Did Solid Surfaces Enable the Origin of Life?
Abstract
:1. Introduction
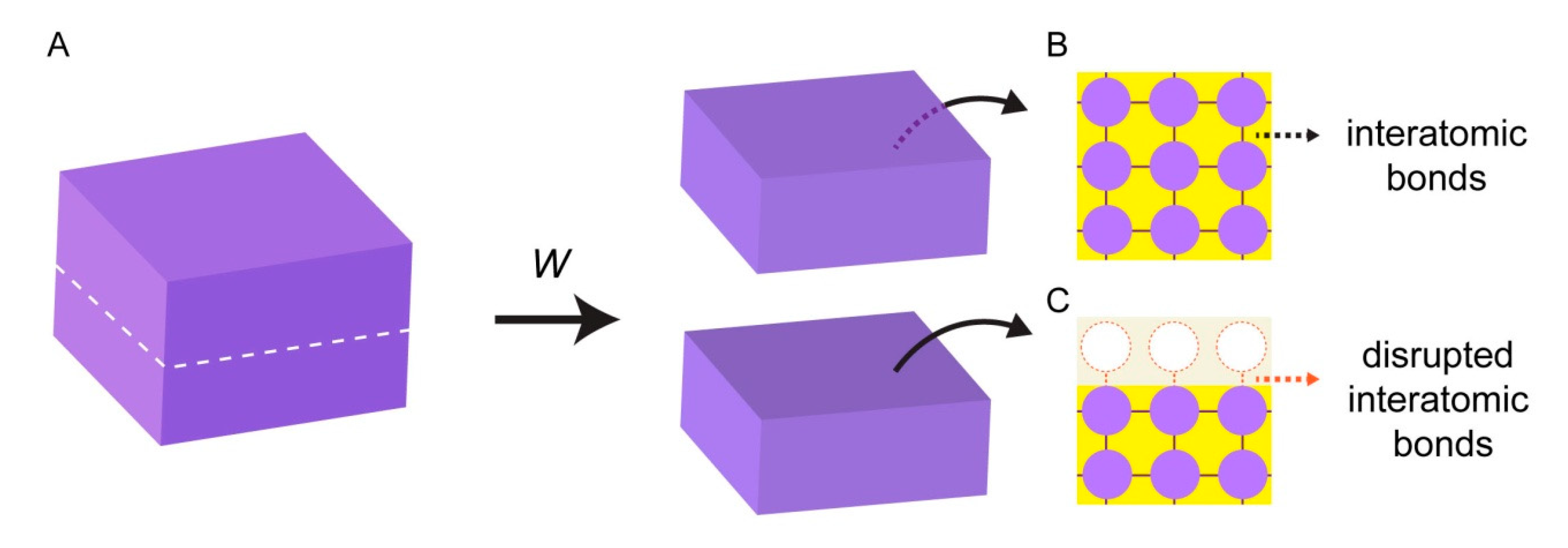
2. Intrinsic Energy of Surfaces
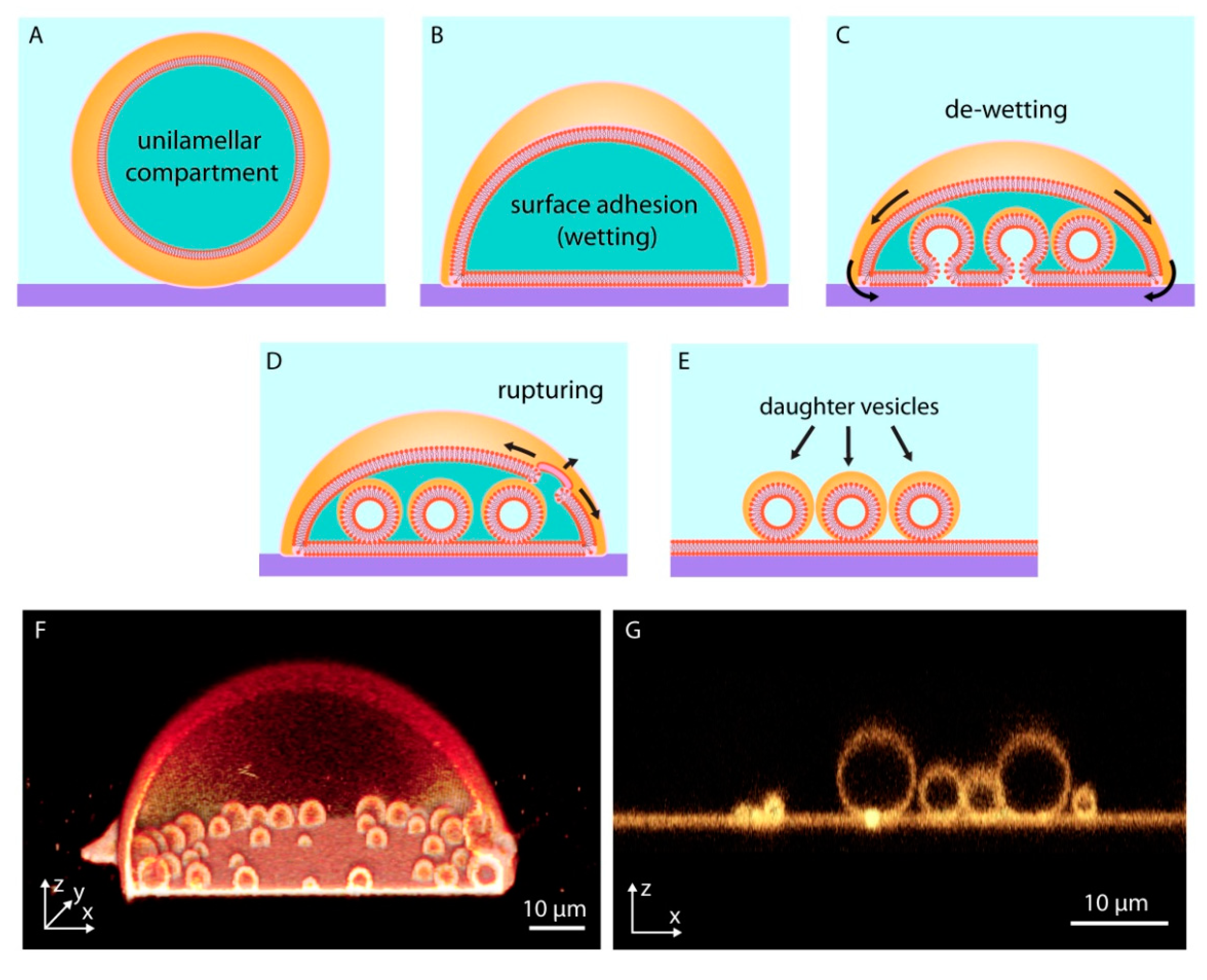
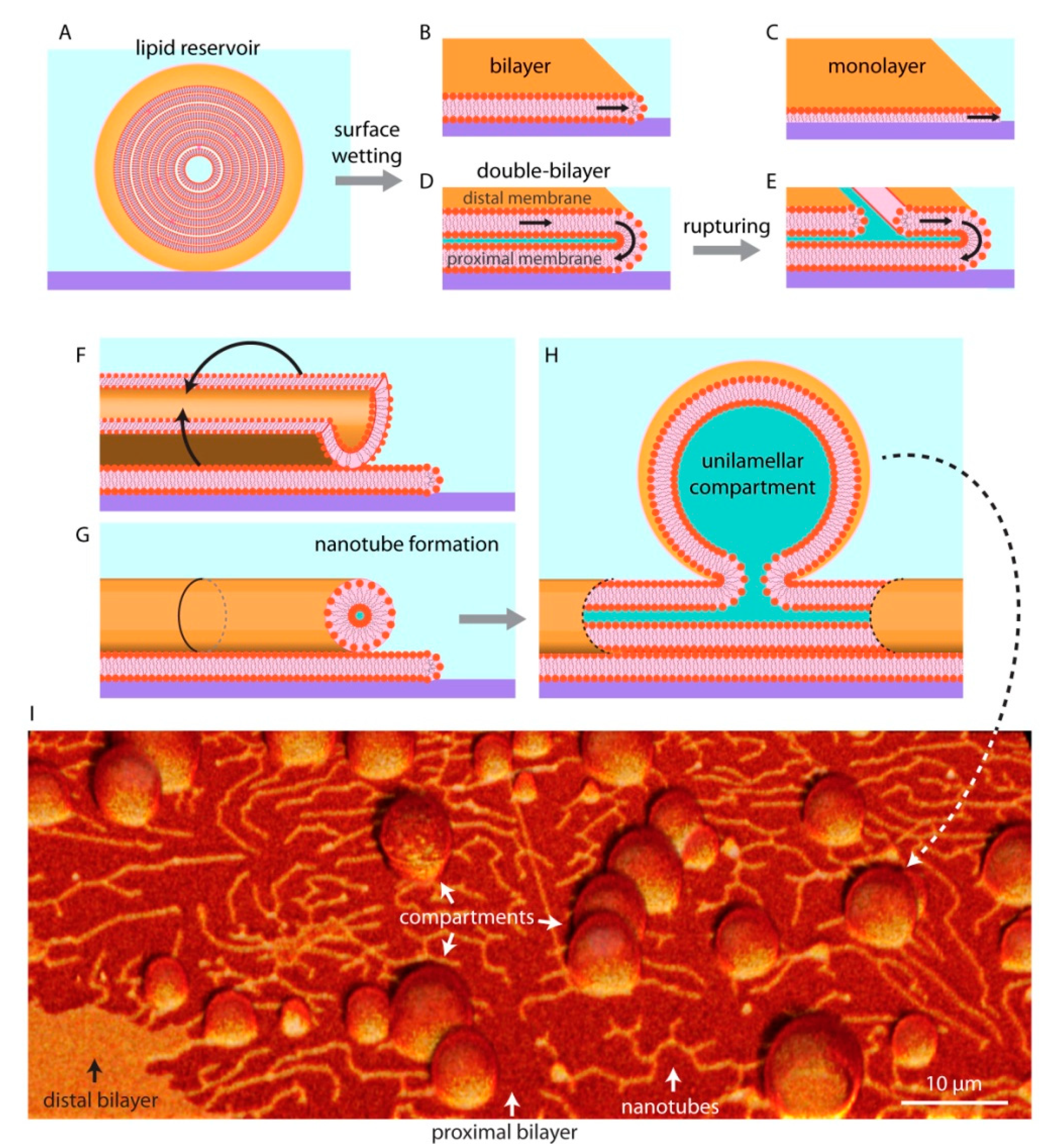
3. Biomembrane Transformations on Solid Surfaces
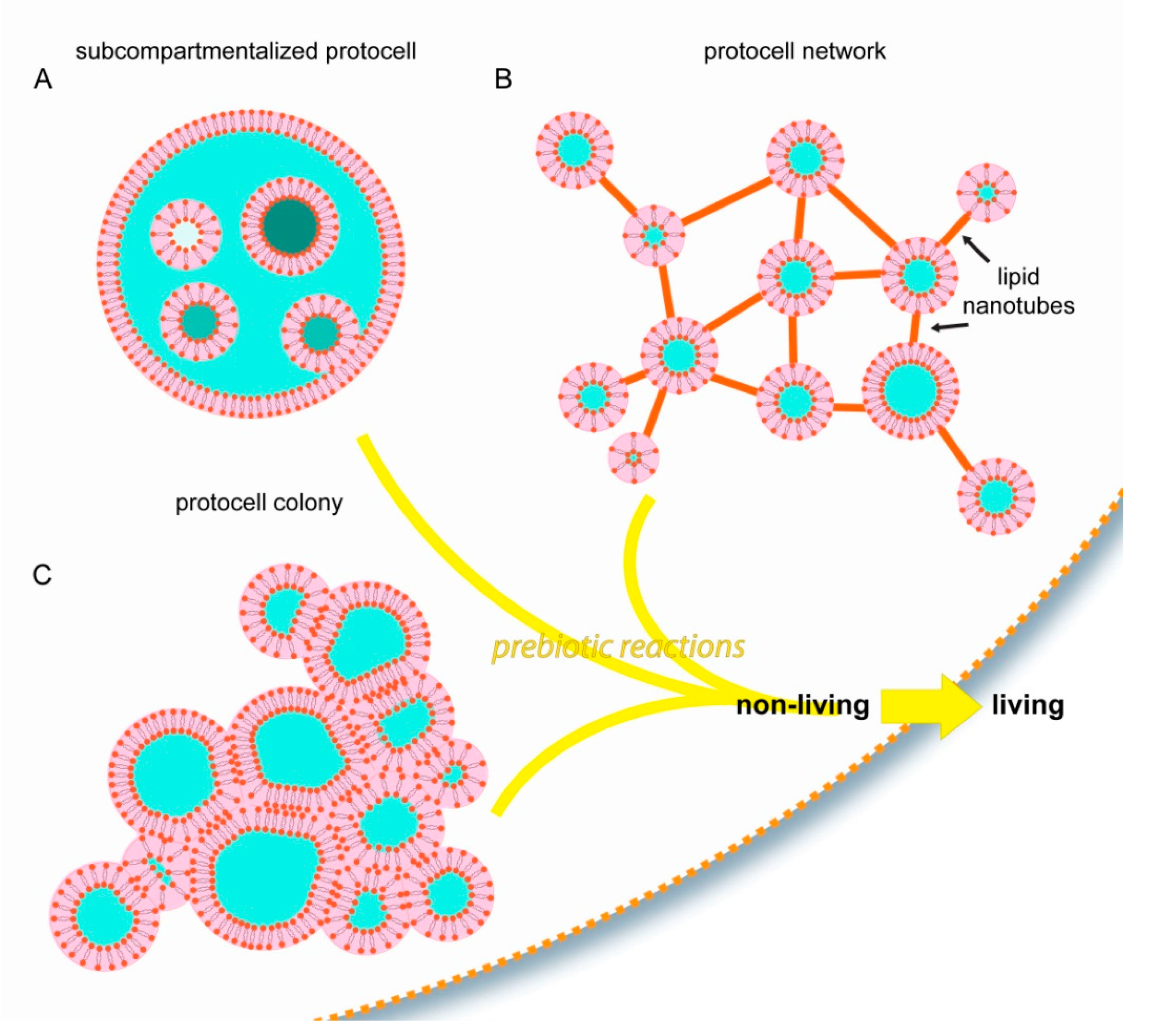
4. Possible Implications of Self-Forming Surface-Based Protocells for the Origin of Life
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Poudyal, R.R.; Pir Cakmak, F.; Keating, C.D.; Bevilacqua, P.C. Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry. Biochemistry 2018, 57, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K.; Cleaves, H.J. Membraneless polyester microdroplets as primordial compartments at the origins of life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, M.; Lipiński, W.P.; Wang, J.; Spruijt, E. Peptide-based coacervates as biomimetic protocells. Chem. Soc. Rev. 2021, 50, 3690–3705. [Google Scholar] [CrossRef] [PubMed]
- Donau, C.; Späth, F.; Sosson, M.; Kriebisch, B.A.K.; Schnitter, F.; Tena-Solsona, M.; Kang, H.-S.; Salibi, E.; Sattler, M.; Mutschler, H.; et al. Active coacervate droplets as a model for membraneless organelles and protocells. Nat. Commun. 2020, 11, 5167. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Walde, P. Current Ideas about Prebiological Compartmentalization. Life 2015, 5, 1239–1263. