Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Determination of mRNA Expression
2.3. Determination of Protein Expression
2.4. Infection Assay
2.5. Spike Protein Immunostaining
2.6. Cell Viability Assay
2.7. Cell Barrier Assay
2.8. Statistics
3. Results
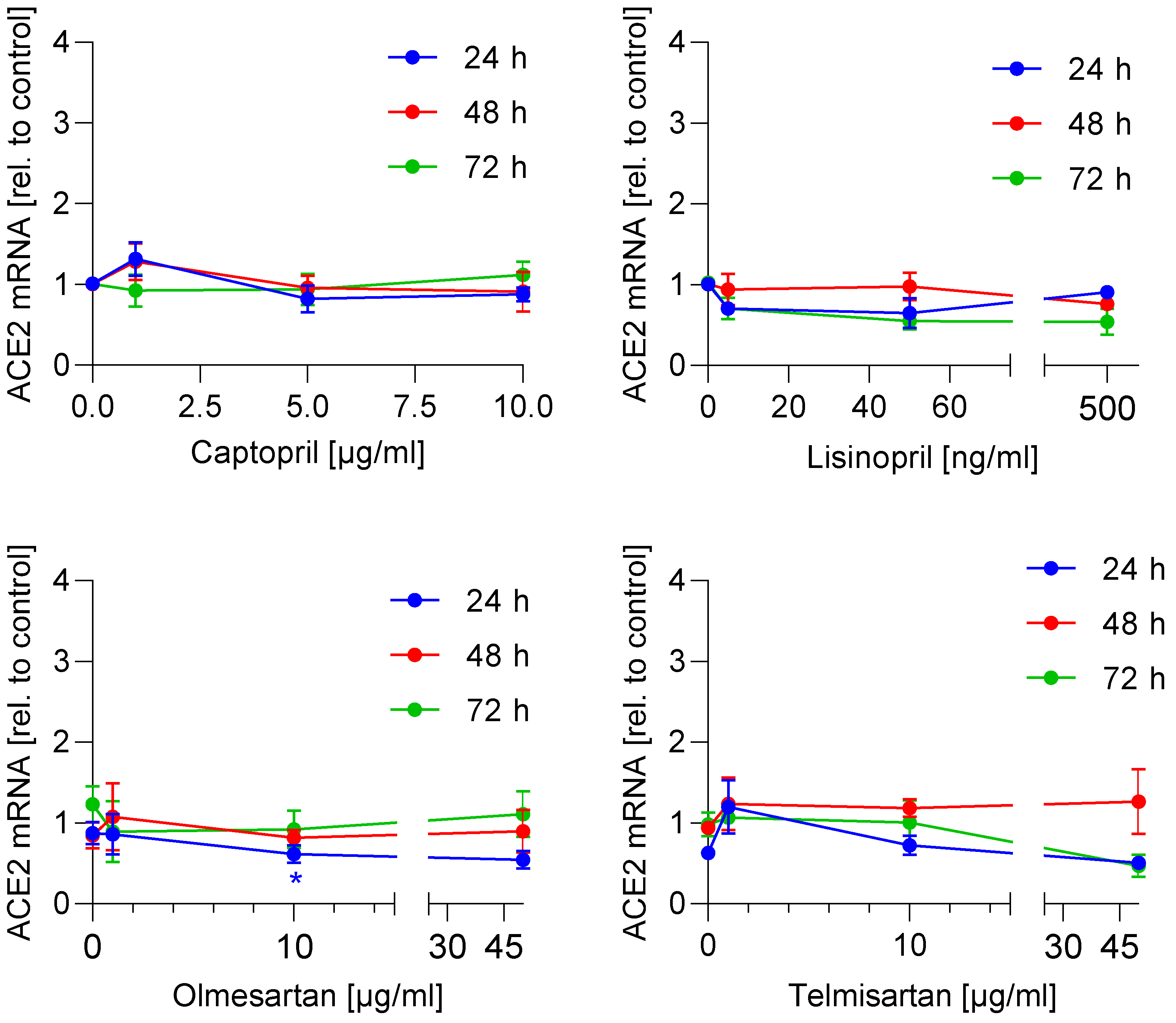
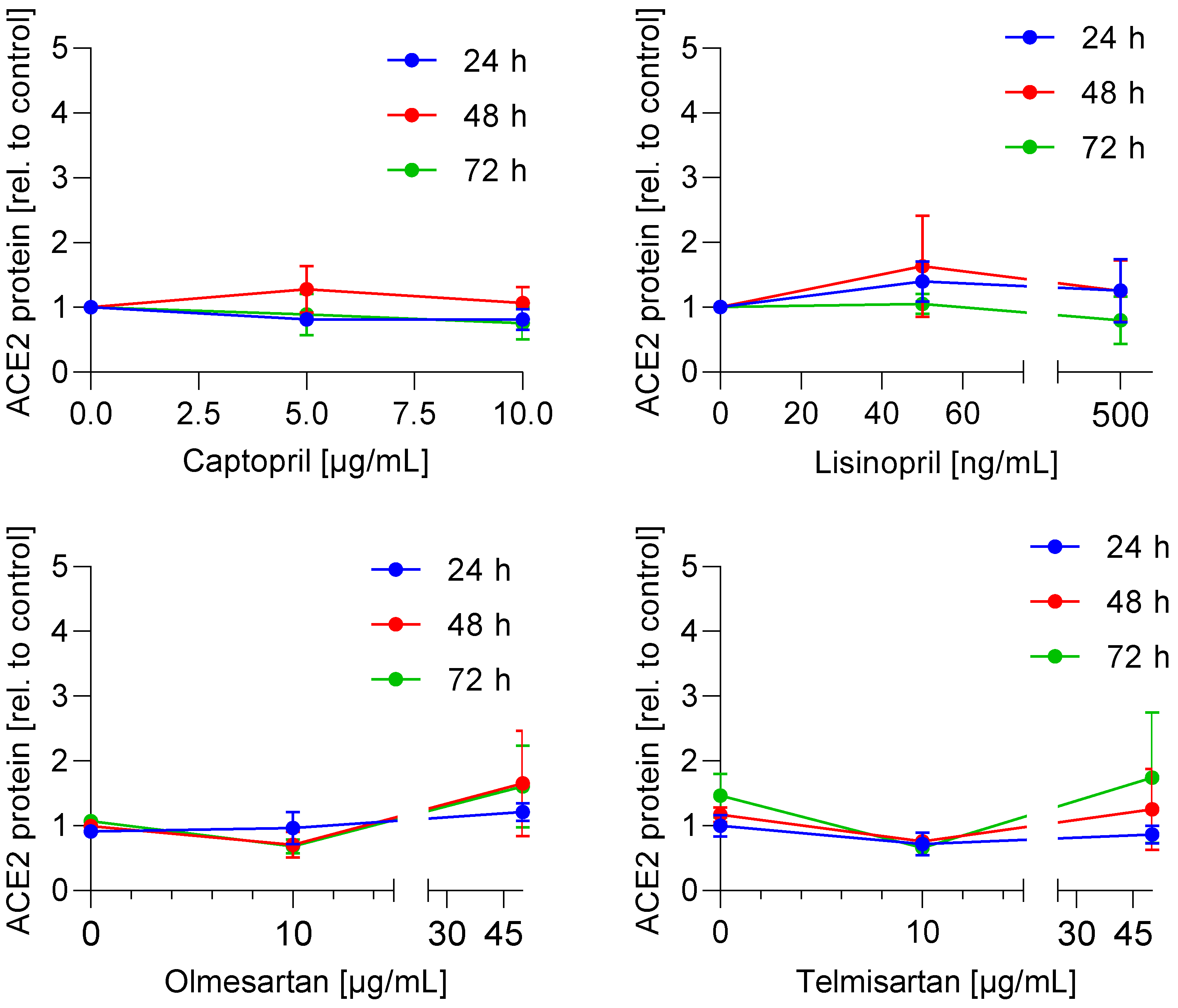
3.1. ACE2 mRNA and Protein Expression Is Not Regulated by ACE Inhibitors and AT1 Antagonists
3.2. ACE Inhibitors and AT1 Antagonists Have No Effect on Caco-2 Cell Viability
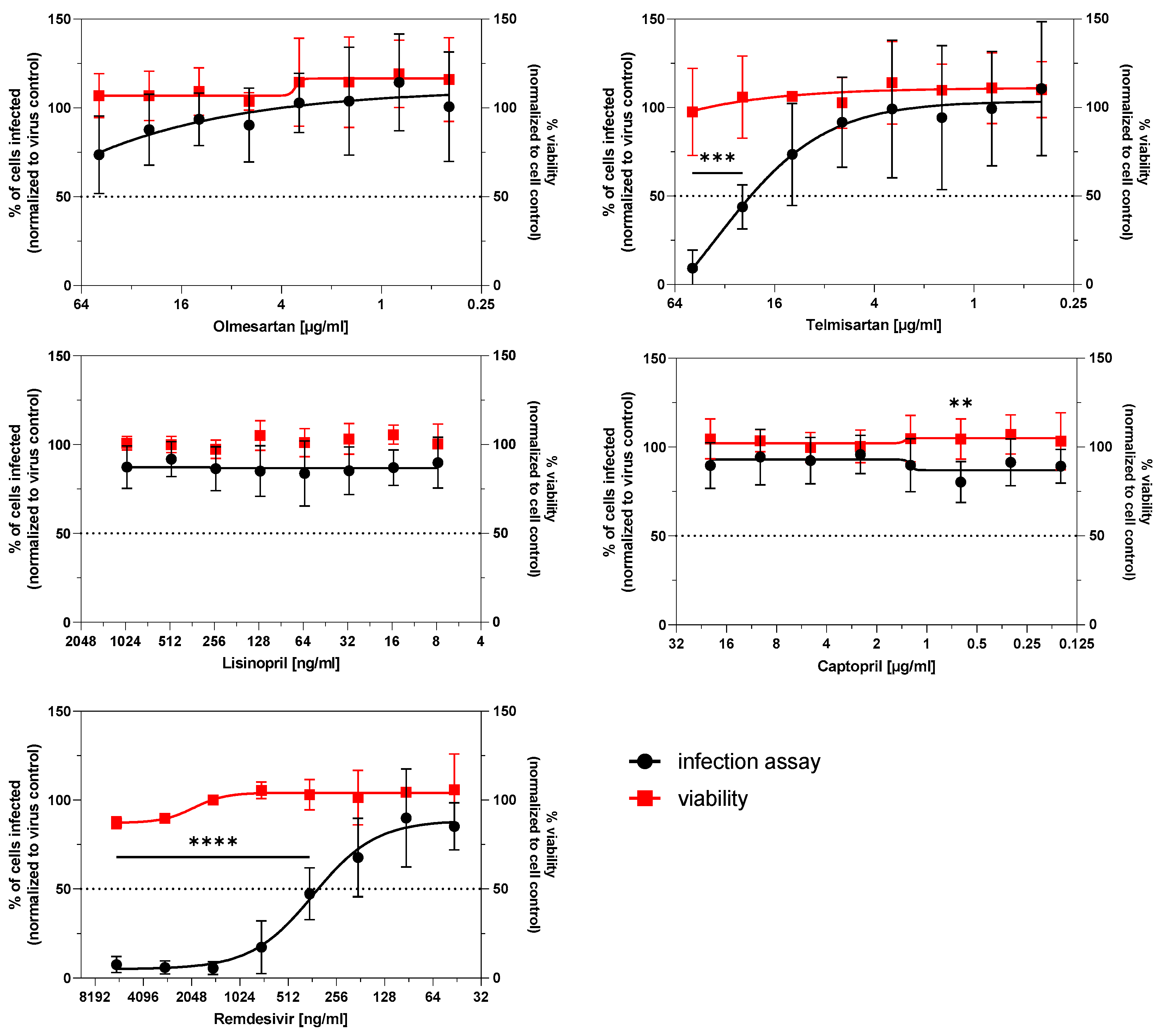
3.3. Infection Potential of SARS-CoV-2 Is Not Affected by ACE Inhibitors and AT1R Antagonists
3.4. SARS-CoV-2-Induced Disintegration of Caco-2 Cell Barrier Is Not Prevented by ACE Inhibitors and AT1R Antagonists
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, J. Preventing a covid-19 pandemic. BJM 2020, 368, m810. [Google Scholar] [CrossRef] [Green Version]
- Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell, C.-R.C.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Deppe, S.; Boger, R.H.; Weiss, J.; Benndorf, R.A. Telmisartan: A review of its pharmacodynamic and pharmacokinetic properties. Expert Opin. Drug Metab. Toxicol. 2010, 6, 863–871. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.; Caffe, S.E.; Michalak, R.A.; Reid, J.L. Losartan, an orally active angiotensin (AT1) receptor antagonist: A review of its efficacy and safety in essential hypertension. Pharmacol. Ther. 1997, 74, 181–194. [Google Scholar] [CrossRef]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Munch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Mori, I.; Dong, L.; Liu, B.; Kimura, Y. Fetal calf serum inhibits virus genome expression in Madin-Darby canine kidney cells persistently infected with influenza A virus. Med. Microbiol. Immunol. 2008, 197, 21–27. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.; Shurbaji, S. Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms. Int. J. Mol. Sci 2021, 22, 6703. [Google Scholar] [CrossRef]
