Protective Effects of L-2-Oxothiazolidine-4-Carboxylate during Isoproterenol-Induced Myocardial Infarction in Rats: In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Biochemical Estimations
2.3. Histopathological Analysis
2.4. RT-qPCR
2.5. Statistical Analysis
3. Results
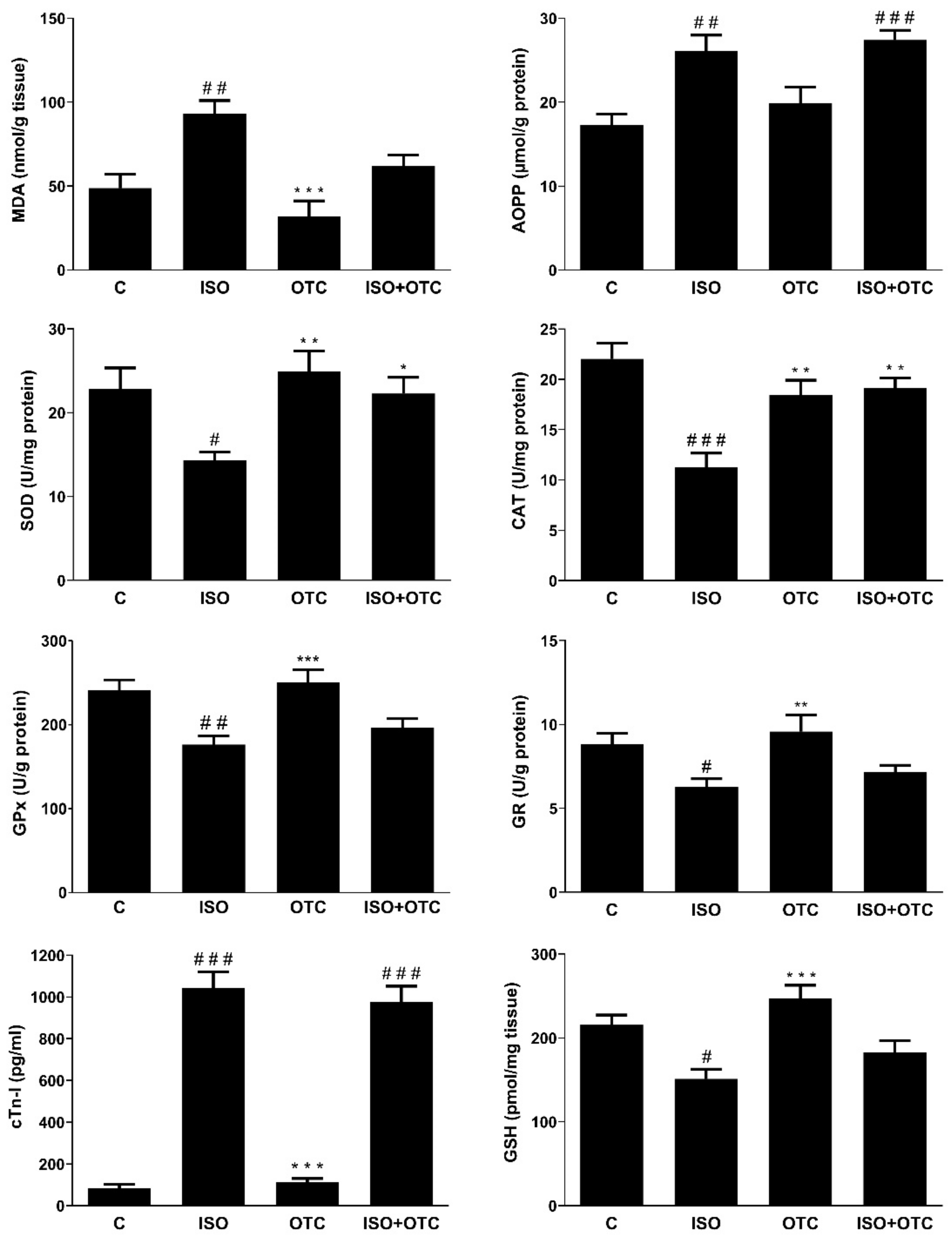
3.1. Biomarker for Myocardial Infarction
3.2. Antioxidant and Oxidative Stress Markers and NF-κB Transcriptional Activity
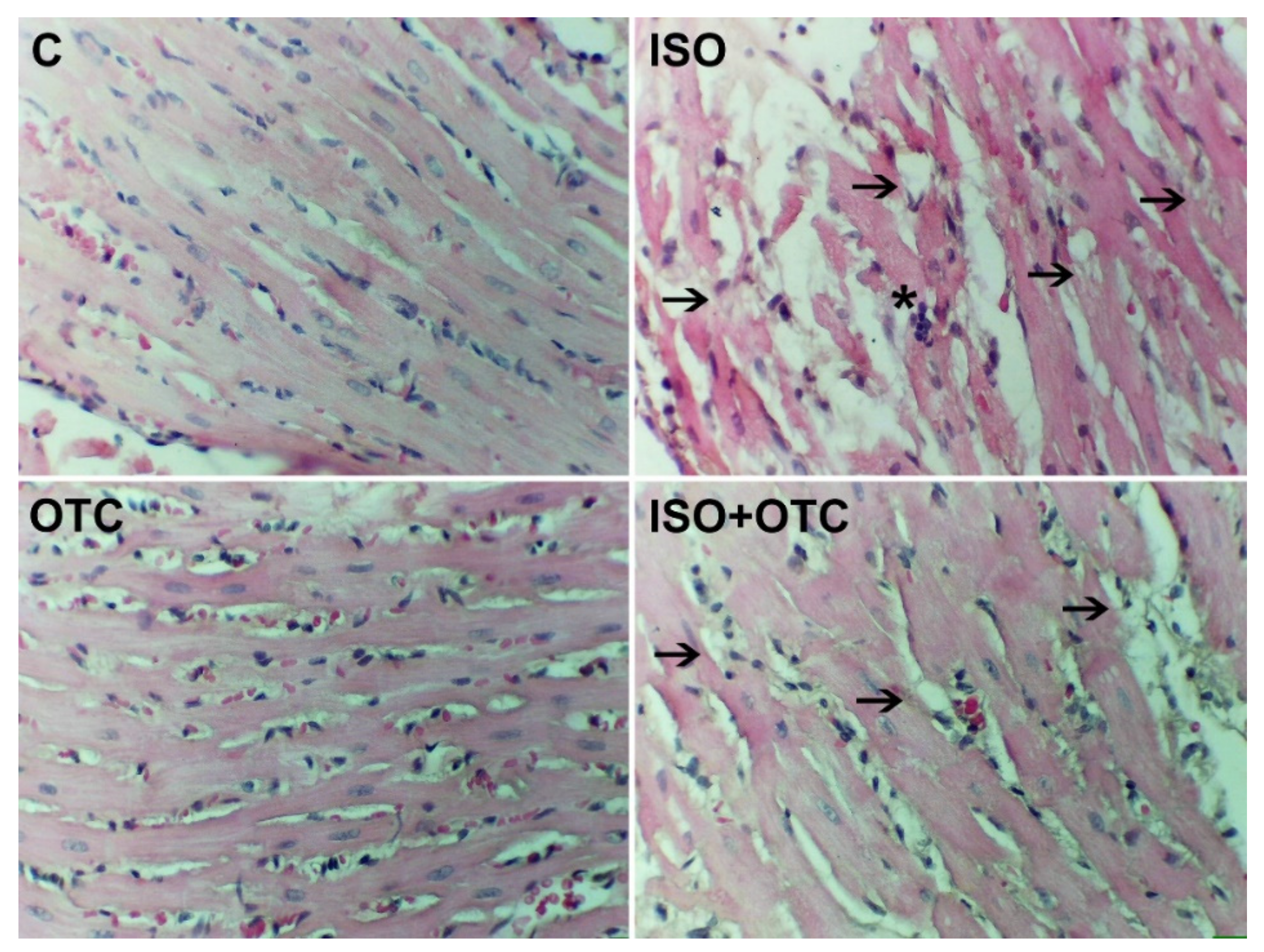
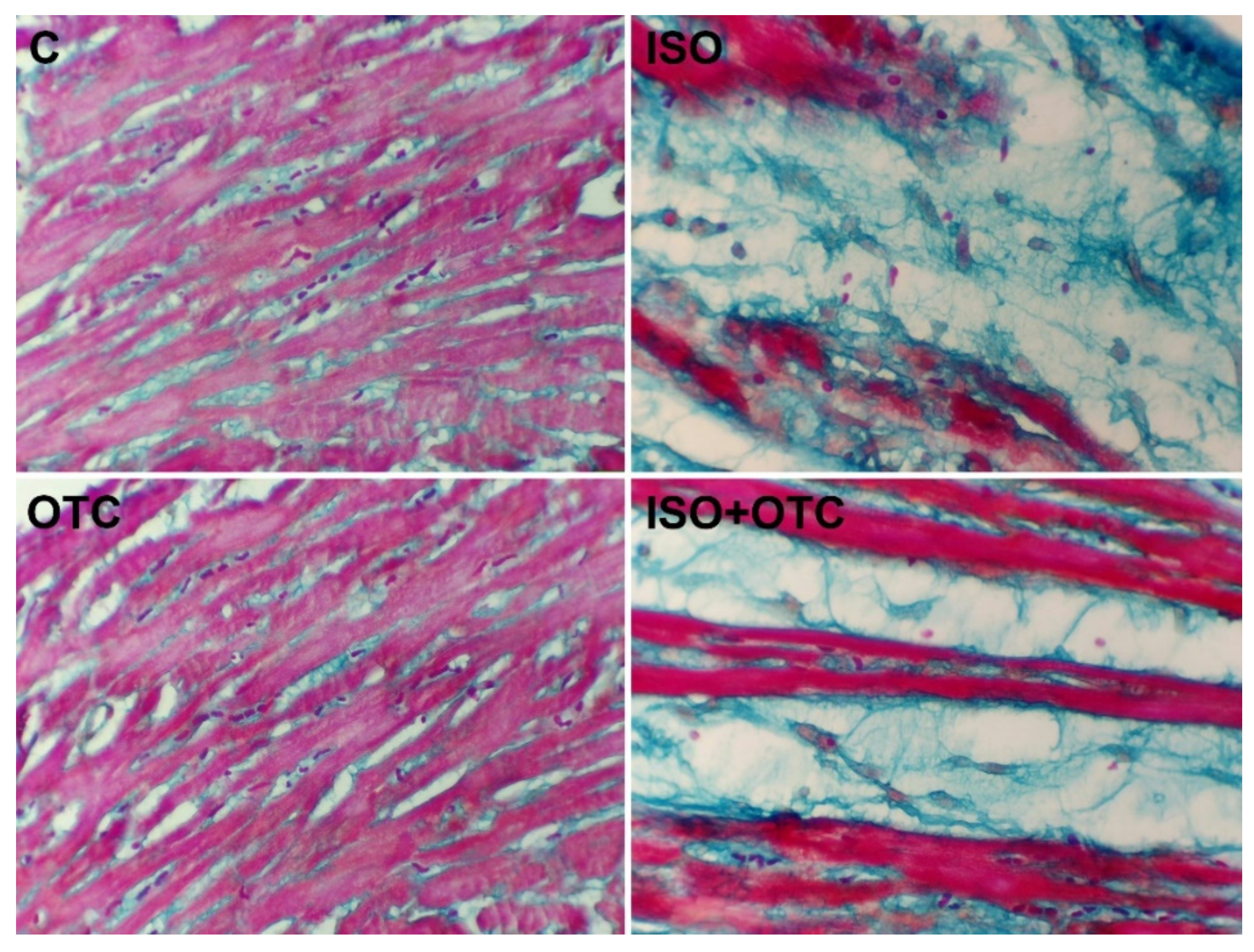
3.3. Histopathological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, 146–603. [Google Scholar]
- Garg, M.; Khanna, D. Exploration of pharmacological interventions to prevent isoproterenol-induced myocardial infarction in experimental models. Ther. Adv. Cardiovasc. Dis. 2014, 8, 155–169. [Google Scholar] [PubMed]
- Fan, Y. Cardioprotective effect of rhapontigenin in isoproterenol-induced myocardial infarction in a rat model. Pharmacology 2019, 103, 291–302. [Google Scholar] [PubMed]
- Shukla, S.K.; Sharma, S.B.; Singh, U.R. β-Adrenoreceptor agonist isoproterenol alters oxidative status, inflammatory signaling, injury markers and apoptotic cell death in myocardium of rats. Indian J. Clin. Biochem. 2015, 30, 27–34. [Google Scholar] [PubMed]
- Rodrigo, R.; Libuy, M.; Feliú, F.; Hasson, D. Molecular basis of cardioprotective effect of antioxidant vitamins in myocardial infarction. Biomed. Res. Int. 2013, 2013, 437613. [Google Scholar] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 2181–2190. [Google Scholar]
- Hori, M.; Nishida, K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc. Res. 2009, 81, 457–464. [Google Scholar]
- Neri, M.; Fineschi, V.; Di Paolo, M.; Pomara, C.; Riezzo, I.; Turillazzi, E.; Cerretani, D. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar]