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Szostak, J.W. Lipid constituents of model protocell membranes. Emerg. Top. Life Sci. 2019, 3, 537–542. [Google Scholar] [PubMed]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of phosphatidylcholine under possible primitive Earth conditions. J. Mol. Evol. 1982, 18, 196–202. [Google Scholar] [CrossRef]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef]
- Dworkin, J.P.; Deamer, D.W.; Sandford, S.A.; Allamandola, L.J. Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. USA 2001, 98, 815. [Google Scholar] [CrossRef] [Green Version]
- Lawless, J.G.; Yuen, G.U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 1979, 282, 396–398. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Czolkos, I.; Jesorka, A.; Orwar, O. Molecular phospholipid films on solid supports. Soft Matter 2011, 7, 4562–4576. [Google Scholar] [CrossRef]
- Lewin, M.; Mey-Marom, A.; Frank, R. Surface free energies of polymeric materials, additives and minerals. Polym. Adv. Technol. 2005, 16, 429–441. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of cellular life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazen, R.M.; Ferry, J.M. Mineral Evolution: Mineralogy in the Fourth Dimension. Elements 2010, 6, 9–12. [Google Scholar] [CrossRef]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- James Cleaves, H., II; Michalkova Scott, A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral–organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brack, A. Chapter 10.4—Clay Minerals and the Origin of Life. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 507–521. [Google Scholar]
