1. Introduction
Organophosphate insecticides (OPs) are commonly used in agriculture to control insect pests [
1]. Increased production and widespread use of pesticides harm the environment, posing immediate risks to all species, including humans [
2].
Chlorpyrifos (CPF), a lipophilic organophosphorus insecticide, is commonly utilized to control foliar and subsurface crop insects [
3]. The pesticide is an acetylcholinesterase (AChE) inhibitor that causes acetylcholine accumulation and neurochemical disruption, leading to death after severe exposure [
4]. Many neurological deficits, such as altered blood–brain barrier integrity, attention deficit hyperactivity disorder, Parkinsonism, and dementia, are linked to consuming even small amounts of CPF [
5].
CPF exhibits toxicity to multiple organs/systems, such as testes [
6], reproductive systems [
7], spermatogenesis, testosterone levels, DNA damage in sperm [
8], hepatotoxicity, immunotoxicity, carcinogenicity, and neurotoxicity [
9]. Furthermore, mechanisms of toxicity include the production of free radicals and proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-), interleukin-1 beta (IL-1), and apoptosis activation [
10].
Olive leaves have been used in conventional medicine throughout Europe and countries in the Mediterranean for centuries [
11]. Many flavonoid and polyphenolic chemicals are found in olive leaves; oleuropein is the most abundant molecule, accounting for 86.9% of all components. This substance has antioxidative stress and anti-inflammatory properties [
12]. Oral treatment with OLE reduced oxidative stress in the midbrain by decreasing free-radical generation and increasing SOD, CAT, and GPx activities [
13]. Neuroprotective properties against neurodegenerative disorders and oxidative stress-induced neurotoxicity, as well as stroke and brain damage prevention, were also observed [
14]. Oleuropein is a neuroprotectant in mice’s focal cerebral ischemia/reperfusion damage [
15].
Treatment with olive leaves also enhanced the quality and quantity of sperm, raising testosterone and luteinizing hormone levels in males. The latter activates testicular cells to produce testosterone [
16]. Additionally, treatment with OLE increased total antioxidant capacity (TAC), sperm parameters, and testis antioxidant status in rat testicular tissue exposed to rotenone [
17].
This research assessed the possible advantages of using OLE to counter CPF-induced neural and reproductive toxicity by evaluating serum gonadotropins, sex hormones, sperm status, oxidative status, acetylcholinesterase, brain neurotransmitters, immunohistochemistry, and histological alterations in the brain and testes of male albino rats.
2. Material and Methods
2.1. Tested Materials
Chlorpyrifos (diethyl 3,5,6-tricholoro-2-pyridyl phosphonothioate, formulation EC48%) was provided. Olive leaves were harvested, dried and powdered before extraction.
2.2. Extraction of Plant Material
Fresh olive leaves were left to dry in the sunlight and ground manually to a powder. Fifteen grams of olive leaf powder in a Soxhlet thimble was extracted for 4 h at 60 °C in 300 mL of 80% ethanol and 20% acetonitrile. The extract was filtered after cooling to room temperature. The filtrate solvent was removed under vacuum in a rotating evaporator at room temperature. Until used, the extract concentrate was refrigerated at 2–8 °C [
18].
2.3. Animals
Thirty-two mature male albino rats weighing 170–200 g were kept in polypropylene cages under strict conditions of −22 °C and a 12 h/12 h light/dark cycle. The methodology followed guidelines set by the Committee of Research Ethics for Laboratory Animal Care at Zagazig University, Faculty of Veterinary Medicine, Zagazig, Egypt; (approval no. ZU-IACUC/2/F/35/2021).
2.4. Experimental Design
Rats were randomly divided into four groups, each with eight animals. Control animals were given distilled water in Group I; positive control animals were given CPF at a dose of 9 mg/kg bw in Group II, negative control animals were given OLE at a dose of 150 mg/kg bw in Group III, and test animals were given CPF and OLE at the above doses in Group IV.
Rats received CPF or OLE or their combination daily for 80 days. Rats had free access to food and water. Rats’ body weights were assessed weekly, and any signs of illness were recorded. Animals were euthanized by decapitation under anesthesia 24 h following the last treatment, and blood was collected for further investigation. Biochemical and hormonal tests and histological and immunohistopathological examinations were performed with brain and test samples. Semen samples were collected for semen analysis.
