Effects of Altitude on Chronic Obstructive Pulmonary Disease Patients: Risks and Care
Abstract
1. Introduction
2. Literature Search
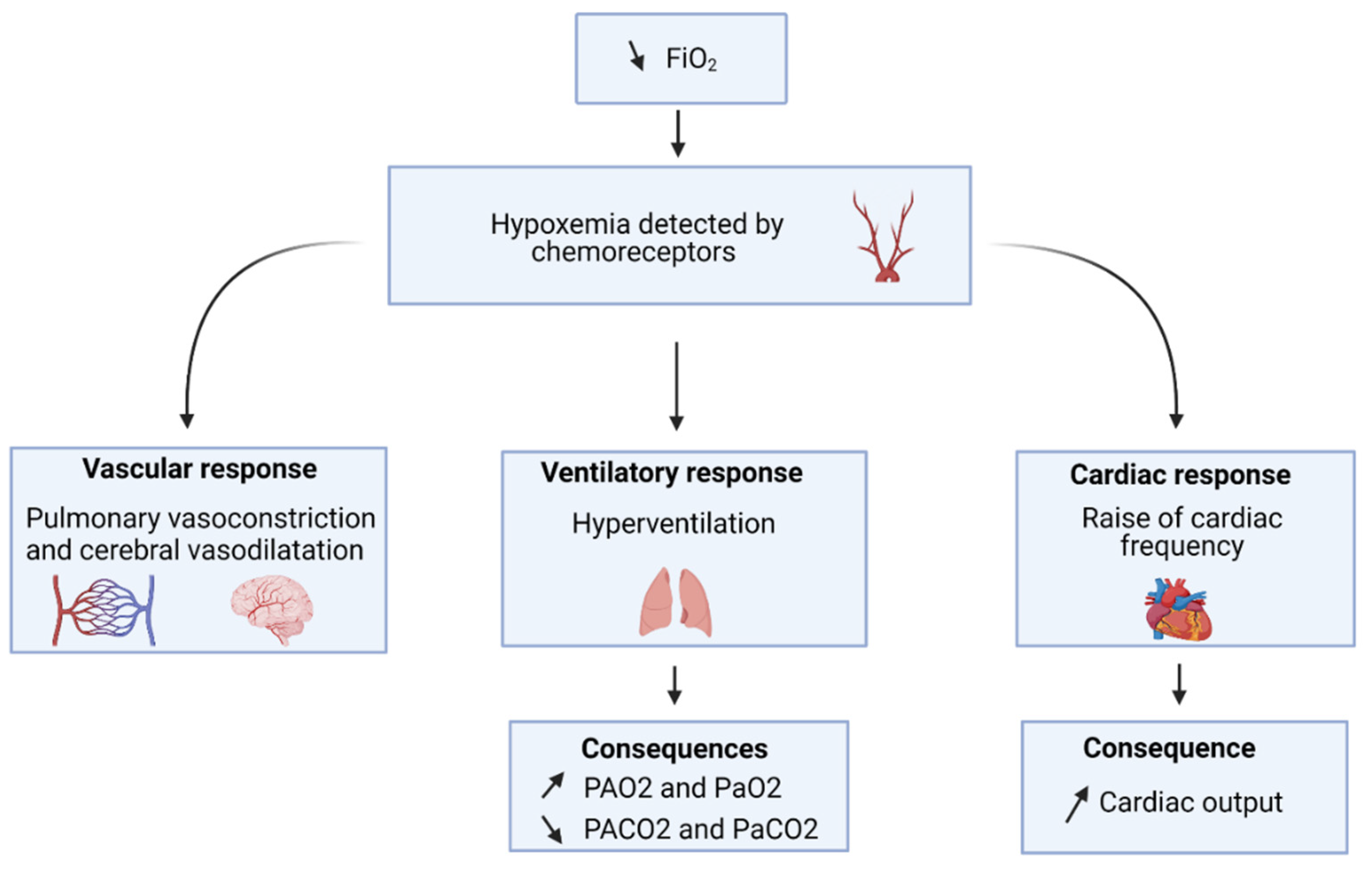
3. Pathophysiology of Altitude
4. Altitude-Hypoxemia Assessment in COPD Patients
4.1. Pulse Oximetry
4.2. Blood Gases
4.3. Pulmonary Function Testing
4.4. 6-min Walking Test
4.5. Equations
4.6. Hypoxic Challenge Testing
5. Others Causes of Dyspnea Induced by Altitude in COPD Patients
5.1. Respiratory Mechanism
5.2. Cardio-Vascular Adaptation
6. Other Considerations
6.1. Sleep
6.2. Venous Thromboembolism
7. Management of COPD Patient Wishing to Travel in Moderate-Altitude
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The World of Air Transport in 2019. Available online: https://www.icao.int/annual-report-2019/Pages/the-world-of-air-transport-in-2019.aspx (accessed on 6 July 2021).
