The Antioxidant and Antitumor Efficiency of Litophyton sp. Extract in DMH-Induced Colon Cancer in Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Litophyton Methanolic Extract (LME)
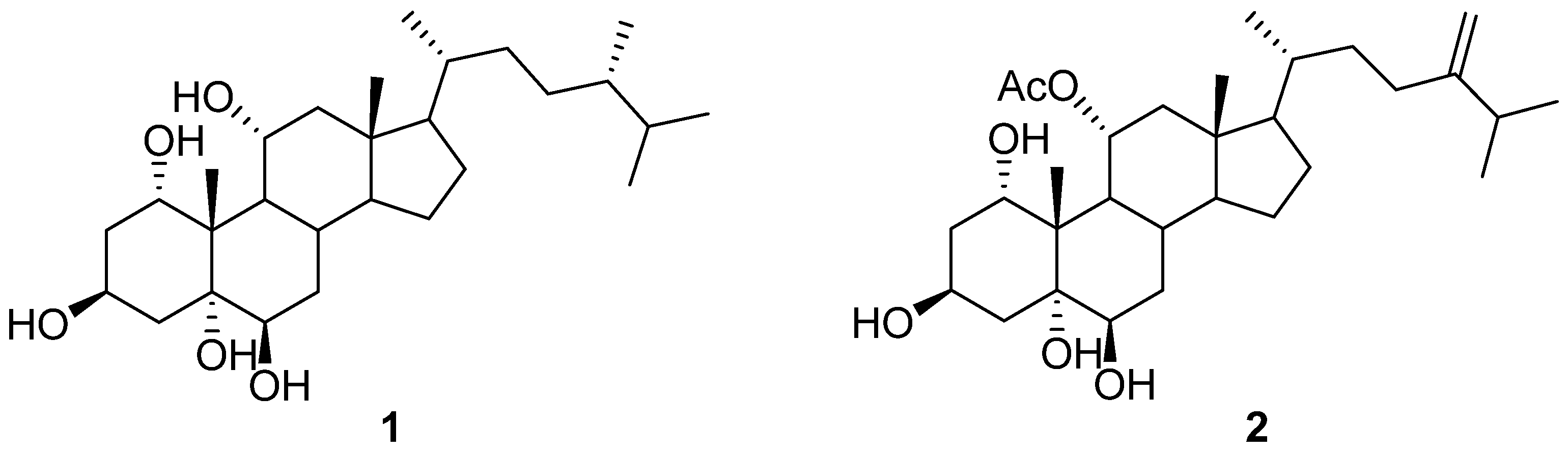
2.3. Purification of Compounds 1 and 2
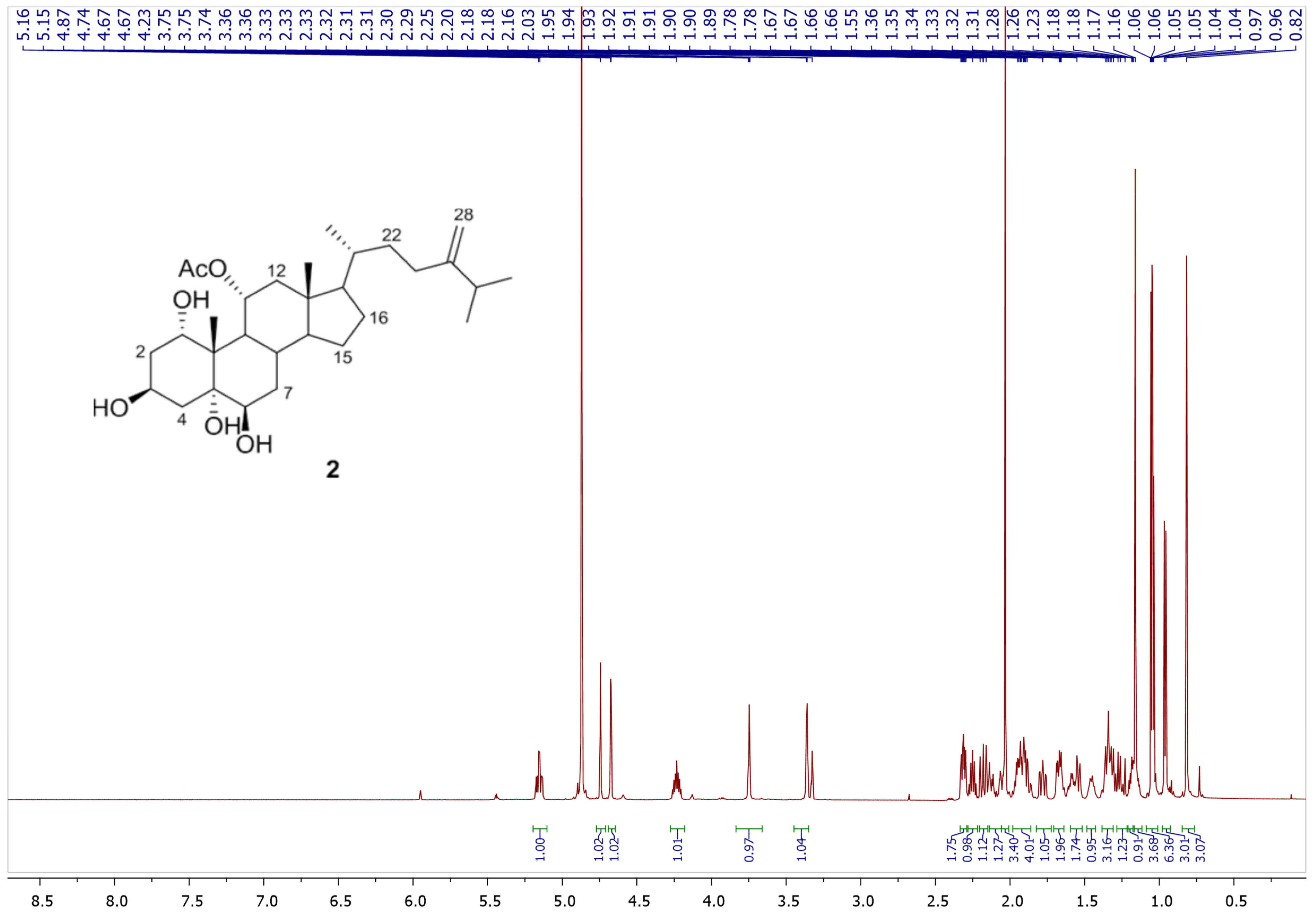
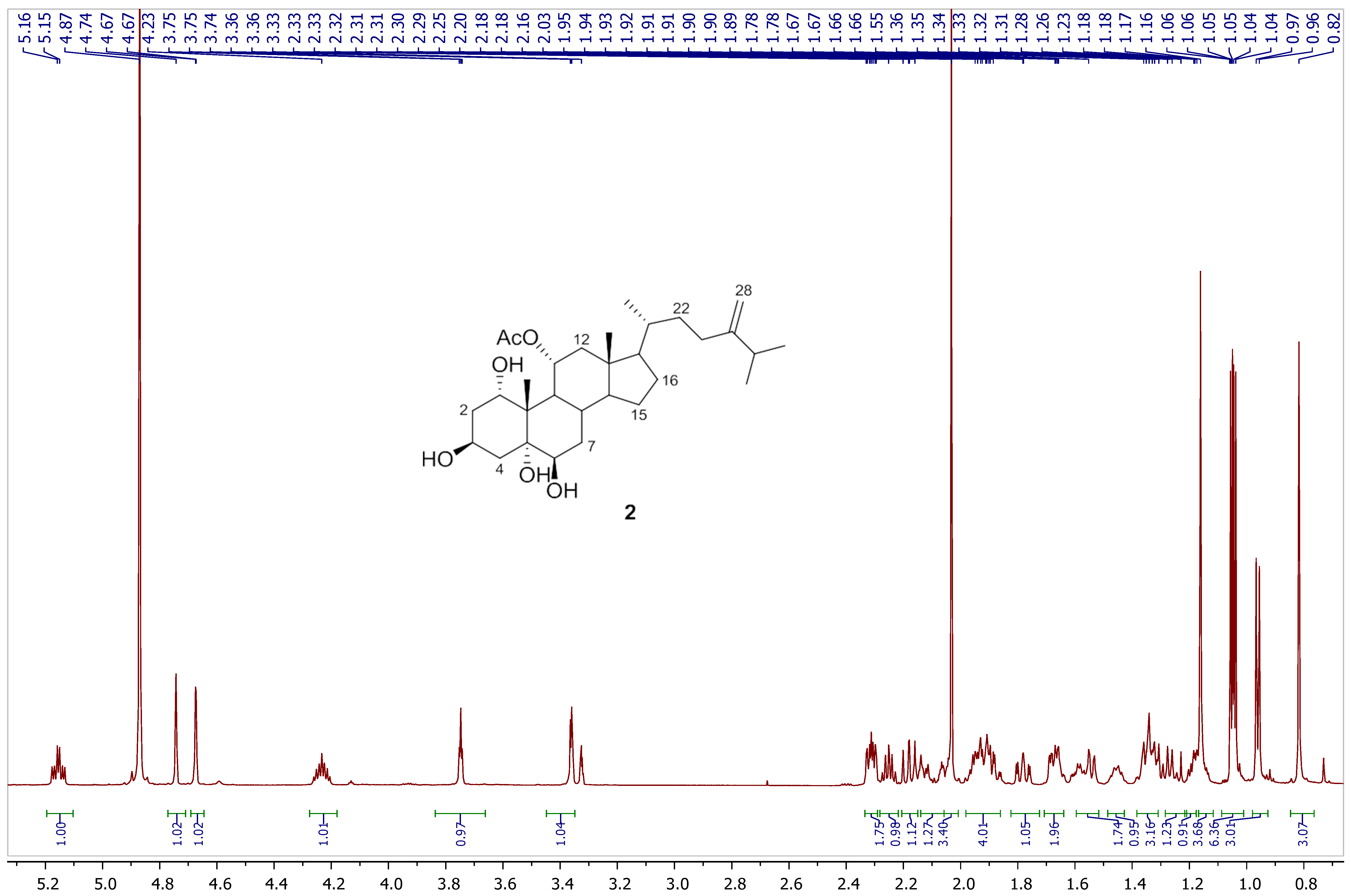
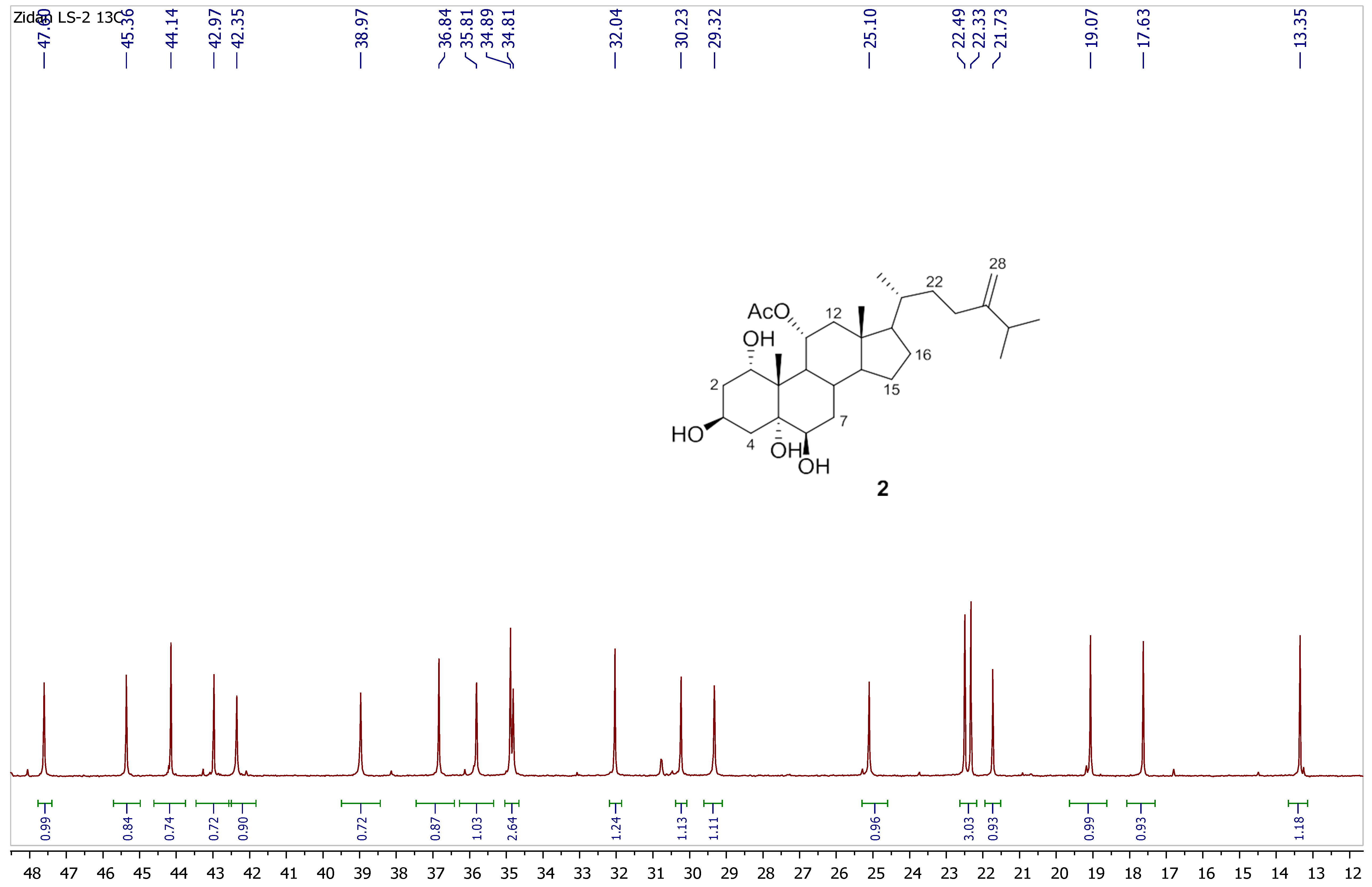
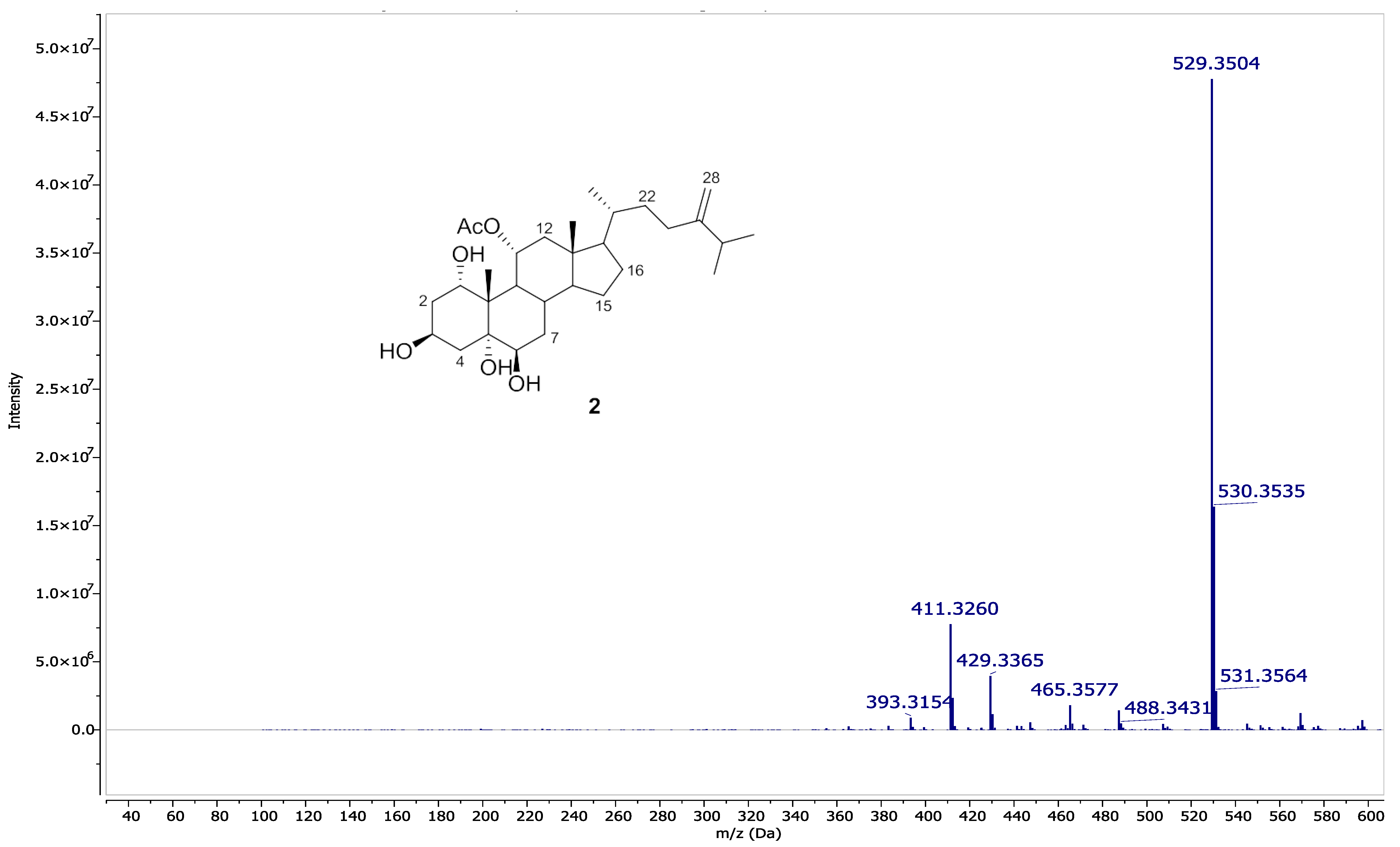
2.4. Spectral Data of Compounds 1 and 2
2.5. Animals
2.6. Induction of Colon Cancer
2.7. Experimental Animals’ Design
2.8. Blood and Tissue Sampling
2.9. Tissue Homogenization
2.10. Biochemical Determinations
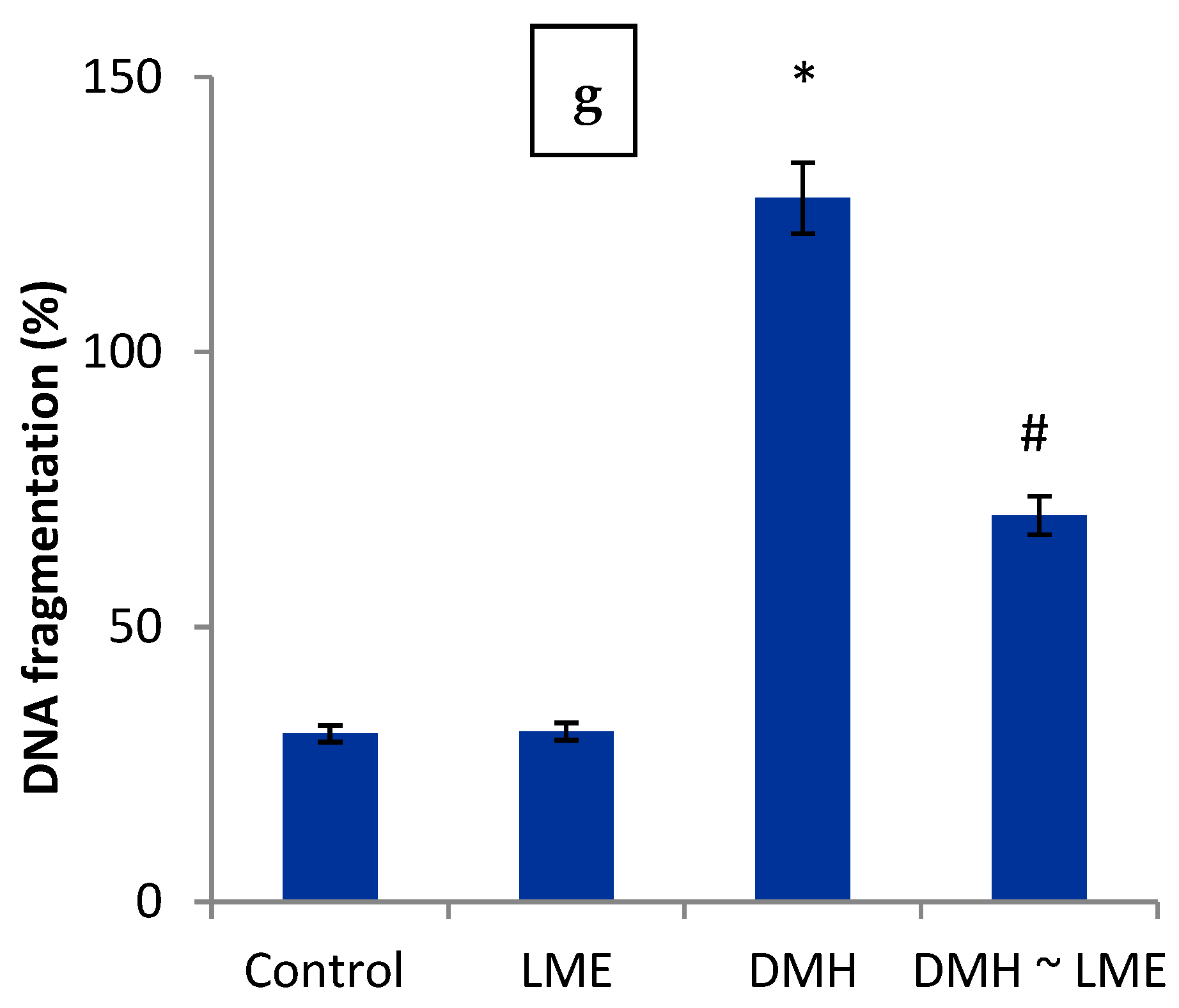
2.11. DNA Fragmentation Percentage
2.12. Biomarker, Immune Cytokines and Apoptotic Biomarker
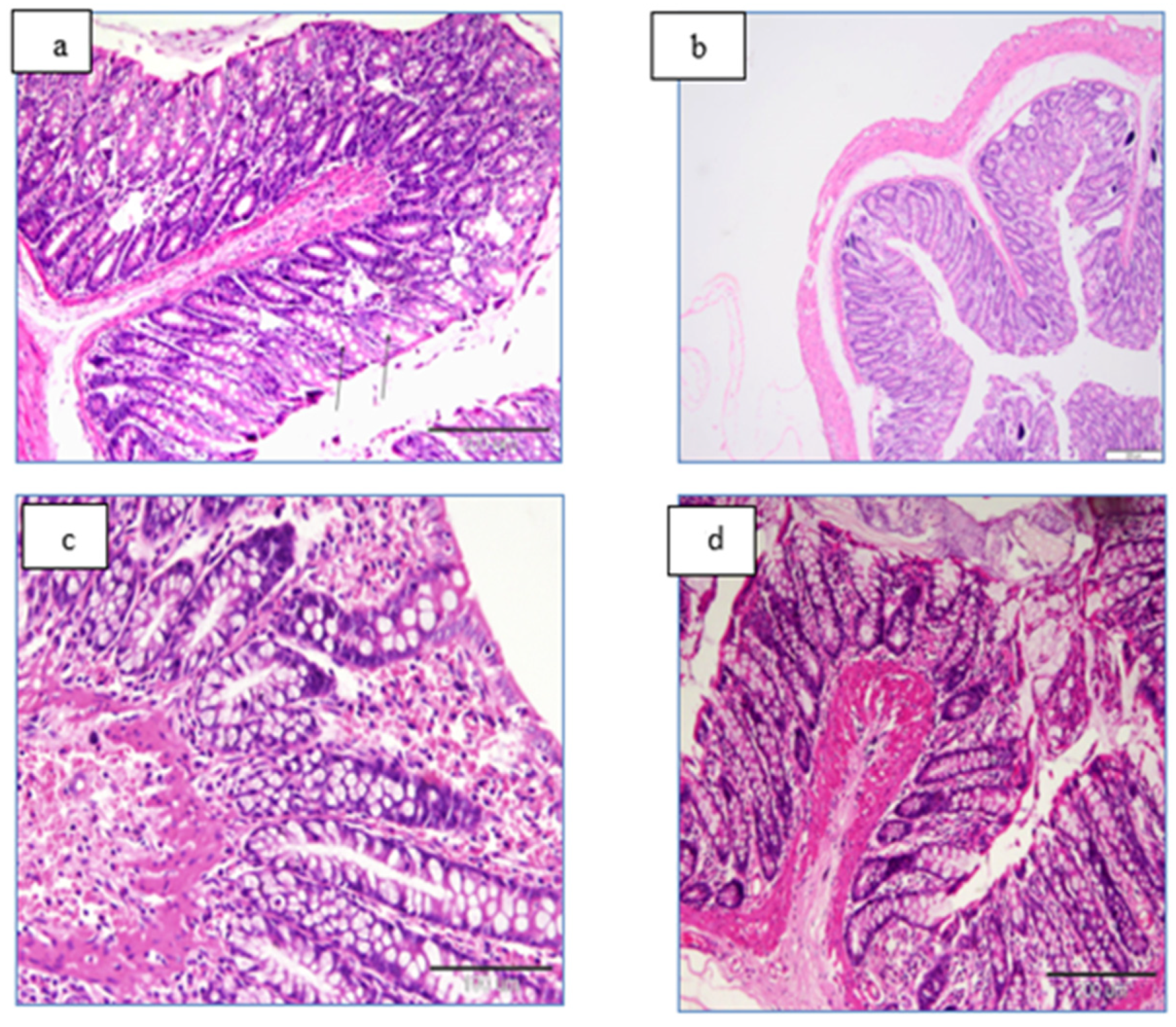
2.13. Histopathology
2.14. Statistical Analysis
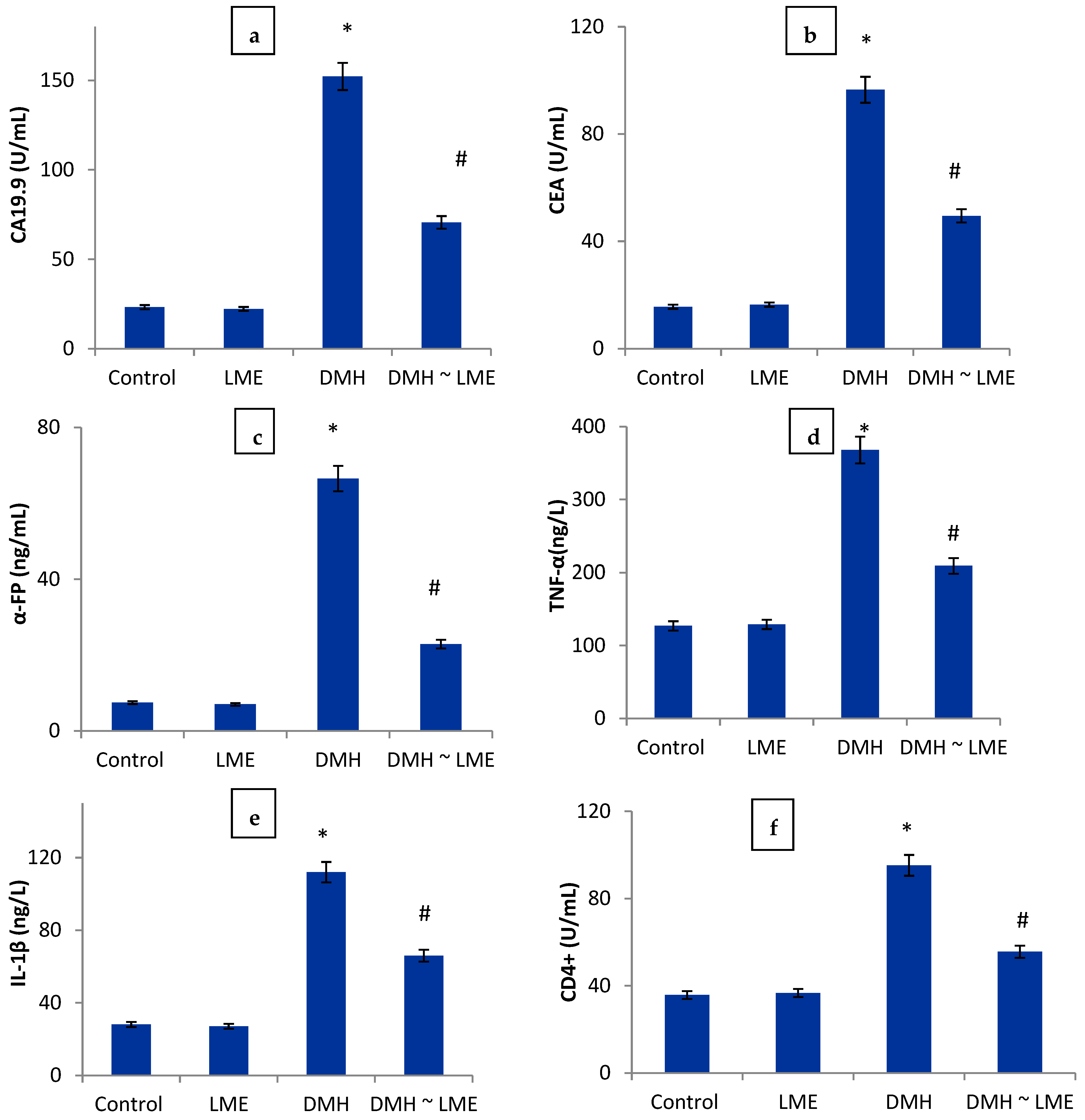
3. Results
| 1 | 2 | |||
|---|---|---|---|---|
| No | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type |
| 1 | 4.12, 1H,t (3.6) | 78.1 d | 3.70, 1H,t (3.6) | 78.0 d |
| 2a | 2.06, 1H, m | 38.1 t | 2.03, 1H, m | 38.9 t |
| 2b | 1.66, 1H, m | 1.76, 1H, m | ||
| 3 | 4.21, 1H, m | 64.6 d | 4.19, 1H, m | 64.3 d |
| 4a | 2.07, 1H, m | 42.1 t | 2.17, 1H, m | 42.3 t |
| 4b | 1.65, 1 H, m | 1.63, 1H, m | ||
| 5 | - | 79.4 s | - | 79.3 s |
| 6 | 3.34, 1H, br.s | 75.5 d | 3.32, 1H, br,d (2.4) | 75.4 d |
| 7a | 1.73, 1H, m | 36.1 t | 1.13, 1H, m | 35.8 t |
| 7b | 1.55, 1H, m | 1.55, 1H, m | ||
| 8 | 1.85, 1H, m | 30.7 d | 1.86, 1H, m | 30.2 d |
| 9 | 1.89, 1H, m | 48.0 d | 2.27 *, 1H | 45.3 d |
| 10 | - | 42.1 s | - | 42.9 s |
| 11 | 3.92, 1H, m | 67.8 d | 5.12, 1 H, ddd (4.8, 10.8, 16.8) | 73.1 d |
| 12a | 2.35, 1 H, dd (12.0,6.6) | 51.7 t | 2.27 *, 1H | 47.6 t |
| 12b | 1.24, 1H, m | 1.30 *, 1H | ||
| 13 | - | 43.2 s | - | 44.1 s |