- Hofmann, H.; Geier, M.; Marzi, A.; Krumbiegel, M.; Peipp, M.; Fey, G.H.; Gramberg, T.; Pohlmann, S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004, 319, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Lubel, J.S.; Herath, C.B.; Velkoska, E.; Casley, D.J.; Burrell, L.M.; Angus, P.W. Angiotensin converting enzyme 2 (ACE2) activity in fetal calf serum: Implications for cell culture research. Cytotechnology 2008, 58, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Trachtman, H.; Frymoyer, A.; Lewandowski, A.; Greenbaum, L.A.; Feig, D.I.; Gipson, D.S.; Warady, B.A.; Goebel, J.W.; Schwartz, G.J.; Lewis, K.; et al. Pharmacokinetics, Pharmacodynamics, and Safety of Lisinopril in Pediatric Kidney Transplant Patients: Implications for Starting Dose Selection. Clin. Pharm. 2015, 98, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.W.; Goldberg, M.R.; McCrea, J.B.; Lu, H.; Furtek, C.I.; Bjornsson, T.D. Pharmacokinetics of losartan, an angiotensin II receptor antagonist, and its active metabolite EXP3174 in humans. Clin. Pharm. 1995, 58, 641–649. [Google Scholar] [CrossRef]
- Stangier, J.; Su, C.A.; Roth, W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J. Int. Med. Res. 2000, 28, 149–167. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.M.; Schoemaker, R.C.; Cohen, A.F.; Luus, H.G.; Seibert-Grafe, M.; Rothig, H.J. Pharmacokinetics, pharmacodynamics and bioavailability of the ACE inhibitor ramipril. Eur. J. Clin. Pharmacol. 1995, 47, 513–518. [Google Scholar] [CrossRef]
- Richer, C.; Giroux, B.; Plouin, P.F.; Maarek, B.; Giudicelli, J.F. Captopril: Pharmacokinetics, antihypertensive and biological effects in hypertensive patients. Br. J. Clin. Pharmacol. 1984, 17, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Laeis, P.; Puchler, K.; Kirch, W. The pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interaction. J. Hypertens Suppl. 2001, 19, S21–S32. [Google Scholar] [CrossRef] [PubMed]