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Varmaghani, M.; Dehghani, M.; Heidari, E.; Sharifi, F.; Moghaddam, S.S.; Farzadfar, F. Global Prevalence of Chronic Obstructive Pulmonary Disease: Systematic Review and Meta-Analysis. East. Mediterr. Health J. 2019, 25, 47–57. [Google Scholar] [CrossRef]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Furian, M.; Flueck, D.; Latshang, T.D.; Scheiwiller, P.M.; Segitz, S.D.; Mueller-Mottet, S.; Murer, C.; Steiner, A.; Ulrich, S.; Rothe, T.; et al. Exercise Performance and Symptoms in Lowlanders with COPD Ascending to Moderate Altitude: Randomized Trial. Int. J. COPD 2018, 13, 3529–3558. [Google Scholar] [CrossRef]
- Furian, M.; Hartmann, S.E.; Latshang, T.D.; Flueck, D.; Murer, C.; Scheiwiller, P.M.; Osmonov, B.; Ulrich, S.; Kohler, M.; Poulin, M.J.; et al. Exercise Performance of Lowlanders with COPD at 2590 m: Data from a Randomized Trial. Respiration 2018, 95, 422–432. [Google Scholar] [CrossRef]
- Ergan, B.; Arıkan, H.; Akgün, M. Are Pulmonologists Well Aware of Planning Safe Air Travel for Patients with COPD? The SAFCOP Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1895–1900. [Google Scholar] [CrossRef]
- West, J.B.; Luks, A. West’s Respiratory Physiology: The Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2021. [Google Scholar]
- Nishihara, F.; Shimada, H.; Saito, S. Rate Pressure Product and Oxygen Saturation in Tourists at Approximately 3000 m above Sea Level. Int. Arch. Occup. Environ. Health 1998, 71, 520–524. [Google Scholar] [CrossRef]
- Samaja, M. Blood Gas Transport at High Altitude. Respiration 1997, 64, 422–428. [Google Scholar] [CrossRef]
- Viscor, G.; Torrella, J.R.; Corral, L.; Ricart, A.; Javierre, C.; Pages, T.; Ventura, J.L. Physiological and Biological Responses to Short-Term Intermittent Hypobaric Hypoxia Exposure: From Sports and Mountain Medicine to New Biomedical Applications. Front. Physiol. 2018, 9, 814. [Google Scholar] [CrossRef]
- Silverman, D.; Gendreau, M. Medical Issues Associated with Commercial Flights. Lancet 2009, 373, 2067–2077. [Google Scholar] [CrossRef]
- IATA Medical Manual, 12th ed.; IATA: Montreal, QC, Canada, 2020; 102p, Available online: https://www.medicaltourismtraining.com/wp-content/uploads/2020/09/IATA-Medical-Manual-2020.pdf (accessed on 8 July 2021).