- Hazen, R.M.; Sverjensky, D.A. Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef] [Green Version]
- Kindt, J.T.; Szostak, J.W.; Wang, A. Bulk self-assembly of giant, unilamellar vesicles. ACS Nano 2020, 14, 14627–14634. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Strazewski, P. Prebiotic Lipidic Amphiphiles and Condensing Agents on the Early Earth. Life 2016, 6, 17. [Google Scholar] [CrossRef]
- Liu, L.; Zou, Y.; Bhattacharya, A.; Zhang, D.; Lang, S.Q.; Houk, K.N.; Devaraj, N.K. Enzyme-free synthesis of natural phospholipids in water. Nat. Chem. 2020, 12, 1029–1034. [Google Scholar] [CrossRef]
- Jõemetsa, S.; Spustova, K.; Kustanovich, K.; Ainla, A.; Schindler, S.; Eigler, S.; Lobovkina, T.; Lara-Avila, S.; Jesorka, A.; Gözen, I. Molecular Lipid Films on Microengineering Materials. Langmuir 2019, 35, 10286–10298. [Google Scholar] [CrossRef] [PubMed]
- Melcrová, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef] [PubMed]
- Lobovkina, T.; Gözen, I.; Erkan, Y.; Olofsson, J.; Weber, S.G.; Orwar, O. Protrusive growth and periodic contractile motion in surface-adhered vesicles induced by Ca2+-gradients. Soft Matter 2010, 6, 268–272. [Google Scholar] [CrossRef]
- Spustova, K.; Köksal, E.S.; Ainla, A.; Gözen, I. Subcompartmentalization and Pseudo-Division of Model Protocells. Small 2021, 17, 2005320. [Google Scholar] [CrossRef] [PubMed]