| 14 | 1.19, 1H, m | 56.2 d | 1.30 *, 1H | 56.5 d |
| 15a | 1.63, 1H, m | 25.3 t | 1.63, 1H, m | 25.1 t |
| 15b | 1.10, 1H, m | 1.14, 1H, m | ||
| 16a | 1.42, 1H, m | 31.6 t | 2.10, 1H, m | 32.0 t |
| 16b | 0.97, 1 H, m | 1.88, 1H, m | ||
| 17 | 1.21, 1H, m | 57.2 d | 1.22, 1H, m | 57.2 d |
| 18 | 0.70, 3H, s | 13.2 q | 0.77, 3H, s | 13.3 q |
| 19 | 1.21, 3H, s | 16.7 q | 1. 12, 3H, s | 17.6 q |
| 20 | 1.37, 1H, m | 37.4 d | 1.42, 1H, m | 36.8 d |
| 21 | 0.97, 3H, d (6.6) | 19.3 q | 0.92, 3H, d (6.6) | 19.0 q |
| 22a | 1.44, 1H, m | 34.8 t | 1.85, 1H, m | 34.8 t |
| 22b | 0.97, 1H, m | 1.50, 1H, m | ||
| 23a | 1.89, 1H, m | 29.3 t | 1.98, 1H | 29.3 t |
| 23b | 1.31, 1H, m | 1.31 *, 1H,\ | ||
| 24 | 1.20, 1H, m | 40.3 d | - | 157.5 s |
| 25 | 1.57, 1H, m | 32.7 d | 2.21 *, 1H | 34.8 d |
| 26 | 0.82, 3H, d (6.6) | 18.0 q | 0.99, 3H, d (6.6) | 22.3 q |
| 27 | 0.87, 3H, d (6.6) | 20.9 q | 1.00, 3H, d (6.6) | 22.4 q |
| 28 | 0.81, 3H, d (6.6) | 15.9 q | - | 106.9 d |
| OAc | 172.2 s | |||
| 1.99, 3H, s | 21.7 q |
Histopathological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Qiao, X.; Wang, J.; Yang, J.; Qiao, Y.; Guan, Y.; Wen, A.; Jiang, L. Amelioration of DMH-induced colon cancer by eupafolin through the reprogramming of apoptosis-associated p53/Bcl2/Bax signaling in rats. Eur. J. Inflamm. 2022, 20, 20587392211069771. [Google Scholar] [CrossRef]
- El-Khadragy, M.F.; Nabil, H.M.; Hassan, B.N.; Tohamy, A.A.; Waaer, H.F.; Yehia, H.M.; Alharbi, A.M.; Moneim, A.E.A. Bone Marrow Cell Therapy on 1,2-Dimethylhydrazine (DMH)-Induced Colon Cancer in Rats. Cell. Physiol. Biochem. 2018, 45, 1072–1083. [Google Scholar] [CrossRef]
- Nabil, H.; Hassan, B.M.; Tohamy, A.A.; Waaer, H.F.; Moneim, A.E.A. Radioprotection of 1, 2-dimethylhydrazine-initiated colon cancer in rats using low-dose γ rays by modulating multidrug resistance-1, cytokeratin 20, and β-catenin expression. Hum. Exp. Toxicol. 2016, 35, 282–292. [Google Scholar] [CrossRef]