- Aerospace Medical Association; Aviation Safety Committee. Civil Aviation Subcommittee Cabin Cruising Altitudes for Regular Transport Aircraft. Aviat. Space Environ. Med. 2008, 79, 433–439. [Google Scholar] [CrossRef]
- Akerø, A.; Christensen, C.C.; Edvardsen, A.; Ryg, M.; Skjønsberg, O.H. Pulse Oximetry in the Preflight Evaluation of Patients with Chronic Obstructive Pulmonary Disease. Aviat. Space Environ. Med. 2008, 79, 518–524. [Google Scholar] [CrossRef]
- Robson, A.G.; Lenney, J.; Innes, J.A. Using Laboratory Measurements to Predict In-Flight Desaturation in Respiratory Patients: Are Current Guidelines Appropriate? Respir. Med. 2008, 102, 1592–1597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ling, I.T.; Singh, B.; James, A.L.; Hillman, D.R. Vital Capacity and Oxygen Saturation at Rest and after Exercise Predict Hypoxaemia during Hypoxic Inhalation Test in Patients with Respiratory Disease. Respirology 2013, 18, 507–513. [Google Scholar] [CrossRef]
- Christensen, C.C.; Ryg, M.; Refvem, O.K.; Skjùnsberg, O.H. Development of Severe Hypoxaemia in Chronic Obstructive Pulmonary Disease Patients at 2438 m (8000 Ft) Altitude. Eur. Respir. J. 2009, 15, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Akerø, A.; Christensen, C.C.; Edvardsen, A.; Skjønsberg, O.H. Hypoxaemia in Chronic Obstructive Pulmonary Disease Patients during a Commercial Flight. Eur. Respir. J. 2005, 25, 725–730. [Google Scholar] [CrossRef]
- Kelly, P.T.; Swanney, M.P.; Stanton, J.D.; Frampton, C.; Peters, M.J.; Beckert, L.E. Resting and Exercise Response to Altitude in Patients with Chronic Obstructive Pulmonary Disease. Aviat. Space Environ. Med. 2009, 80, 102–107. [Google Scholar] [CrossRef]
- Dellweg, D. Impact of Hypobaric Flight Simulation on Walking Distance and Oxygenation in COPD Patients. Respir. Physiol. 2008, 79, 518–524. [Google Scholar] [CrossRef]
- ATS. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef]
- Chetta, A.; Castagnetti, C.; Aiello, M.; Sergio, F.; Fabiano, N.; Tzani, P.; Marangio, E.; Olivieri, D. Walking Capacity and Fitness to Fly in Patients with Chronic Respiratory Disease. Aviat. Space Environ. Med. 2007, 78, 789–792. [Google Scholar]
- Edvardsen, A.; Akerø, A.; Christensen, C.C.; Ryg, M.; Skjønsberg, O.H. Air Travel and Chronic Obstructive Pulmonary Disease: A New Algorithm for Pre-FLight Evaluation. Thorax 2012, 67, 964–969. [Google Scholar] [CrossRef]
- Dillard, T.A.; Rosenberg, A.P.; Berg, B.W. Hypoxemia During Altitude Exposure: A Meta-Analysis of Chronic Obstructive Pulmonary Disease. Chest 1993, 103, 422–425. [Google Scholar] [CrossRef][Green Version]
- Bradi, A.C.; Faughnan, M.E.; Stanbrook, M.B.; Rrt, E.D.-L.; Chapman, K.R. Predicting the Need for Supplemental Oxygen during Airline Flight in Patients with Chronic Pulmonary Disease: A Comparison of Predictive Equations and Altitude Simulation. Can. Respir. J. 2009, 16, 119–124. [Google Scholar] [CrossRef]
- Martin, S.E.; Bradley, J.M.; Buick, J.B.; Bradbury, I.; Elborn, J.S. Flight Assessment in Patients with Respiratory Disease: Hypoxic Challenge Testing vs. Predictive Equations. QJM 2007, 100, 361–367. [Google Scholar] [CrossRef]