2.5. Serum Sex Hormones
Blood samples were collected and centrifuged at 5000 rpm for 10 min at 4 °C to separate serum for sex hormone estimation. Rat testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) levels were estimated immediately in serum using enzyme-linked immunosorbent assay (ELISA) kits (Cusabio, Houston, TX, USA) following the manufacturer’s instructions.
2.6. Sperm Parameters
Sperm examination criteria (motility, viability, count, and anomalies) were examined using previously described protocols [
19]. Sperm were examined under sterile conditions. The cauda epididymis was chopped in 2 mL physiological saline and incubated at 37 °C for 5 min to allow dispersion of spermatozoa.
Sperm motility: A drop of the prepared suspension was checked for spermatozoa motility with light microscopy; 200 spermatozoa were inspected in five microscope fields to record the proportion of motile sperm.
Sperm viability: First, 20 µL of epididymal sperm suspension was mixed with 20 µL of eosin-nigrosin combination stain in a sterile test tube. Spermatozoa were counted randomly using light microscopy (oil immersion lens at 1000×). Sperm without a crimson or pink head was regarded as viable, and percentages of these spermatozoa were recorded [
20].
Sperm count: A standard hemocytometer was used to determine the epididymal sperm count. Formol saline was diluted 1:4 in formen suspensions. Ten microliters of the dilution was introduced to each of five counting chambers of hemocytometers (HBG, Hamburg, Germany). Cells were settled and counted using light microscopy at 400×. The number of sperm per milliliter was reported.
Sperm abnormalities: Freshly prepared alkaline methyl violet stain was to stain several semen samples films by soaking for 5 min. Films were removed from the stain and cleaned with a few drops of water before drying. In various fields, 200 spermatozoa were randomly checked for abnormalities (head or tail), and the overall anomaly percentage was calculated [
21].
2.7. Oxidative Stress and Antioxidant Status Markers Determination
Malondialdehyde (MDA) is used as a marker of lipid peroxidation. A tissue homogenizer was used to prepare the brain and testicular tissues (10% w/v) in potassium phosphate buffer solution (pH 7.4) to assess MDA levels. We centrifuged homogenized samples for 15 min at 3000 rpm.
Homogenates were treated with thiobarbituric acid using the technique described in [
22]. Antioxidant enzyme activity in the brain and testicular tissues was measured as oxidant and antioxidant status indicators. Superoxide dismutase (SOD) activity was determined using nitro blue tetrazolium reduction [
23]. Reduced glutathione (GSH) concentration was assessed as previously described [
24]. TAC in the homogenate was evaluated following Koracevic et al. [
25].
2.8. Serum Acetylcholine Esterase Determination
Serum acetylcholine esterase (AChE) activity was determined quantitatively using ELISA specialized kits (Cusabio, Houston, TX, USA) following the manufacturer’s protocol. Data are expressed in units of pg/mg tissue.
2.9. Determination of Monoamines (Serotonin and Dopamine)
Serotonin and dopamine in brain tissue extracts were assessed with ELISA kits (Bioassay laboratory technology, Shanghai, China and ELISA Genie, Dublin, Ireland, respectively) following the manufacturer’s guidelines. Data are expressed in units of pg/mg tissue.
2.10. Gene Expression
Brain and tissue samples were obtained from all rats, and 30 mg of tissue was rinsed with cold saline, snap-frozen in liquid nitrogen, and stored at −80 °C for further RT-PCR analysis. Extraction of total RNA was performed using Trizol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). As previously described [
26,
27,
28], cDNA was synthesized using a HiSenScriptTM RH (-) cDNA Synthesis Kit (iNtRON Biotechnology Co., Seoul, Korea). RT-PCR was performed in a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) using TOPrealTM qPCR 2X PreMIX SYBR Green (Enzynomics, Seoul, Korea) following gene expression estimation guidelines. RT-PCR cycling conditions were as follows: initial denaturation for 15 min at 95 °C, followed by 40 cycles of denaturation for 20 s at 95 °C, annealing for 30 s at 60 °C, and extension for 30 s at 72 °C. Primer sequences used to determine
Bax and
Bcl-2 gene expression were developed by Sangon Biotech (Beijing, China) (
Table 1). The expression levels of target genes were compared to GAPDH, and relative fold changes in gene expression were estimated using the 2
−ΔΔCT method [
29].