- Cremer, P.S.; Boxer, S.G. Formation and Spreading of Lipid Bilayers on Planar Glass Supports. J. Phys. Chem. B 1999, 103, 2554–2559. [Google Scholar] [CrossRef]
- Nissen, J.; Jacobs, K.; Rädler, J.O. Interface Dynamics of Lipid Membrane Spreading on Solid Surfaces. Phys. Rev. Lett. 2001, 86, 1904–1907. [Google Scholar] [CrossRef]
- Czolkos, I.; Guan, J.; Orwar, O.; Jesorka, A. Flow control of thermotropic lipid monolayers. Soft Matter 2011, 7, 6926–6933. [Google Scholar] [CrossRef]
- Raedler, J.; Strey, H.; Sackmann, E. Phenomenology and Kinetics of Lipid Bilayer Spreading on Hydrophilic Surfaces. Langmuir 1995, 11, 4539–4548. [Google Scholar] [CrossRef]
- Gözen, I.; Dommersnes, P.; Czolkos, I.; Jesorka, A.; Lobovkina, T.; Orwar, O. Fractal avalanche ruptures in biological membranes. Nat. Mater. 2010, 9, 908–912. [Google Scholar] [CrossRef]
- Köksal, E.S.; Liese, S.; Kantarci, I.; Olsson, R.; Carlson, A.; Gözen, I. Nanotube-Mediated Path to Protocell Formation. ACS Nano 2019, 13, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Karatekin, E.; Sandre, O.; Guitouni, H.; Borghi, N.; Puech, P.-H.; Brochard-Wyart, F. Cascades of Transient Pores in Giant Vesicles: Line Tension and Transport. Biophys. J. 2003, 84, 1734–1749. [Google Scholar] [CrossRef] [Green Version]
- Põldsalu, I.; Köksal, E.S.; Gözen, I. Mixed fatty acid-phospholipid protocell networks. BioRxiv 2021. [Google Scholar] [CrossRef]