- Gong, H.; Tashkin, D.P.; Lee, E.Y.; Simmons, M.S. Hypoxia-Altitude Simulation Test. Am. Rev. Respir. Dis. 1984, 130, 980–986. [Google Scholar] [CrossRef]
- Dillard, T.A.; Moores, L.K.; Bilello, K.L.; Phillips, Y.Y. The Preflight Evaluation. A Comparison of the Hypoxia Inhalation Test with Hypobaric Exposure. Chest 1995, 107, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; Swanney, M.P.; Frampton, C.; Seccombe, L.M.; Peters, M.J.; Beckert, L.E. Normobaric Hypoxia Inhalation Test vs. Response to Airline Flight in Healthy Passengers. Aviat. Space Environ. Med. 2006, 77, 1143–1147. [Google Scholar]
- Ahmedzai, S.; Balfour-Lynn, I.M.; Bewick, T.; Buchdahl, R.; Coker, R.K.; Cummin, A.R.; Gradwell, D.P.; Howard, L.; Innes, J.A.; Johnson, A.O.C.; et al. Managing Passengers with Stable Respiratory Disease Planning Air Travel: British Thoracic Society Recommendations. Thorax 2011, 66, i1–i30. [Google Scholar] [CrossRef]
- Conférences d’experts. Voyages aériens et maladies respiratoires. Rev. Mal. Respir. 2007, 24 (Suppl. S3), 71. [Google Scholar] [CrossRef]
- Ergan, B.; Akgun, M.; Pacilli, A.M.G.; Nava, S. Should I Stay or Should I Go? COPD and Air Travel. Eur. Respir. Rev. 2018, 27, 180030. [Google Scholar] [CrossRef]
- Edvardsen, A. High Prevalence of Respiratory Symptoms during Air Travel in Patients with COPD. Respir. Med. 2011, 105, 7. [Google Scholar] [CrossRef][Green Version]
- Edvardsen, A.; Ryg, M.; Akerø, A.; Christensen, C.C.; Skjønsberg, O.H. COPD and Air Travel: Does Hypoxia-Altitude Simulation Testing Predict in-Flight Respiratory Symptoms? Eur. Respir. J. 2013, 42, 1216–1223. [Google Scholar] [CrossRef]
- Dillard, T.A.; Rajagopal, K.R.; Slivka, W.A.; Berg, B.W.; Mehm, W.J.; Lawless, N.P. Lung Function during Moderate Hypobaric Hypoxia in Normal Subjects and Patients with Chronic Obstructive Pulmonary Disease. Aviat. Space Environ. Med. 1998, 69, 979–985. [Google Scholar]
- Harding, R.M.; Mills, F.J. Aviation Medicine. Problems of Altitude I: Hypoxia and Hyperventilation. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1408–1410. [Google Scholar] [CrossRef]
- Casaburi, R.; Rennard, S.I. Exercise Limitation in Chronic Obstructive Pulmonary Disease. The O’Donnell Threshold. Am. J. Respir. Crit. Care Med. 2015, 191, 873–875. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R. Travel to High Altitude with Pre-Existing Lung Disease. Eur. Respir. J. 2007, 29, 770–792. [Google Scholar] [CrossRef]
- Harding, R.M.; Mills, F.J. Problems of Altitude. II: Decompression Sickness and Other Effects of Pressure Changes. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1498–1500. [Google Scholar] [CrossRef][Green Version]
- Stream, J.O.; Luks, A.M.; Grissom, C.K. Lung Disease at High Altitude. Expert Rev. Respir. Med. 2009, 3, 635–650. [Google Scholar] [CrossRef]
- Thabut, G.; Dauriat, G.; Stern, J.B.; Logeart, D.; Lévy, A.; Marrash-Chahla, R.; Mal, H. Pulmonary Hemodynamics in Advanced COPD Candidates for Lung Volume Reduction Surgery or Lung Transplantation. Chest 2005, 127, 1531–1536. [Google Scholar] [CrossRef]