- Gil, A.; van der Pol, A.; van der Meer, P.; Bischoff, R. LC-MS analysis of key components of the glutathione cycle in tissues and body fluids from mice with myocardial infarction. J. Pharm. Biomed. Anal. 2018, 160, 289–296. [Google Scholar]
- Singh, A.; Lee, K.J.; Lee, C.Y.; Goldfarb, R.D.; Tsan, M.F. Relation between myocardial glutathione content and extent of ischemia-reperfusion injury. Circulation 1989, 80, 1795–1804. [Google Scholar]
- Van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [PubMed]
- Adamy, C.; Mulder, P.; Khouzami, L.; Andrieu-Abadie, N.; Defer, N.; Candiani, G.; Pavoine, C.; Caramelle, P.; Souktani, R.; Le Corvoisier, P.; et al. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J. Mol. Cell. Cardiol. 2007, 43, 344–353. [Google Scholar] [PubMed]
- Basha, R.H.; Priscilla, D.H. An in vivo and in vitro study on the protective effects of N-acetylcysteine on mitochondrial dysfunction in isoproterenol treated myocardial infarcted rats. Exp. Toxicol. Pathol. 2013, 65, 7–14. [Google Scholar]
- Giam, B.; Chu, P.; Kuruppu, S.; Smith, I.; Horlock, D.; Kiriazis, H.; Du, X.; Kaye, D.M.; Rajapakse, N.W. N-acetylcysteine attenuates the development of cardiac fibrosis and remodeling in a mouse model of heart failure. Physiol. Rep. 2016, 4, e12757. [Google Scholar] [PubMed]
- Angelovski, M.; Hadzi-Petrushev, N.; Mitrokhin, V.; Kamkin, A.; Mladenov, M. Myocardial infarction and oxidative damage in animal models: Objective and expectations from the application of cysteine derivatives. Toxicol. Mech. Methods 2022, 10, 1–17. [Google Scholar]
- Shug, A. Protection of the ischemic rat heart by procysteine and amino acids. J. Nutr. Biochem. 1994, 5, 356–359. [Google Scholar]