- Alkhuriji, A.F.; Alsaiari, S.G.; Alomar, S.Y.; Alnafjan, A.A.; Alobaid, H.; El-Khadragy, M.F. Effect of mesenchymal stem cells on cytochrome-c release and inflammation in colon cancer induced by 1,2-dimethylhydrazine in Wistar albino rats. Biosci. Rep. 2021, 41, BSR20204356. [Google Scholar] [CrossRef]
- Du, W.; Elemento, O. Cancer systems biology: Embracing complexity to develop better anticancer therapeutic strategies. Oncogene 2015, 34, 3215–3225. [Google Scholar] [CrossRef]
- Abdelaleem, E.R.; Samy, M.N.; Ali, T.F.S.; Mustafa, M.; Ibrahim, M.A.A.; Bringmann, G.; Ahmed, S.A.; Abdelmohsen, U.R.; Desoukey, S.Y. NS3 helicase inhibitory potential of the marine sponge Spongia irregularis. RSC Adv. 2022, 12, 2992–3002. [Google Scholar] [CrossRef]
- Elkhouly, H.B.; Attia, E.Z.; Khedr, A.I.M.; Samy, M.N.; Fouad, M.A. Recent updates on Sinularia soft coral. Mini Rev. Med. Chem. 2022, 22, 1152–1196. [Google Scholar] [CrossRef]
- Daly, M.; Brugler, M.R.; Cartwright, P.; Collins, A.G.; Dawson, M.N.; Fautin, D.G.; France, S.; McFadden, C.S.; Opresko, D.M.; Rodriguez, E.; et al. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 2007, 1668, 127–182. [Google Scholar] [CrossRef]
- Mahmoud, A.H.; Zidan, S.A.H.; Samy, M.N.; Alian, A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S.; Matsunami, K. Cytotoxicity and chemical profiling of the Red Sea soft corals Litophyton arboreum. Nat. Prod. Res. 2021, 36, 1–5. [Google Scholar] [CrossRef]
- Hu, J.; Yang, B.; Lin, X.; Zhou, X.; Yang, X.; Long, L.; Liu, Y. Chemical and Biological Studies of Soft Corals of the Nephtheidae Family. Chem. Biodivers. 2011, 8, 1011–1032. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Fahim, J.R.; Desoukey, S.Y.; Kamel, M.S.; Abdelmohsen, U.R. Recent Updates on Corals from Nephtheidae. Chem. Biodivers. 2019, 16, e1800692. [Google Scholar] [CrossRef]