- Lichtblau, M.; Latshang, T.D.; Furian, M.; Müller-Mottet, S.; Küest, S.; Tanner, F.; Grünig, E.; Bloch, K.E.; Ulrich, S. Right and Left Heart Function in Lowlanders with COPD at Altitude: Data from a Randomized Study. Respiration 2019, 97, 125–134. [Google Scholar] [CrossRef]
- Lichtblau, M.; Furian, M.; Aeschbacher, S.S.; Bisang, M.; Sheraliev, U.; Mademilov, M.; Marazhapov, N.H.; Ulrich, S.; Sooronbaev, T.; Bloch, K.E.; et al. Right-to-Left Shunts in Lowlanders with COPD Traveling to Altitude: A Randomized Controlled Trial with Dexamethasone. J. Appl. Physiol. 2020, 128, 117–126. [Google Scholar] [CrossRef]
- Bärtsch, P.; Gibbs, J.S.R. Effect of Altitude on the Heart and the Lungs. Circulation 2007, 116, 2191–2202. [Google Scholar] [CrossRef]
- Roversi, S.; Fabbri, L.M.; Sin, D.D.; Hawkins, N.M.; Agustí, A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. Am. J. Respir. Crit. Care Med. 2016, 194, 1319–1336. [Google Scholar] [CrossRef]
- Rossi, V.A.; Schmied, C.; Niebauer, J.; Niederseer, D. Cardiovascular Effects and Risks of Recreational Alpine Skiing in the Elderly. J. Sci. Med. Sport 2019, 22 (Suppl. S1), S27–S33. [Google Scholar] [CrossRef]
- Schmid, J.-P.; Noveanu, M.; Gaillet, R.; Hellige, G.; Wahl, A.; Saner, H. Safety and Exercise Tolerance of Acute High Altitude Exposure (3454 m) among Patients with Coronary Artery Disease. Heart 2006, 92, 921–925. [Google Scholar] [CrossRef]
- Wyss, C.A.; Koepfli, P.; Fretz, G.; Seebauer, M.; Schirlo, C.; Kaufmann, P.A. Influence of Altitude Exposure on Coronary Flow Reserve. Circulation 2003, 108, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, G.; Fantoni, C.; Anzà, C.; Cattaneo, P.; Rossi, A.; Montenero, A.S.; Baravelli, M. Arrhythmic Risk Evaluation during Exercise at High Altitude in Healthy Subjects: Role of Microvolt T-Wave Alternans. Pacing Clin. Electrophysiol. 2008, 31, 1277–1283. [Google Scholar] [CrossRef]
- Bisang, M.; Latshang, T.D.; Aeschbacher, S.S.; Huber, F.; Flueck, D.; Lichtblau, M.; Ulrich, S.; Hasler, E.D.; Scheiwiller, P.M.; Ulrich, S.; et al. Nocturnal Heart Rate and Cardiac Repolarization in Lowlanders with Chronic Obstructive Pulmonary Disease at High Altitude: Data from a Randomized, Placebo-Controlled Trial of Nocturnal Oxygen Therapy. Front. Med. 2021, 8, 557369. [Google Scholar] [CrossRef]
- Carta, A.F.; Bitos, K.; Furian, M.; Mademilov, M.; Sheraliev, U.; Marazhapov, N.H.; Lichtblau, M.; Schneider, S.R.; Sooronbaev, T.; Bloch, K.E.; et al. ECG Changes at Rest and during Exercise in Lowlanders with COPD Travelling to 3100 m. Int. J. Cardiol. 2021, 324, 173–179. [Google Scholar] [CrossRef]