2.11. Tissue Preparation for Histopathological and Immunohistochemical Analyses
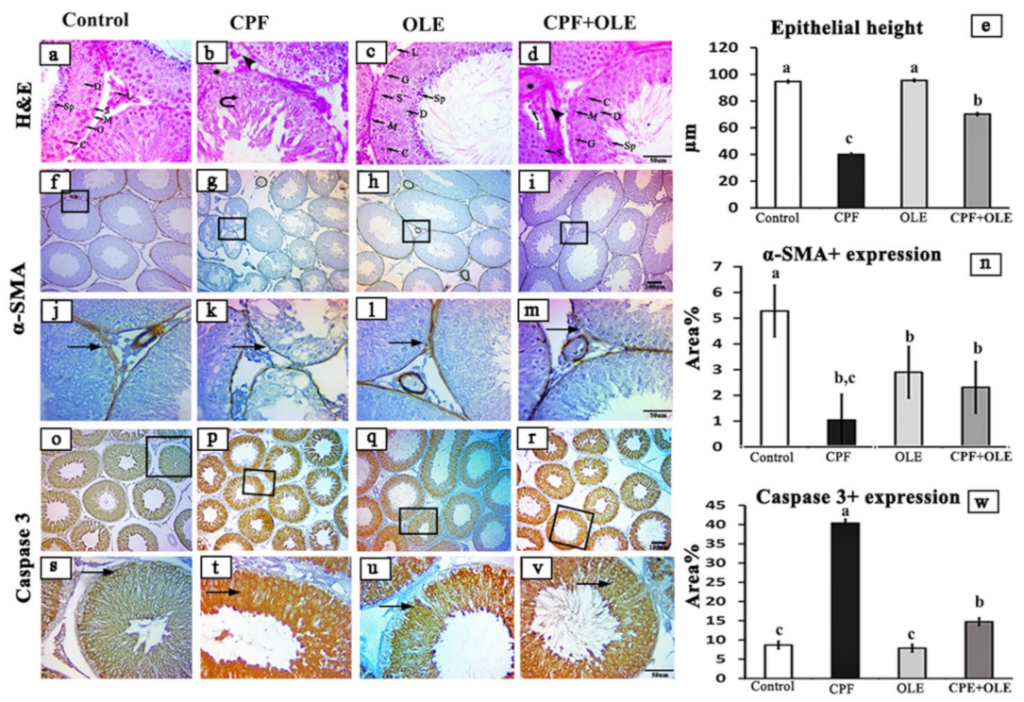
Testes were submerged in Bouin’s solution for 24 h, and the brains were fixed in a 10% neutral buffered formalin solution. Tissues were dried and encased in paraffin blocks. Under a light microscope, 5 µm thick hematoxylin and eosin (H&E)-stained sections were examined [
30]. At least one deparaffinized and hydrated section from animals in each group was used to assess immune expression of caspase-3 (Catalog No. RB-1197-R7 Thermo Fisher Scientific, Waltham, MA, USA), alpha-smooth muscle actin (α-SMA) (Catalog No. ab5694, Abcam, Cambridge, UK), and Ki-67 (Catalog No. MA5-14520, Thermo Fisher Scientific, Waltham, MA, USA). As mentioned, an avidin–biotin–peroxidase technique was used [
31,
32]. In brief, endogenous peroxidase was inhibited by incubation for 30 min at 4 °C with 3% H
2O
2 in absolute methanol, and then washed with phosphate-buffered saline (PBS). A 10% normal blocking serum for 60 min at room temperature was used to prevent nonspecific reactions (Sigma-Aldrich, Cat: A9647, St. Louis, MO, USA).
