G.C.; Vaquerizo-Villar, F.; Barroso-García, V.; Moreno, F.; Arroyo, C.A.; Ruiz, T.; Hornero, R. A machine learning-based test for adult sleep apnoea screening at home using oximetry and airflow. Sci. Rep. 2020, 10, 5332. [Google Scholar] [CrossRef]
- Mendonça, F.; Mostafa, S.S.; Morgado-Dias, F.; Ravelo-García, A.G. An oximetry based wireless device for sleep apnea detection. Sensors 2020, 20, 888. [Google Scholar] [CrossRef]
- Haidar, R.; Koprinska, I.; Jeffries, B. Sleep apnea event detection from nasal airflow using convolutional neural networks. In Proceedings of the International Conference on Neural Information Processing, Guangzhou, China, 14–18 November 2017; pp. 819–827. [Google Scholar]
- Yue, H.; Lin, Y.; Wu, Y.; Wang, Y.; Li, Y.; Guo, X.; Huang, Y.; Wen, W.; Zhao, G.; Pang, X. Deep learning for diagnosis and classification of obstructive sleep apnea: A nasal airflow-based multi-resolution residual network. Nat. Sci. Sleep 2021, 13, 361. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Wu, H.-T.; Hsu, C.-A.; Huang, P.-C.; Huang, Y.-H.; Lo, Y.-L. Sleep apnea detection based on thoracic and abdominal movement signals of wearable piezoelectric bands. IEEE J. Biomed. Health Inform. 2016, 21, 1533–1545. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Yang, T.; Ji, S.; Wang, H.; Wang, J.; Wang, Y.; Wu, Q. Classification of sleep apnea based on EEG sub-band signal characteristics. Sci. Rep. 2021, 11, 5824. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Saha, S.; Fattah, S.A.; Zhu, W.-P.; Ahmad, M.O. Sleep apnea detection based on rician modeling of feature variation in multiband EEG signal. IEEE J. Biomed. Health Inform. 2018, 23, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Fattah, S.A.; Zhu, W.-P.; Ahmad, M.O. VMD-RiM: Rician modeling of temporal feature variation extracted from variational mode decomposed EEG signal for automatic sleep apnea detection. IEEE Access 2018, 6, 77440–77453. [Google Scholar] [CrossRef]
- Taran, S.; Bajaj, V. Sleep apnea detection using artificial bee colony optimize hermite basis functions for EEG signals. IEEE Trans. Instrum. Meas. 2019, 69, 608–616. [Google Scholar] [CrossRef]
- Penzel, T.; Moody, G.B.; Mark, R.G.; Goldberger, A.L.; Peter, J.H. The apnea-ECG database. Proc. Comput. Cardiol. 2000, 27, 255–258. [Google Scholar]
- Penzel, T.; Kantelhardt, J.W.; Grote, L.; Peter, J.-H.; Bunde, A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans. Biomed. Eng. 2003, 50, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Janbakhshi, P.; Shamsollahi, M.B. ECG-derived respiration estimation from single-lead ECG using gaussian process and phase space reconstruction methods. Biomed. Signal Processing Control 2018, 45, 80–90. [Google Scholar] [CrossRef]
- Guilleminault, C.; Winkle, R.; Connolly, S.; Melvin, K.; Tilkian, A. Cyclical variation of the heart rate in sleep apnoea syndrome: Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet 1984, 323, 126–131. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wang, Y.-W.; Setiawan, F.; Trang, N.T.H.; Lin, C.-W. Sleep apnea classification algorithm development using a machine-learning framework and bag-of-features derived from electrocardiogram spectrograms. J. Clin. Med. 2021, 11, 192. [Google Scholar] [CrossRef]
- Qin, H.; Liu, G. A dual-model deep learning method for sleep apnea detection based on representation learning and temporal dependence. Neurocomputing 2022, 473, 24–36. [Google Scholar] [CrossRef]