- Nussbaumer-Ochsner, Y.; Schuepfer, N.; Siebenmann, C.; Maggiorini, M.; Bloch, K.E. High Altitude Sleep Disturbances Monitored by Actigraphy and Polysomnography. High Alt. Med. Biol. 2011, 12, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Latshang, T.D.; Lo Cascio, C.M.; Stöwhas, A.-C.; Grimm, M.; Stadelmann, K.; Tesler, N.; Achermann, P.; Huber, R.; Kohler, M.; Bloch, K.E. Are Nocturnal Breathing, Sleep, and Cognitive Performance Impaired at Moderate Altitude (1630–2590 m)? Sleep 2013, 36, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Latshang, T.D.; Tardent, R.P.M.; Furian, M.; Flueck, D.; Segitz, S.D.; Mueller-Mottet, S.; Kohler, M.; Ulrich, S.; Bloch, K.E. Sleep and Breathing Disturbances in Patients with Chronic Obstructive Pulmonary Disease Traveling to Altitude: A Randomized Trial. Sleep 2019, 42, zsy203. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; Andreas, S.; Arzt, M.; Bloch, K.E.; Brack, T.; Buyse, B.; De Backer, W.; Eckert, D.J.; Grote, L.; et al. Definition, Discrimination, Diagnosis and Treatment of Central Breathing Disturbances during Sleep. Eur. Respir. J. 2017, 49, 1600959. [Google Scholar] [CrossRef]
- Tan, L.; Latshang, T.D.; Aeschbacher, S.S.; Huber, F.; Flueck, D.; Lichtblau, M.; Ulrich, S.; Hasler, E.D.; Scheiwiller, P.M.; Ulrich, S.; et al. Effect of Nocturnal Oxygen Therapy on Nocturnal Hypoxemia and Sleep Apnea Among Patients with Chronic Obstructive Pulmonary Disease Traveling to 2048 Meters: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e207940. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.C.; Jha, S.K.; Saha, A.; Sharma, V.; Adya, C.M. Thrombosis as a Complication of Extended Stay at High Altitude. Natl. Med. J. India 2001, 14, 197–201. [Google Scholar]
- Hodkinson, P.D.; Hunt, B.J.; Parmar, K.; Ernsting, J. Is Mild Normobaric Hypoxia a Risk Factor for Venous Thromboembolism? J. Thromb. Haemost. 2003, 1, 2131–2133. [Google Scholar] [CrossRef]
- Sawka, M.N.; Convertino, V.A.; Eichner, E.R.; Schnieder, S.M.; Young, A.J. Blood Volume: Importance and Adaptations to Exercise Training, Environmental Stresses, and Trauma/Sickness. Med. Sci. Sports Exerc. 2000, 32, 332–348. [Google Scholar] [CrossRef]
- Ge, R.-L.; Witkowski, S.; Zhang, Y.; Alfrey, C.; Sivieri, M.; Karlsen, T.; Resaland, G.K.; Harber, M.; Stray-Gundersen, J.; Levine, B.D. Determinants of Erythropoietin Release in Response to Short-Term Hypobaric Hypoxia. J. Appl. Physiol. 2002, 92, 2361–2367. [Google Scholar] [CrossRef]
- Lapostolle, F.; Surget, V.; Borron, S.W.; Desmaizières, M.; Sordelet, D.; Lapandry, C.; Cupa, M.; Adnet, F. Severe Pulmonary Embolism Associated with Air Travel. N. Engl. J. Med. 2001, 345, 779–783. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, E.; Jiménez, D.; Díaz, G.; Pérez-Walton, I.; Luque, M.; Guillén, C.; Mañas, E.; Yusen, R.D. Incidence of Air Travel-Related Pulmonary Embolism at the Madrid-Barajas Airport. Arch. Intern. Med. 2003, 163, 2766–2770. [Google Scholar] [CrossRef]
- Schwarz, T.; Siegert, G.; Oettler, W.; Halbritter, K.; Beyer, J.; Frommhold, R.; Gehrisch, S.; Lenz, F.; Kuhlisch, E.; Schroeder, H.-E.; et al. Venous Thrombosis after Long-Haul Flights. Arch. Intern. Med. 2003, 163, 2759–2764. [Google Scholar] [CrossRef]