- Mahmoud, A.H.; Zidan, S.A.; Samy, M.N.; Alian, A.; Fouad, M.A.; Kamel, M.S.; Matsunami, K. Phytochemical and biological investigation of Litophyton arboreum. J. Pharmacogn. Phytochem. 2022, 11, 12–15. [Google Scholar] [CrossRef]
- Ruiz-Larrea, M.B.; Maleal, A.; Liza, M.; Lacort, M.; De Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 1994, 59, 383–388. [Google Scholar]
- Perandones, C.E.; Illera, V.A.; Peckham, D.; Stunz, L.L.; Ashman, R.F. Regulation of apoptosis in vitro in mature murine spleen T cells. J. Immunol. 1993, 151, 3521–3529. [Google Scholar]
- Carleton, H.M.; Drury, R.A.B.; Wallington, E.A. Carleton’s Histological Technique; Oxford University Press: Cary, NC, USA, 1980. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1980. [Google Scholar]
- Su, C.-C.; Lin, J.-G.; Li, T.-M.; Chung, J.G.; Yang, J.S.; Ip, S.-W.; Lin, W.-C.; Chen, G.-W. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006, 26, 4379–4389. [Google Scholar]
- Gonzalez-Pons, M.; Cruz-Correa, M. Colorectal Cancer Biomarkers: Where Are We Now? Biomed. Res. Int. 2015, 2015, 149014. [Google Scholar] [CrossRef]
- Abdel-Hamid, O.M.; Nafee, A.A.; Emam, M.A.; Elshimaa, M.A. The ameliorative effect of Vitamin C in experimentally induced colon cancer in rats. Benha Veter. Med. J. 2018, 34, 329–343. [Google Scholar] [CrossRef]
- Muthu, R.; Selvaraj, N.; Vaiyapuri, M. Anti-inflammatory and proapoptotic effects of umbelliferone in colon carcinogenesis. Hum. Exp. Toxicol. 2016, 35, 1041–1054. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, M.Y.; Park, J.H.; Park, D.I.; Sohn, C.I.; Choi, K.; Jung, Y.S. Serum CEA and CA 19-9 Levels are Associated with the Presence and Severity of Colorectal Neoplasia. Yonsei Med. J. 2017, 58, 918–924. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Mauer, J.; Denson, J.L.; Brüning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015, 36, 92–101. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. OncoImmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Garza-Treviño, E.N.; Said-Fernández, S.L.; Martínez-Rodríguez, H.G. Understanding the colon cancer stem cells and perspectives on treatment. Cancer Cell Int. 2015, 15, 2. [Google Scholar] [CrossRef]
- Spina, A.; De Pasquale, V.; Cerulo, G.; Cocchiaro, P.; Della Morte, R.; Avallone, L.; Pavone, L.M. HGF/c-MET Axis in Tumor Microenvironment and Metastasis Formation. Biomedicines 2015, 3, 71–88. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; El Shamy, A.I.; Aziz, M.; Paré, P.W. Molecular Architecture and Biomedical Leads of Terpenes from Red Sea Marine Invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef]
- Ellithey, M.S.; Ahmed, H.H. Bioactive marine-derived compounds as potential anticancer candidates. Asian J. Pharm. Clin. Res. 2018, 11, 464–466. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, J.; Decker, E.A.; Zhang, G. Roles of Lipid Peroxidation-Derived Electrophiles in Pathogenesis of Colonic Inflammation and Colon Cancer. Front. Cell Dev. Biol. 2021, 9, 665591. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Sulkowski, S.; Koda, M.; Zalewski, B.; Kanczuga-Koda, L.; Sulkowska, M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef]
- Ying, L.; Hofseth, L.J.; Das, S.; Hahn, Y.; Nagata, S.; Willingham, M.C.; Bera, T.K.; Lee, B.; Pastan, I. An Emerging Role for Endothelial Nitric Oxide Synthase in Chronic Inflammation and Cancer. Cancer Res. 2007, 67, 1407–1410. [Google Scholar] [CrossRef]
- Wink, D.A.; Vodovotz, Y.; Laval, J.; Laval, F.; Dewhirst, M.W.; Mitchell, J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis 1998, 19, 711–721. [Google Scholar] [CrossRef]