Subsequently, depending on the species, primary antibodies were incubated for 60 min with biotin-conjugated goat anti-rabbit IgG antiserum or rabbit anti-goat IgG antiserum (Histofine kit, Nichirei Corporation, Tsukiji, Tokyo, Japan). A solution of 3,3-diaminobenzidine tetrahydrochloride (DAB)–H2O2 pH 7.0 was incubated with the streptavidin–biotin complex for 3 min. After washing with distilled water, the slices were stained with Mayer’s hematoxylin counterstain. Negative control sections were generated through incubation with PBS instead of primary antibodies. A standard light microscope was used to examine stained sections, and images were captured using an AmScope (MU1403B) Digital Imaging System. ImageJ Analyzer software was used to assess the epithelial heights of 20 round seminiferous tubules from each rat and to measure the intensity of brown staining (ImageJ, NIH-Bethesda, MD, USA). Microscopic fields were selected at random and analyzed at 100× magnification.
2.12. Statistical Analysis
Data are expressed as means and standard deviations using the statistical package application, SPSS version 20.0 (2011, SPSS Inc., IBM, Armonk, NY, USA). Data from multiple evaluations were analyzed using one-way ANOVA and Duncan’s post hoc test. Results were deemed significant at p ≤ 0.05 compared to controls.
4. Discussion
The excessive use of the organophosphorus pesticide CPF can induce adverse effects, such as reproductive deficits [
33]. CPF exposure is also linked to nervous system disruption in both central and peripheral systems [
34]. The agent inhibits AChE activity, increasing acetylcholine concentrations in synapses and causing overactivation of neurons [
35]. Therefore, an urgent need exists to evaluate the neural and reproductive impacts of CPF and characterize OLE as a protective natural product to counter CPF-induced toxicity.
Chen et al. [
36] found that CPF reduced sex hormone production, inhibiting spermatogenesis and inducing infertility. CPF is classified as an endocrine disruptor because of its adverse impact on reproduction via altering the pituitary–thyroid and pituitary–adrenal axes [
9].
Chronic CPF exposure significantly negatively affected the adult male rat reproductive function, as evidenced by reduced sperm count, motility, and viability. Additionally, a significantly higher percentage of immotile and morphologically abnormal sperm was found after CPF exposure. These observations are consistent with previous reports [
8,
33,
37,
38,
39,
40]. Notably, co-administration of OLE largely restored sperm count and motility and decreased the incidence of immotile and morphologically abnormal sperm compared to CPF-intoxicated rats. Similar findings have been reported [
17,
41].
A decline in serum LH might be due to decreased testosterone levels. LH is involved in spermatogenesis by targeting testicular Leydig cells to inhibit testosterone synthesis [
33,
42]. Furthermore, active sulfur atoms, CPF metabolites, inhibit activation of cytochrome P450 3A4 (CYP3A4) [
43], which participates in testosterone metabolism [
44]. CPF also downregulates genes essential for gonadotropin production and steroidogenesis, which may explain its effect on FSH and LSH levels [
45]. Since FSH is essential for spermatogenesis, low sperm counts after CPF treatment might be due to reduced FSH levels [
42]. Concurrent administration of CPF + OLE produces a dramatic elevation in serum testosterone, LH, and FSH. Our results are consistent with [
16,
46]. Secretion of GnRH and testosterone is increased by oleuropein found in OLE [
47]. Moreover, acidic molecules in OLE can inhibit aromatase enzyme activation, thus increasing androgens, testosterone, and dihydrotestosterone [
48,
49].
CPF-treated animals exhibit an oxidant/antioxidant imbalance in testis tissues, as evidenced by elevated MDA levels and decreased GSH, SOD, and TAC. The authors of [
50] reported similar results. Reactive oxygen species (ROS) adversely affect the genetic content of spermatozoa and, thus, impair sperm survival and function. Furthermore, oxidative stress leads to DNA strand breaks that trigger programmed cell death [
51]. Our results indicate that OLE treatment of CPF-intoxicated rats largely restored redox balance, evidenced by reduced tissue MDA and increased GSH, SOD, and TAC [
16,
41]. The authors of [
17] reported that OLE increased TAC and decreased MDA levels in the testes of rats exposed to rotenone. High flavonoid and polyphenol content in OLE scavenges free radicals, which increases TAC concentration [
52]. This content also reduces oxidative stress, upregulates SOD, and lowers MDA levels [
53].