- Schwarz, T.; Langenberg, K.; Oettler, W.; Halbritter, K.; Beyer, J.; Siegert, G.; Gehrisch, S.; Schroeder, H.E.; Schellong, S.M. Deep Vein and Isolated Calf Muscle Vein Thrombosis Following Long-Haul Flights: Pilot Study. Blood Coagul. Fibrinolysis 2002, 13, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Chevallier, T.; Chapelier, A.; Baudouy, M. Travel as a Risk Factor for Venous Thromboembolic Disease: A Case-Control Study. Chest 1999, 115, 440–444. [Google Scholar] [CrossRef]
- Mercer, A.; Brown, J.D. Venous Thromboembolism Associated with Air Travel: A Report of 33 Patients. Aviat. Space Environ. Med. 1998, 69, 154–157. [Google Scholar] [PubMed]
- Lenferink, A.; Brusse-Keizer, M.; van der Valk, P.D.; Frith, P.A.; Zwerink, M.; Monninkhof, E.M.; van der Palen, J.; Effing, T.W. Self-Management Interventions Including Action Plans for Exacerbations versus Usual Care in Patients with Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 2017, 8, CD011682. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Flüge, T.; Gerken, F.; Hamilton, A.; Webb, K.; Aguilaniu, B.; Make, B.; Magnussen, H. Effects of Tiotropium on Lung Hyperinflation, Dyspnoea and Exercise Tolerance in COPD. Eur. Respir. J. 2004, 23, 832–840. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Sciurba, F.; Celli, B.; Mahler, D.A.; Webb, K.A.; Kalberg, C.J.; Knobil, K. Effect of Fluticasone Propionate/Salmeterol on Lung Hyperinflation and Exercise Endurance in COPD. Chest 2006, 130, 647–656. [Google Scholar] [CrossRef]
- Melani, A.S.; Bonavia, M.; Cilenti, V.; Cinti, C.; Lodi, M.; Martucci, P.; Serra, M.; Scichilone, N.; Sestini, P.; Aliani, M.; et al. Inhaler Mishandling Remains Common in Real Life and Is Associated with Reduced Disease Control. Respir. Med. 2011, 105, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Lichtblau, M.; Furian, M.; Aeschbacher, S.S.; Bisang, M.; Ulrich, S.; Saxer, S.; Sheraliev, U.; Marazhapov, N.H.; Osmonov, B.; Estebesova, B.; et al. Dexamethasone Improves Pulmonary Hemodynamics in COPD-Patients Going to Altitude: A Randomized Trial. Int. J. Cardiol. 2019, 283, 159–164. [Google Scholar] [CrossRef]
- Furian, M.; Lichtblau, M.; Aeschbacher, S.S.; Estebesova, B.; Emilov, B.; Sheraliev, U.; Marazhapov, N.H.; Mademilov, M.; Osmonov, B.; Bisang, M.; et al. Effect of Dexamethasone on Nocturnal Oxygenation in Lowlanders with Chronic Obstructive Pulmonary Disease Traveling to 3100 Meters: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e190067. [Google Scholar] [CrossRef]
- Grissom, C.K.; Roach, R.C.; Sarnquist, F.H.; Hackett, P.H. Acetazolamide in the Treatment of Acute Mountain Sickness: Clinical Efficacy and Effect on Gas Exchange. Ann. Intern. Med. 1992, 116, 461–465. [Google Scholar] [CrossRef]
- Swenson, E.R.; Hughes, J.M. Effects of Acute and Chronic Acetazolamide on Resting Ventilation and Ventilatory Responses in Men. J. Appl. Physiol. 1993, 74, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, R.S.; Cain, S.M. Effects of Acetazolamide and Hypoxia on Cerebrospinal Fluid Bicarbonate. J. Appl. Physiol. 1968, 24, 17–20. [Google Scholar] [CrossRef]
- Adamson, R.; Swenson, E.R. Acetazolamide Use in Severe Chronic Obstructive Pulmonary Disease. Pros and Cons. Ann. Am. Thorac. Soc. 2017, 14, 1086–1093. [Google Scholar] [CrossRef]