- Udupa, E.G.; Rao, N.M. Inhibition of angiotensin converting enzyme from sheep tissues by captopril, lisinopril and enalapril. Indian J. Biochem. Biophys. 1997, 34, 524–528. [Google Scholar]
- Le, M.T.; Pugsley, M.K.; Vauquelin, G.; Van Liefde, I. Molecular characterisation of the interactions between olmesartan and telmisartan and the human angiotensin II AT1 receptor. Br. J. Pharm. 2007, 151, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.H. Telmisartan Inhibits Nitric Oxide Production and Vessel Relaxation via Protein Phosphatase 2A-mediated Endothelial NO Synthase-Ser(1179) Dephosphorylation. J. Korean Med. Sci. 2019, 34, e266. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, S.; Kawai, T.; Yamamoto, K.; Yibin, H.; Yamamoto, H.; Kakino, A.; Takeshita, H.; Nozato, Y.; Fujimoto, T.; Hongyo, K.; et al. RAGE ligands stimulate angiotensin II type I receptor (AT1) via RAGE/AT1 complex on the cell membrane. Sci. Rep. 2021, 11, 5759. [Google Scholar] [CrossRef]
- Gayathri, E.; Punnagai, K.; Chellathai, D.D. Evaluation of Anticancer Activity of Olmesartan and Ramipril on A549 Cell Line. Biomed. Pharmcol. J. 2018, 11. [Google Scholar] [CrossRef]
- Ellinger, B.; Bojkova, D.; Zaliani, A.; Cinatl, J.; Claussen, C.; Westhaus, S.; Keminer, O.; Reinshagen, J.; Kuzikov, M.; Wolf, M.; et al. A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection. Sci. Data 2021, 8, 70. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, Y.; Gallagher, P.E.; Averill, D.B.; Tallant, E.A.; Brosnihan, K.B.; Ferrario, C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004, 43, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, M.A.; Valenzuela, R.; Garrido, P.; Labandeira, C.M.; Navarro, G.; Franco, R.; Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I. Experimental data using candesartan and captopril indicate no double-edged sword effect in COVID-19. Clin. Sci. 2021, 135, 465–481. [Google Scholar] [CrossRef]