- Köksal, E.S.; Liese, S.; Xue, L.; Ryskulov, R.; Viitala, L.; Carlson, A.; Gözen, I. Rapid Growth and Fusion of Protocells in Surface-Adhered Membrane Networks. Small 2020, 16, 2002569. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.S.; Põldsalu, I.; Friis, H.; Mojzsis, S.; Bizzarro, M.; Gözen, I. Spontaneous formation of prebiotic compartment colonies on Hadean Earth and pre-Noachian Mars. BioRxiv 2021. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The RNA World and the Origins of Life, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Gözen, İ. A Hypothesis for Protocell Division on the Early Earth. ACS Nano 2019, 13, 10869–10871. [Google Scholar] [CrossRef]
- Ganti, T. The Principles of Life; OUP Oxford: Oxford, UK, 2003; p. 201. [Google Scholar]
- Diekmann, Y.; Pereira-Leal, J.B. Evolution of intracellular compartmentalization. Biochem. J. 2012, 449, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, E.; Abreu, N.; Komeili, A. Compartmentalization and organelle formation in bacteria. Curr. Opin. Cell Biol. 2014, 26, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Seufferheld, M.J.; Kim, K.M.; Whitfield, J.; Valerio, A.; Caetano-Anollés, G. Evolution of vacuolar proton pyrophosphatase domains and volutin granules: Clues into the early evolutionary origin of the acidocalcisome. Biol. Direct 2011, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Tran, Q.P.; Adam, Z.R.; Fahrenbach, A.C. Prebiotic Reaction Networks in Water. Life 2020, 10, 352. [Google Scholar] [CrossRef]