Additionally, CPF-treated rats displayed oxidative stress in brain tissues via remarkable increases in MDA concentrations and significant declines in GSH, SOD, and TAC. The authors of [
4] reported similar results where CPF neurotoxicity was characterized by diminished cellular antioxidants, such as GSH, SOD, and CAT, and elevated lipid peroxidation levels. The lipophilic CPF easily penetrates the blood–brain barrier. Consequently, CPF disrupts brain integrity, promotes permeability and free-radical production, and ultimately causes CNS morphology and function alterations [
54]. Changes influence the CPF-generated oxidant/antioxidant imbalance in numerous processes. For instance, antioxidant enzyme degradation is furthered by increased ROS synthesis, depletion of antioxidants, damage to DNA, and protein and lipid peroxidation [
55].
Rats treated with OLE showed marked reversal in these adverse effects, as shown by decreases in brain tissue MDA and increases in GSH, SOD, and TAC. This OLE property is supported by [
13], who demonstrated that rats treated with OLE exhibited dramatic elevation in SOD, CAT, and GPx activity and reduced MDA levels in the midbrain of intoxicated rats. These same findings were confirmed by others, suggesting that OLE is a potent antioxidant [
56,
57]. This property is likely associated with flavonoids and polyphenols, two key bioactive components in OLE [
58]. Oleuropein efficiently suppresses MDA production in the hippocampus of an Alzheimer’s disease animal model [
57].
AChE is critical to acetylcholine-mediated neurotransmission. This enzyme rapidly hydrolyzes acetylcholine released into cholinergic and neuromuscular synapses [
59]. The current study recorded a marked decline in serum AChE and brain neurotransmitters (dopamine and serotonin) in CPF-treated rats versus untreated rats. However, supplementation with OLE significantly relieved AChE inhibition [
60]. Furthermore, treatment with CPF significantly inhibited AChE in the cerebrum and cerebellum tissue in adult male rats. CPF causes cholinergic neurotoxicity by irreversibly inhibiting AChE activity, resulting in the accumulation of acetylcholine in the synaptic cleft, which overstimulates muscarinic and nicotinic receptors. Consequently, this process alters the maturation of neural pathways and synapses essential for the development of the nervous system [
61]. This failure in development leads to neurobehavioral alternations and cognitive dysfunction [
35]. Excess acetylcholine also suppresses anterior pituitary gland function and secondary neurotransmitter secretion, particularly dopamine and gonadotrophins [
62,
63,
64]. Moreover, our results are consistent with previous findings that oral OLE co-administered with profenofos significantly protects against AChE inhibition [
65].
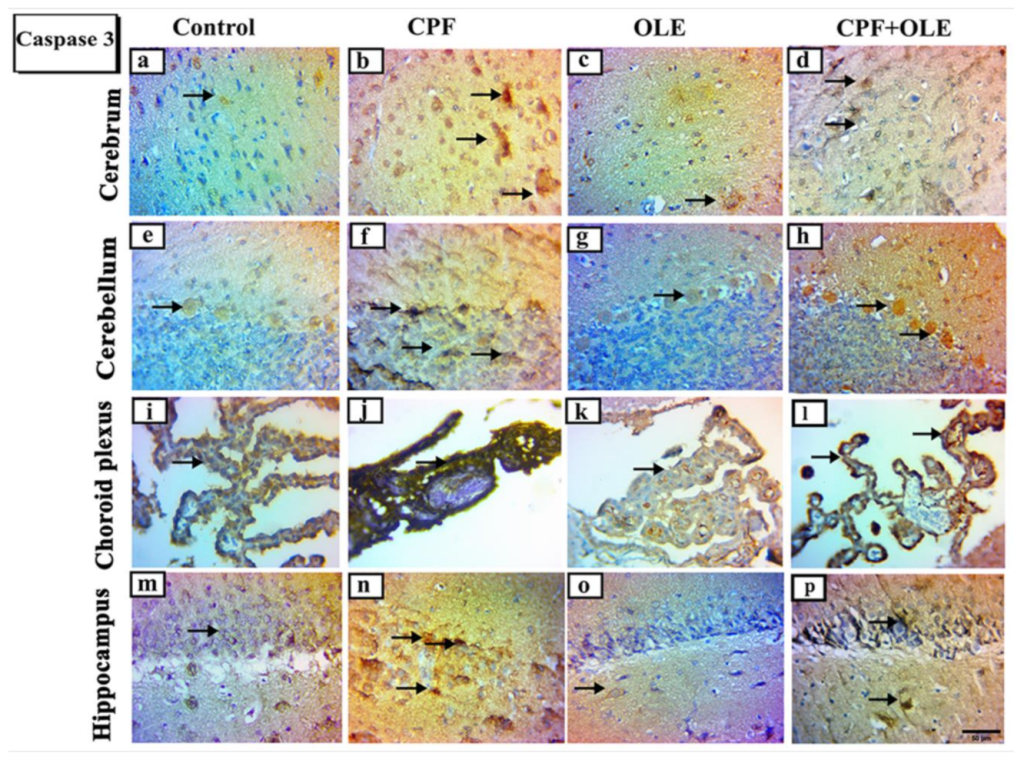
Apoptosis is programmed cell death defined by morphological and genomic alterations in the cell. This process occurs in normal and pathological conditions, such as chemically induced cell death. CPF induces apoptosis, encouraging the production of ROS, which in turn alters mitochondrial membrane permeability. This change causes cytochrome c release into the cytosol and activates caspase-3, thus initiating an apoptosis cascade [