- Femia, A.P.; Luceri, C.; Toti, S.; Giannini, A.; Dolara, P.; Caderni, G. Gene expression profile and genomic alterations in colonic tumours induced by 1,2-dimethylhydrazine (DMH) in rats. BMC Cancer 2010, 10, 194. [Google Scholar] [CrossRef]
- Cianchi, F.; Cuzzocrea, S.; Vinci, M.; Messerini, L.; Comin, C.; Navarra, G.; Perigli, G.; Centorrino, T.; Marzocco, S.; Lenzi, E.; et al. Heterogeneous expression of cyclooxygenase-2 and inducible nitric oxide synthase within colorectal tumors: Correlation with tumor angiogenesis. Dig. Liver Dis. 2010, 42, 20–27. [Google Scholar] [CrossRef]
- Barth, S.; Fähndrich, C.; Bub, A.; Dietrich, H.; Watzl, B.; Will, F.; Briviba, K.; Rechkemmer, G. Cloudy apple juice decreases DNA damage, hyperproliferation and aberrant crypt foci development in the distal colon of DMH-initiated rats. Carcinogenesis 2005, 26, 1414–1421. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, L.-J.; Xu, F.; Zhao, X.; Liu, B.; Cai, K.-L.; Wang, G.-B. Estrogen Inhibits Colon Polyp Formation by Reducing Angiogenesis in a Carcinogen-Induced Rat Model. Int. J. Endocrinol. 2013, 2013, 453898. [Google Scholar] [CrossRef]
- Abd-Elmoneim, M.A.; Bakar, A.A.; Awad, I.M.; Moharib, S.A.; Mohamed, E.M. Anticarcinogenic Effect of Raphanus sativus on 1, 2 Dimethylhydrazine (DMH) Induced Colon Cancer in Rats. Egypt. J. Hosp. Med. 2013, 51, 473–486. [Google Scholar]
- Garba, S.; Adelaiye, A.; Mshelia, L. Histopathological and biochemical changes in the rats kidney following exposure to a pyrethroid based mosquito coil. J. Appl. Sci. Res. 2007, 3, 1788–1793. [Google Scholar]
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic, Cytostatic and HIV-1 PR Inhibitory Activities of the Soft Coral Litophyton arboreum. Mar. Drugs 2013, 11, 4917–4936. [Google Scholar] [CrossRef]