- Carrara, P.; Stano, P.; Luisi, P.L. Giant Vesicles “Colonies”: A Model for Primitive Cell Communities. ChemBioChem 2012, 13, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, L.; Du, H.; Li, M.; Mu, W.; Drinkwater, B.W.; Han, X.; Mann, S. Chemical communication in spatially organized protocell colonies and protocell/living cell micro-arrays. Chem. Sci. 2019, 10, 9446–9453. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Mansy, S.S.; Szostak, J.W. Mineral Surface Directed Membrane Assembly. Orig. Life Evol. Biosph. 2007, 37, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Sahai, N.; Kaddour, H.; Dalai, P.; Wang, Z.; Bass, G.; Gao, M. Mineral Surface Chemistry and Nanoparticle-aggregation Control Membrane Self-Assembly. Sci. Rep. 2017, 7, 43418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The Lipid World. Orig. Life Evol. Biosph. 2001, 31, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gözen, İ. Did Solid Surfaces Enable the Origin of Life? Life 2021, 11, 795. https://doi.org/10.3390/life11080795
Gözen İ. Did Solid Surfaces Enable the Origin of Life? Life. 2021; 11(8):795. https://doi.org/10.3390/life11080795
Chicago/Turabian StyleGözen, İrep. 2021. "Did Solid Surfaces Enable the Origin of Life?" Life 11, no. 8: 795. https://doi.org/10.3390/life11080795
APA StyleGözen, İ. (2021). Did Solid Surfaces Enable the Origin of Life? Life, 11(8), 795. https://doi.org/10.3390/life11080795