- Poon, B.Y.; Goddard, C.M.; Leaf, C.D.; Russell, J.A.; Walley, K.R. L-2-oxothiazolidine-4-carboxylic acid prevents endotoxin-induced cardiac dysfunction. Am. J. Respir. Crit. Care Med. 1998, 158, 1109–1113. [Google Scholar]
- Choi, J.; Park, K.-H.; Kim, S.Z.; Shin, J.H.; Jang, S.-I. The ameliorative effects of L-2-oxothiazolidine-4-carboxylate on acetaminophen-induced hepatotoxicity in mice. Molecules 2013, 18, 3467–3478. [Google Scholar]
- Hadzi-Petrushev, N.; Jankulovski, N.; Milev, M.; Filipovska, P.; Gagov, H.; Hjorgievska, E.; Mitrov, D.; Sopi, R.; Hristov, K.; Mladenov, M. L-2-oxothiazolidine-4-carboxylate influence on age- and heat exposure-dependent peroxidation in rat’s liver and kidney. J. Therm. Biol. 2012, 37, 361–365. [Google Scholar]
- Park, S.J.; Lee, K.S.; Lee, S.J.; Kim, S.R.; Park, S.Y.; Jeon, M.S.; Lee, H.B.; Lee, Y.C. L-2-Oxothiazolidine-4-carboxylic acid or α-lipoic acid attenuates airway remodeling: Involvement of nuclear factor-κB (NF-κB), nuclear factor erythroid 2p45-related factor-2 (Nrf2), and hypoxia-inducible factor (HIF). Int. J. Mol. Sci. 2012, 13, 7915–7937. [Google Scholar]
- Promsote, W.; Veeranan-Karmegam, R.; Ananth, S.; Shen, D.; Chan, C.-C.; Lambert, N.A.; Ganapathy, V.; Martin, P.M. L-2-oxothiazolidine-4-carboxylic acid attenuates oxidative stress and inflammation in retinal pigment epithelium. Mol. Vis. 2014, 20, 73–88. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [PubMed]
- Taylor, E.L.; Armstrong, K.R.; Perrett, D.; Hattersley, A.; Winyard, P.G. Optimisation of an advanced oxidation protein products assay: Its application to studies of oxidative stress in diabetes mellitus. Oxid. Med. Cell Longev. 2015, 2015, 496271. [Google Scholar] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [PubMed]