- Swenson, E.R.; Robertson, H.T.; Hlastala, M.P. Effects of Carbonic Anhydrase Inhibition on Ventilation-Perfusion Matching in the Dog Lung. J. Clin. Invest. 1993, 92, 702–709. [Google Scholar] [CrossRef]
- Teppema, L.J.; Rochette, F.; Demedts, M. Ventilatory Effects of Acetazolamide in Cats during Hypoxemia. J. Appl. Physiol. 1992, 72, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.U.; Scheuermann, B.W. Effect of Acetazolamide on Respiratory Muscle Fatigue in Humans. Respir. Physiol. Neurobiol. 2013, 185, 386–392. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 2015, 23, CD003793. [Google Scholar] [CrossRef]
- Farkas, G.A.; Roussos, C. Diaphragm in Emphysematous Hamsters: Sarcomere Adaptability. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 1635–1640. [Google Scholar] [CrossRef]
- Levine, S.; Kaiser, L.; Leferovich, J.; Tikunov, B. Cellular Adaptations in the Diaphragm in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 1997, 337, 1799–1806. [Google Scholar] [CrossRef]
- Clanton, T.L.; Levine, S. Respiratory Muscle Fiber Remodeling in Chronic Hyperinflation: Dysfunction or Adaptation? J. Appl. Physiol. 2009, 107, 324–335. [Google Scholar] [CrossRef][Green Version]
- Geddes, E.L.; O’Brien, K.; Reid, W.D.; Brooks, D.; Crowe, J. Inspiratory Muscle Training in Adults with Chronic Obstructive Pulmonary Disease: An Update of a Systematic Review. Respir. Med. 2008, 102, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.; De Vos, J.; van den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of Inspiratory Muscle Training in Patients with COPD: What Is the Evidence? Eur. Respir. J. 2011, 37, 416–425. [Google Scholar] [CrossRef]
- Lomax, M. Inspiratory Muscle Training, Altitude, and Arterial Oxygen Desaturation: A Preliminary Investigation. Aviat. Space Environ. Med. 2010, 81, 498–501. [Google Scholar] [CrossRef]
- EFA Booklet: Enabling Air Travel with Oxygen in Europe. Available online: https://www.efanet.org/images/2015/20151118_EFA_Booklet_Enabling_Air_Travel_with_Oxygen_Updated_2015_FINAL.pdf (accessed on 8 July 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georges, T.; Le Blanc, C.; Ferreol, S.; Menu, P.; Dauty, M.; Fouasson-Chailloux, A. Effects of Altitude on Chronic Obstructive Pulmonary Disease Patients: Risks and Care. Life 2021, 11, 798. https://doi.org/10.3390/life11080798
Georges T, Le Blanc C, Ferreol S, Menu P, Dauty M, Fouasson-Chailloux A. Effects of Altitude on Chronic Obstructive Pulmonary Disease Patients: Risks and Care. Life. 2021; 11(8):798. https://doi.org/10.3390/life11080798
Chicago/Turabian StyleGeorges, Thomas, Camille Le Blanc, Sophie Ferreol, Pierre Menu, Marc Dauty, and Alban Fouasson-Chailloux. 2021. "Effects of Altitude on Chronic Obstructive Pulmonary Disease Patients: Risks and Care" Life 11, no. 8: 798. https://doi.org/10.3390/life11080798
APA StyleGeorges, T., Le Blanc, C., Ferreol, S., Menu, P., Dauty, M., & Fouasson-Chailloux, A. (2021). Effects of Altitude on Chronic Obstructive Pulmonary Disease Patients: Risks and Care. Life, 11(8), 798. https://doi.org/10.3390/life11080798