- Hikmet, F.; Mear, L.; Edvinsson, A.; Micke, P.; Uhlen, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Alnajjar, R.; Mostafa, A.; Kandeil, A.; Al-Karmalawy, A.A. Molecular docking, molecular dynamics, and in vitro studies reveal the potential of angiotensin II receptor blockers to inhibit the COVID-19 main protease. Heliyon 2020, 6, e05641. [Google Scholar] [CrossRef]
- Wang, Y.; Tse, G.; Li, G.; Lip, G.Y.H.; Liu, T. ACE Inhibitors and Angiotensin II Receptor Blockers May Have Different Impact on Prognosis of COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2041. [Google Scholar] [CrossRef]
- Sisignano, M.; Angioni, C.; Park, C.K.; Meyer Dos Santos, S.; Jordan, H.; Kuzikov, M.; Liu, D.; Zinn, S.; Hohman, S.W.; Schreiber, Y.; et al. Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain. Proc. Natl. Acad. Sci. USA 2016, 113, 12544–12549. [Google Scholar] [CrossRef] [Green Version]
- Bentz, J.; O’Connor, M.P.; Bednarczyk, D.; Coleman, J.; Lee, C.; Palm, J.; Pak, Y.A.; Perloff, E.S.; Reyner, E.; Balimane, P.; et al. Variability in P-glycoprotein inhibitory potency (IC(5)(0)) using various in vitro experimental systems: Implications for universal digoxin drug-drug interaction risk assessment decision criteria. Drug Metab. Dispos. 2013, 41, 1347–1366. [Google Scholar] [CrossRef] [Green Version]
- Rothlin, R.P.; Vetulli, H.M.; Duarte, M.; Pelorosso, F.G. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev. Res. 2020, 81, 768–770. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients with Hypertension Hospitalized with COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Rossi, L.; Malagoli, A.; Biagi, A.; Zanni, A.; Sticozzi, C.; Comastri, G.; Pannone, L.; Gandolfi, S.; Vergara, P.; Villani, G.Q. Renin-angiotensin system inhibitors and mortality in patients with COVID-19. Infection 2020, 49, 287–294. [Google Scholar] [CrossRef]
- THE COVID-19 RISk and Treatments (CORIST) Collaboration. RAAS inhibitors are not associated with mortality in COVID-19 patients: Findings from an observational multicenter study in Italy and a meta-analysis of 19 studies. Vasc. Pharm. 2020, 135, 106805. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Touyz, R.M. A new look at the renin-angiotensin system-focusing on the vascular system. Peptides 2011, 32, 2141–2150. [Google Scholar] [CrossRef]