66]. Caspase-3 is an aspartic acid cysteine protease recognized as the last enzyme in the regulation of the apoptotic process [
67]. Likewise,
Bax is recognized as a proapoptotic protein that penetrates the outer mitochondrial membrane causing the release of apoptogenic proteins into the cytoplasm. Conversely,
Bcl-2 is an antiapoptotic and antioxidant molecule located in the outer mitochondrial membrane. This protein supports cell survival and counteracts
Bax, thus protecting mitochondrial membrane integrity [
68].
The current study confirms that CPF causes apoptosis by
Bcl-2 downregulation,
Bax upregulation, and increased immune expression of caspase-3 in neuronal and testicular cells. In contrast, OLE treatment significantly suppressed
Bax expression and caspase-3 immuno-expression, as well as upregulated
Bcl-2 levels. Hence, OLE counters apoptosis in the brain and testes. Similarly, CPF-induced cell death in neurons and tests is associated with upregulation of
Bax at the mRNA level, upregulating caspase-3 expression, and downregulating
Bcl-2 levels [
4,
69]. Conversely, OLE significantly upregulates
Bcl-2, downregulates
Bax, and decreases caspase 3 expression as protection against apoptosis in neural tissue [
15,
70]. Furthermore, OLE is an antiapoptotic agent in the testicular tissue of treated rats [
16,
41].
Our histological examinations revealed CPF-induced alterations in seminiferous tubules, including degenerative and necrotic alterations. The authors of [
6,
71] confirmed these findings. Furthermore, testes histological structures were largely rescued with co-administration of OLE. Our findings are consistent with [
16,
46,
72]. CPF caused some remarkable histological aberrations in brain tissues. Similar observations were reported by [
4,
73,
74,
75]. However, supplementation with OLE largely restored normal histology. The authors of [
13,
76] observed similar phenomena.
Ki-67 is a marker of cellular proliferation [
77], associated with nuclear antigens and detected in cells throughout various cell-cycle phases [
78]. CPF treatment induced a decrease in Ki-67 protein-positive nuclei in the cerebrum, cerebellum, choroid plexus, and hippocampus. However, supplementation with OLE preserved neurogenic Ki-67 cell distribution. Similar results were obtained by [
79,
80], who reported that immunoreactive Ki-67 nuclear proteins were reduced, indicating a loss of neurogenic characteristics.
Alpha smooth muscle actin (α-SMA) is a component of the cytoskeleton and a specific marker for myoepithelial and smooth muscle cells [
81,
82]. It affects nearly all tissues in the body, such as the liver, lung, testis, kidney, heart, and pancreas [
83].
The increased use of chlorpyrifos to control insects necessitates using a natural product such as olive leaf extract to mitigate its harmful effect on the brain and testes due to its powerful antioxidant and antiapoptotic effect.