- Yeh, C.-Y.; Chang, H.-Y.; Hu, J.-Y.; Lin, C.-C. Contribution of Different Subbands of ECG in Sleep Apnea Detection Evaluated Using Filter Bank Decomposition and a Convolutional Neural Network. Sensors 2022, 22, 510. [Google Scholar] [CrossRef]
- Feng, K.; Qin, H.; Wu, S.; Pan, W.; Liu, G. A sleep apnea detection method based on unsupervised feature learning and single-lead electrocardiogram. IEEE Trans. Instrum. Meas. 2020, 70, 1–12. [Google Scholar] [CrossRef]
- Sheta, A.; Turabieh, H.; Thaher, T.; Too, J.; Mafarja, M.; Hossain, M.S.; Surani, S.R. Diagnosis of obstructive sleep apnea from ecg signals using machine learning and deep learning classifiers. Appl. Sci. 2021, 11, 6622. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Yeh, C.-Y.; Lee, C.-T.; Lin, C.-C. A sleep apnea detection system based on a one-dimensional deep convolution neural network model using single-lead electrocardiogram. Sensors 2020, 20, 4157. [Google Scholar] [CrossRef]
- Zarei, A.; Asl, B.M. Automatic classification of apnea and normal subjects using new features extracted from HRV and ECG-derived respiration signals. Biomed. Signal Processing Control 2020, 59, 101927. [Google Scholar] [CrossRef]
- Bozkurt, F.; Uçar, M.K.; Bozkurt, M.R.; Bilgin, C. Detection of abnormal respiratory events with single channel ECG and hybrid machine learning model in patients with obstructive sleep apnea. Irbm 2020, 41, 241–251. [Google Scholar] [CrossRef]
- Viswabhargav, C.S.; Tripathy, R.; Acharya, U.R. Automated detection of sleep apnea using sparse residual entropy features with various dictionaries extracted from heart rate and EDR signals. Comput. Biol. Med. 2019, 108, 20–30. [Google Scholar] [CrossRef]
- Zarei, A.; Asl, B.M. Automatic detection of obstructive sleep apnea using wavelet transform and entropy-based features from single-lead ECG signal. IEEE J. Biomed. Health Inform. 2018, 23, 1011–1021. [Google Scholar] [CrossRef]
- Singh, S.A.; Majumder, S. A novel approach osa detection using single-lead ECG scalogram based on deep neural network. J. Mech. Med. Biol. 2019, 19, 1950026. [Google Scholar] [CrossRef]
- Li, K.; Pan, W.; Li, Y.; Jiang, Q.; Liu, G. A method to detect sleep apnea based on deep neural network and hidden Markov model using single-lead ECG signal. Neurocomputing 2018, 294, 94–101. [Google Scholar] [CrossRef]
- Sharma, M.; Agarwal, S.; Acharya, U.R. Application of an optimal class of antisymmetric wavelet filter banks for obstructive sleep apnea diagnosis using ECG signals. Comput. Biol. Med. 2018, 100, 100–113. [Google Scholar] [CrossRef]
- Tripathy, R. Application of intrinsic band function technique for automated detection of sleep apnea using HRV and EDR signals. Biocybern. Biomed. Eng. 2018, 38, 136–144. [Google Scholar] [CrossRef]
- Surrel, G.; Aminifar, A.; Rincón, F.; Murali, S.; Atienza, D. Online obstructive sleep apnea detection on medical wearable sensors. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.R.; Haque, M.A. An expert system for automated identification of obstructive sleep apnea from single-lead ECG using random under sampling boosting. Neurocomputing 2017, 235, 122–130. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, K. An algorithm for sleep apnea detection from single-lead ECG using Hermite basis functions. Comput. Biol. Med. 2016, 77, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, K.; Zhang, X.; Chen, L.; Xian, X. An obstructive sleep apnea detection approach using a discriminative hidden Markov model from ECG signals. IEEE Trans. Biomed. Eng. 2015, 63, 1532–1542. [Google Scholar] [CrossRef]