- Ramírez-Rico, G.; Drago-Serrano, M.E.; León-Sicairos, N.; de la Garza, M. Lactoferrin: A Nutraceutical with Activity against Colorectal Cancer. Front. Pharmacol. 2022, 13, 855852. [Google Scholar] [CrossRef]
- Aziza, S.A.H.; Abdel-Aal, S.; Mady, H. Chemo-preventive effect of curcumin on oxidative stress, antioxidant status, DNA fragmentation and caspase-9 gene expression in 1, 2-dimethylhydrazine-induced colon cancer in rats. Am. J. Biochem. Mol. Biol. 2014, 4, 22–34. [Google Scholar] [CrossRef]
- Yazar, S. The subchronic toxic effects of plant growth promoters in mice. Ank. Üniversitesi Vet. Fakültesi Derg. 2008, 55, 17–21. [Google Scholar]
- Swenberg, J.; Cooper, H.K.; Bücheler, J.; Kleihues, P. 1,2-Dimethylhydrazine-induced methylation of DNA bases in various rat organs and the effect of pretreatment with disulfiram. Cancer Res. 1979, 39, 465–467. [Google Scholar]
- El-Kassem, L.T.A.; Hawas, U.W.; El-Desouky, S.K.; Al-Farawati, R. Sesquiterpenes from the Saudi Red Sea: Litophyton arboreum with their cytotoxic and antimicrobial activities. Z. Nat. C 2018, 73, 9–14. [Google Scholar] [CrossRef]





| Control | LME | DMH | DMH~LME | |
|---|---|---|---|---|
| ALAT (U/L) | 59.2 ± 7.1 | 57.8 ± 5.1 | 109.2 ± 6.1 * | 67.6 ± 5.4 # |
| ASAT (U/L) | 73.2 ± 3.7 | 71.9 ± 4.9 | 141.5 ± 10.9 * | 80.9 ± 9.1 # |
| Creatinine (mg/dL) | 0.91 ± 4.1 | 0.89 ± 2.1 | 1.67 ± 3.9 * | 1.16 ± 5.1 # |
| Urea (mg/dL) | 45.2 ± 3.2 | 44.2 ± 2.9 | 60.3 ± 4.1 * | 49.4 ± 2.2 # |
| Control | LME | DMH | DMH~LME | |
|---|---|---|---|---|
| CHO (mg/dL) | 140 ± 2.5 | 135.1 ± 6.1 | 225.3 ± 10.5 * | 171.4 ± 5.66 # |
| TRG (mg/dL) | 120.1 ± 4.1 | 123.3 ± 4.44 | 215.5 ± 9.4 * | 151.9 ± 6.99 # |
| HDL (mg/dL) | 44.1 ± 1.01 | 45.01 ± 3.9 | 34.1 ± 1.66 * | 42.1 ± 4.8 # |
| LDL (mg/dL) | 72.1 ± 3.5 | 65.4 ± 2.01 | 148.5 ± 3.2 * | 98.8 ± 2.88 # |
| Control | LME | DMH | DMH~LME | |