- Lemarie, C.A.; Schiffrin, E.L. The angiotensin II type 2 receptor in cardiovascular disease. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 19–31. [Google Scholar] [CrossRef]
- Schuster, F.; Huber, G.; Stolting, I.; Wing, E.E.; Saar, K.; Hubner, N.; Banks, W.A.; Raasch, W. Telmisartan prevents diet-induced obesity and preserves leptin transport across the blood-brain barrier in high-fat diet-fed mice. Pflug. Arch. Eur. J. Physiol. 2018, 470, 1673–1689. [Google Scholar] [CrossRef]
- Suwannasual, U.; Lucero, J.; Davis, G.; McDonald, J.D.; Lund, A.K. Mixed Vehicle Emissions Induces Angiotensin II and Cerebral Microvascular Angiotensin Receptor Expression in C57Bl/6 Mice and Promotes Alterations in Integrity in a Blood-Brain Barrier Coculture Model. Toxicol. Sci. 2019, 170, 525–535. [Google Scholar] [CrossRef]
- Martinez-Lopez, D.G.; Fahey, M.; Coburn, J. Responses of human endothelial cells to pathogenic and non-pathogenic Leptospira species. PLoS Negl. Trop. Dis. 2010, 4, e918. [Google Scholar] [CrossRef] [Green Version]
- Wildhaber, B.E.; Yang, H.; Haxhija, E.Q.; Spencer, A.U.; Teitelbaum, D.H. Intestinal intraepithelial lymphocyte derived angiotensin converting enzyme modulates epithelial cell apoptosis. Apoptosis 2005, 10, 1305–1315. [Google Scholar] [CrossRef]
- Howell, S.; Brewis, I.A.; Hooper, N.M.; Kenny, A.J.; Turner, A.J. Mosaic expression of membrane peptidases by confluent cultures of Caco-2 cells. FEBS Lett. 1993, 317, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sun, L.; Xiao, W.; Yang, H. Essential role of angiotensin receptors in the modulation of intestinal epithelial cell apoptosis. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 562–569. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernandez Garcia, C.