- Varon, C.; Caicedo, A.; Testelmans, D.; Buyse, B.; Van Huffel, S. A novel algorithm for the automatic detection of sleep apnea from single-lead ECG. IEEE Trans. Biomed. Eng. 2015, 62, 2269–2278. [Google Scholar] [CrossRef]
- Hassan, A.R. Automatic screening of obstructive sleep apnea from single-lead electrocardiogram. In Proceedings of the 2015 International Conference on Electrical Engineering and Information Communication Technology (ICEEICT), Dhaka, Bangladesh, 21–23 May 2015; pp. 1–6. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Zeiler, A.; Faltermeier, R.; Keck, I.R.; Tomé, A.M.; Puntonet, C.G.; Lang, E.W. Empirical mode decomposition—An introduction. In Proceedings of the 2010 International Joint Conference on Neural Networks (IJCNN), Barcelona, Spain, 18–23 July 2010; pp. 1–8. [Google Scholar]
- Rioul, O.; Duhamel, P. Fast algorithms for discrete and continuous wavelet transforms. IEEE Trans. Inf. Theory 1992, 38, 569–586. [Google Scholar] [CrossRef]
- Goldberger, J.; Hinton, G.E.; Roweis, S.; Salakhutdinov, R.R. Neighbourhood components analysis. Adv. Neural Inf. Processing Syst. 2004, 17, 513–520. [Google Scholar]
- Yang, W.; Wang, K.; Zuo, W. Neighborhood component feature selection for high-dimensional data. J. Comput. 2012, 7, 161–168. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 770–778. [Google Scholar]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-validation. Encycl. Database Syst. 2009, 5, 532–538. [Google Scholar]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Zhao, A.; Qi, L.; Dong, J.; Yu, H. Dual channel LSTM based multi-feature extraction in gait for diagnosis of Neurodegenerative diseases. Knowl.-Based Syst. 2018, 145, 91–97. [Google Scholar] [CrossRef]
- Shen, Q.; Qin, H.; Wei, K.; Liu, G. Multiscale deep neural network for obstructive sleep apnea detection using RR interval from single-lead ECG signal. IEEE Trans. Instrum. Meas. 2021, 70, 1–13. [Google Scholar] [CrossRef]
- Azimi, H.; Xi, P.; Bouchard, M.; Goubran, R.; Knoefel, F. Machine learning-based automatic detection of central sleep apnea events from a pressure sensitive mat. IEEE Access 2020, 8, 173428–173439. [Google Scholar] [CrossRef]
- Van Steenkiste, T.; Groenendaal, W.; Deschrijver, D.; Dhaene, T. Automated sleep apnea detection in raw respiratory signals using long short-term memory neural networks. IEEE J. Biomed. Health Inform. 2018, 23, 2354–2364. [Google Scholar] [CrossRef]
- Hassan, A.R. Computer-aided obstructive sleep apnea detection using normal inverse Gaussian parameters and adaptive boosting. Biomed. Signal Processing Control 2016, 29, 22–30. [Google Scholar] [CrossRef]
- Langley, P.; Bowers, E.J.; Murray, A. Principal component analysis as a tool for analyzing beat-to-beat changes in ECG features: Application to ECG-derived respiration. IEEE Trans. Biomed. Eng. 2009, 57, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Lee, Y.J.; Jeong, D.-U.; Park, K.S. Apnea–hypopnea index prediction using electrocardiogram acquired during the sleep-onset period. IEEE Trans. Biomed. Eng. 2016, 64, 295–301. [Google Scholar]
- Mendez, M.; Corthout, J.; Van Huffel, S.; Matteucci, M.; Penzel, T.; Cerutti, S.; Bianchi, A.M. Automatic screening of obstructive sleep apnea from the ECG based on empirical mode decomposition and wavelet analysis. Physiol. Meas. 2010, 31, 273. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).