|---|---|---|---|---|
| MDA (μmol/g) | 6.2 ± 0.33 | 6.01 ± 0.54 | 18.4 ± 1.22 * | 9.45 ± 1.02 # |
| NO (μmol/g) | 80.3 ± 8.9 | 78.5 ± 10.2 | 156.4 ± 15.4 * | 92.5 ± 6.8 # |
| GSH (nmol/g) | 252 ± 25.6 | 261 ± 24.5 | 110.5 ± 18.55 * | 221.4 ± 15.4 # |
| SOD (U/g) | 375 ± 16.5 | 386 ± 31.2 | 164.5 ± 12.4 * | 295 ± 18.4 # |
| GPx (U/g) | 305 ± 14.2 | 302 ± 24.1 | 130.4 ± 16.5 * | 245 ± 21.6 # |
| CAT(U/g) | 15.4 ± 0.94 | 16.4 ± 0.65 | 7.55 ± 1.1 * | 11.6 ± 2.1 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashry, M.; Askar, H.; Alian, A.; Zidan, S.A.H.; El-Sahra, D.G.; Abdel-Wahhab, K.G.; Lamlom, S.F.; Abdelsalam, N.R.; Abd El-Hack, M.E.; Gomaa, H.F. The Antioxidant and Antitumor Efficiency of Litophyton sp. Extract in DMH-Induced Colon Cancer in Male Rats. Life 2022, 12, 1470. https://doi.org/10.3390/life12101470
Ashry M, Askar H, Alian A, Zidan SAH, El-Sahra DG, Abdel-Wahhab KG, Lamlom SF, Abdelsalam NR, Abd El-Hack ME, Gomaa HF. The Antioxidant and Antitumor Efficiency of Litophyton sp. Extract in DMH-Induced Colon Cancer in Male Rats. Life. 2022; 12(10):1470. https://doi.org/10.3390/life12101470
Chicago/Turabian StyleAshry, Mahmoud, Hussam Askar, Abdallah Alian, Sabry A. H. Zidan, Doaa G. El-Sahra, Khaled G. Abdel-Wahhab, Sobhi F. Lamlom, Nader R. Abdelsalam, Mohamed E. Abd El-Hack, and Heba F. Gomaa. 2022. "The Antioxidant and Antitumor Efficiency of Litophyton sp. Extract in DMH-Induced Colon Cancer in Male Rats" Life 12, no. 10: 1470. https://doi.org/10.3390/life12101470
APA StyleAshry, M., Askar, H., Alian, A., Zidan, S. A. H., El-Sahra, D. G., Abdel-Wahhab, K. G., Lamlom, S. F., Abdelsalam, N. R., Abd El-Hack, M. E., & Gomaa, H. F. (2022). The Antioxidant and Antitumor Efficiency of Litophyton sp. Extract in DMH-Induced Colon Cancer in Male Rats. Life, 12(10), 1470. https://doi.org/10.3390/life12101470