- Racker, E. Glutathione reductase from bakers’ yeast and beef liver. J. Biol. Chem. 1955, 217, 855–865. [Google Scholar]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar]
- Bottero, V.; Imbert, V.; Frelin, C.; Formento, J.-L.; Peyron, J.-F. Monitoring NF-kappa B transactivation potential via real-time PCR quantification of I kappa B-alpha gene expression. Mol. Diagn. 2003, 7, 187–194. [Google Scholar]
- Patel, V.; Upaganlawar, A.; Zalawadia, R.; Balaraman, R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010, 644, 160–168. [Google Scholar]
- Hill, M.F.; Singal, P.K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 1996, 148, 291–300. [Google Scholar]
- Verma, V.K.; Malik, S.; Narayanan, S.P.; Mutneja, E.; Sahu, A.K.; Bhatia, J.; Arya, D.S. Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Mol. Biol. Rep. 2019, 46, 1139–1148. [Google Scholar]
- Saravanan, G.; Prakash, J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J. Ethnopharmacol. 2004, 94, 155–158. [Google Scholar] [PubMed]
- Godugu, C.; Kumari, P.; Khurana, A. Nanoyttria attenuates isoproterenol-induced cardiac injury. Nanomedicine 2018, 13, 2961–2980. [Google Scholar] [PubMed]
- Rajadurai, M.; Stanely Mainzen Prince, P. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: Biochemical and histopathological evidences. Toxicology 2006, 228, 259–268. [Google Scholar] [PubMed]
- Sun, L.; Hu, Y.; Mishra, A.; Sreeharsha, N.; Moktan, J.B.; Kumar, P.; Wang, L. Protective role of poly(lactic-co-glycolic) acid nanoparticle loaded with resveratrol against isoproterenol-induced myocardial infarction. Biofactors 2020, 46, 421–431. [Google Scholar]