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, B.; Ma, J.; Zhang, S. Safety profile of the antiviral drug remdesivir: An update. Biomed. Pharm. 2020, 130, 110532. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.P.K.; Dangerfield, T.L.; Taylor, D.W.; Johnson, K.A. Remdesivir is a delayed translocation inhibitor of SARS-CoV-2 replication. Mol. Cell 2021, 81, 1548–1552. [Google Scholar] [CrossRef]
- Takayama, K. In Vitro and Animal Models for SARS-CoV-2 research. Trends Pharm. Sci. 2020, 41, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Bafna, K.; White, K.; Harish, B.; Rosales, R.; Ramelot, T.A.; Acton, T.B.; Moreno, E.; Kehrer, T.; Miorin, L.; Royer, C.A.; et al. Hepatitis C Virus Drugs That Inhibit the SARS-CoV-2 Papain-Like Protease Synergize with Remdesivir to Suppress Viral Replication in Cell Culture. Cell Rep. 2021, 35, 109133. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reus, P.; Schneider, A.-K.; Ulshöfer, T.; Henke, M.; Bojkova, D.; Cinatl, J.; Ciesek, S.; Geisslinger, G.; Laux, V.; Grättinger, M.; et al. Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model. Life 2021, 11, 810. https://doi.org/10.3390/life11080810
Reus P, Schneider A-K, Ulshöfer T, Henke M, Bojkova D, Cinatl J, Ciesek S, Geisslinger G, Laux V, Grättinger M, et al. Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model. Life. 2021; 11(8):810. https://doi.org/10.3390/life11080810
Chicago/Turabian StyleReus, Philipp, Ann-Kathrin Schneider, Thomas Ulshöfer, Marina Henke, Denisa Bojkova, Jindrich Cinatl, Sandra Ciesek, Gerd Geisslinger, Volker Laux, Mira Grättinger, and et al. 2021. "Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model" Life 11, no. 8: 810. https://doi.org/10.3390/life11080810
APA StyleReus, P., Schneider, A.-K., Ulshöfer, T., Henke, M., Bojkova, D., Cinatl, J., Ciesek, S., Geisslinger, G., Laux, V., Grättinger, M., Gribbon, P., & Schiffmann, S. (2021). Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model. Life, 11(8), 810. https://doi.org/10.3390/life11080810