- Van der Pol, A.; Gil, A.; Silljé, H.H.W.; Tromp, J.; Ovchinnikova, E.S.; Vreeswijk-Baudoin, I.; Hoes, M.; Domian, I.J.; van de Sluis, B.; van Deursen, J.M.; et al. Accumulation of 5-oxoproline in myocardial dysfunction and the protective effects of OPLAH. Sci. Transl. Med. 2017, 9, eaam8574. [Google Scholar]
- Priscilla, D.H.; Prince, P.S. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009, 179, 118–124. [Google Scholar]
- Lu, L.; Zhang, J.Q.; Ramires, F.J.; Sun, Y. Molecular and cellular events at the site of myocardial infarction: From the perspective of rebuilding myocardial tissue. Biochem. Biophys. Res. Commun. 2004, 320, 907–913. [Google Scholar]
- Nakamura, K.; Fushimi, K.; Kouchi, H.; Mihara, K.; Miyazaki, M.; Ohe, T.; Namba, M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation 1998, 98, 794–799. [Google Scholar]
- Wu, X.Y.; Luo, A.Y.; Zhou, Y.R.; Ren, J.H. N-acetylcysteine reduces oxidative stress, nuclear factor-κB activity and cardiomyocyte apoptosis in heart failure. Mol. Med. Rep. 2014, 10, 615–624. [Google Scholar]
- Lee, S.; Moon, S.-O.; Kim, W.; Sung, M.J.; Kim, D.H.; Kang, K.P.; Jang, Y.B.; Lee, J.E.; Jang, K.Y.; Lee, S.Y.; et al. Protective role of L-2-oxothiazolidine-4-carboxylic acid in cisplatin-induced renal injury. Nephrol. Dial. Transplant. 2006, 21, 2085–2095. [Google Scholar] [PubMed]
- Hayıroğlu, M.İ.; Bozbeyoglu, E.; Yıldırımtürk, Ö.; İlker Tekkeşin, A.; Pehlivanoğlu, S. Effect of acute kidney injury on long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention in a high-volume tertiary center. Turk. Kardiyol. Dern. Ars. 2020, 48, 1–9. [Google Scholar] [PubMed]
- Hayıroğlu, M.; Keskin, M.; Uzun, A.O.; Yıldırım, D.I.; Kaya, A.; Çinier, G.; Bozbeyoğlu, E.; Yıldırımtürk, Ö.; Kozan, Ö.; Pehlivanoğlu, S. Predictors of in-hospital mortality in patients with ST-segment elevation myocardial infarction complicated with cardiogenic shock. Heart Lung Circ. 2019, 28, 237–244. [Google Scholar] [PubMed]
| Group | ΔCt (Mean ± SEM) | Fold Change (Normalized Relative to C) |
|---|---|---|
| C | 9.071 ± 0.413 | 1 (0.751–1.332) |
| ISO | 6.516 ± 0.710 1 | 5.877 (3.592–9.617) |
| OTC | 8.783 ± 0.908 | 1.221 (0.650–2.291) |
| ISO+OTC | 7.972 ± 0.575 | 2.141 (1.438–3.189) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelovski, M.; Hadzi-Petrushev, N.; Atanasov, D.; Nikodinovski, A.; Mitrokhin, V.; Avtanski, D.B.; Mladenov, M. Protective Effects of L-2-Oxothiazolidine-4-Carboxylate during Isoproterenol-Induced Myocardial Infarction in Rats: In Vivo Study. Life 2022, 12, 1466. https://doi.org/10.3390/life12101466
Angelovski M, Hadzi-Petrushev N, Atanasov D, Nikodinovski A, Mitrokhin V, Avtanski DB, Mladenov M. Protective Effects of L-2-Oxothiazolidine-4-Carboxylate during Isoproterenol-Induced Myocardial Infarction in Rats: In Vivo Study. Life. 2022; 12(10):1466. https://doi.org/10.3390/life12101466
Chicago/Turabian StyleAngelovski, Marija, Nikola Hadzi-Petrushev, Dino Atanasov, Aleksandar Nikodinovski, Vadim Mitrokhin, Dimiter B. Avtanski, and Mitko Mladenov. 2022. "Protective Effects of L-2-Oxothiazolidine-4-Carboxylate during Isoproterenol-Induced Myocardial Infarction in Rats: In Vivo Study" Life 12, no. 10: 1466. https://doi.org/10.3390/life12101466
APA StyleAngelovski, M., Hadzi-Petrushev, N., Atanasov, D., Nikodinovski, A., Mitrokhin, V., Avtanski, D. B., & Mladenov, M. (2022). Protective Effects of L-2-Oxothiazolidine-4-Carboxylate during Isoproterenol-Induced Myocardial Infarction in Rats: In Vivo Study. Life, 12(10), 1466. https://doi.org/10.3390/life12101466







