High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. The Relationship between hsCRP Level and Age, Gender, and Selected Anthropometric and Laboratory Parameters
3.2. The Relationship between the Concentration of Hscrp and the Occurrence of Selected Diseases, the Number of Elements of the Metabolic Syndrome, and the Number of Risk Factors for Cardiovascular Disease Was Observed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quispe, R.; Michos, E.D.; Martin, S.S.; Puri, R.; Toth, P.P.; Al Suwaidi, J.; Banach, M.; Virani, S.S.; Blumenthal, R.S.; Jones, S.R.; et al. High-Sensitivity C-Reactive Protein Discordance With Atherogenic Lipid Measures and Incidence of Atherosclerotic Cardiovascular Disease in Primary Prevention: The ARIC Study. J. Am. Hear. Assoc. 2020, 9, e013600. [Google Scholar] [CrossRef]
- Gallacher, J.E. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Kim-Mitsuyama, S.; Soejima, H.; Yasuda, O.; Node, K.; Jinnouchi, H.; Yamamoto, E.; Sekigami, T.; Ogawa, H.; Matsui, K. Reduction in hsCRP levels is associated with decreased incidence of cardiovascular events in Japanese hypertensive women but not in men. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int. J. Cardiol. 2013, 168, 5126–5134. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.-B.; Gao, P.-C.; Chen, Y.-Y.; Xia, Y.; Ke, X.-S.; Shao, X.-F.; Xiong, C.-X.; Chen, H.-S.; Xiao, H.; Ning, J.; et al. High-Sensitivity C-Reactive Protein Leads to Increased Incident Metabolic Syndrome in Women but Not in Men: A Five-Year Follow-Up Study in a Chinese Population. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adukauskienė, D.; Čiginskienė, A.; Adukauskaitė, A.; Pentiokinienė, D.; Šlapikas, R.; Ceponiene, I. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina 2016, 52, 1–10. [Google Scholar] [CrossRef]
- Salazar, J.; Martínez, M.S.; Chávez, M.; Toledo, A.; Añez, R.; Torres, Y.; Apruzzese, V.; Silva, C.; Rojas, J.; Bermúdez, V. C-Reactive Protein: Clinical and Epidemiological Perspectives. Cardiol. Res. Pr. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; de Lacerda, A.P. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev. Port. Cardiol. 2012, 31, 733–745. [Google Scholar] [CrossRef]
- Members, W.C.; Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: Executive sum-mary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010, 122, 2748–2764. [Google Scholar] [CrossRef]
- Araújo, J.P.; Lourenço, P.; Azevedo, A.; Friões, F.; Rocha-Gonçalves, F.; Ferreira, A.; Bettencourt, P. Prognostic Value of High-Sensitivity C-Reactive Protein in Heart Failure: A Systematic Review. J. Card. Fail. 2009, 15, 256–266. [Google Scholar] [CrossRef]
- Ridker, P.M.; Koenig, W.; Kastelein, J.J.; Mach, F.; Lüscher, T.F. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur. Hear. J. 2018, 39, 4109–4111. [Google Scholar] [CrossRef]
- Ridker, P.M.; Glynn, R.J.; Hennekens, C.H. C-Reactive Protein Adds to the Predictive Value of Total and HDL Cholesterol in Determining Risk of First Myocardial Infarction. Circulation 1998, 97, 2007–2011. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Yoshinaga, R.; Doi, Y.; Ayukawa, K.; Ishikawa, S. High-sensitivity C reactive protein as a predictor of inhospital mortality in patients with cardiovascular disease at an emergency department: A retrospective cohort study. BMJ Open 2017, 7, e015112. [Google Scholar] [CrossRef]
- Mazidi, M.; Toth, P.P.; Banach, M. C-reactive Protein Is Associated With Prevalence of the Metabolic Syndrome, Hypertension, and Diabetes Mellitus in US Adults. Angiology 2017, 69, 438–442. [Google Scholar] [CrossRef]
- Suhett, L.G.; Hermsdorff, H.H.M.; Rocha, N.P.; Silva, M.A.; Filgueiras, M.; Milagres, L.C.; Peluzio, M.D.C.G.; De Novaes, J.F. Increased C-Reactive Protein in Brazilian Children: Association with Cardiometabolic Risk and Metabolic Syndrome Components (PASE Study). Cardiol. Res. Pr. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Yang, S.K.; Kim, J.; Lee, D.-C. Association between C-Reactive Protein and Metabolic Syndrome in Korean Adults. Korean J. Fam. Med. 2019, 40, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, R.; Kusunoki, T.; Abe, M.; Kohara, K.; Miki, T. An association between body mass index and high-sensitivity C-reactive protein concentrations is influenced by age in community-dwelling persons. Ann. Clin. Biochem. Int. J. Lab. Med. 2013, 50, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Chen, Z.; Zhang, L.; Zhu, M. Distribution of High-Sensitivity C-Reactive Protein and Its Relationship with Other Cardiovascular Risk Factors in the Middle-Aged Chinese Population. Int. J. Environ. Res. Public Health 2016, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Chae, J.; Kang, R.; Kwon, N.; Lee, S.-H.; Lee, J. Effect of age on atherogenicity of LDL and inflammatory markers in healthy women. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 967–972. [Google Scholar] [CrossRef]
- McCabe, E.L.; Larson, M.G.; Lunetta, K.L.; Newman, A.B.; Cheng, S.; Murabito, J.M. Association of an Index of Healthy Aging With Incident Cardiovascular Disease and Mortality in a Community-Based Sample of Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Premanath, M.; Basavanagowdappa, H.; Mahesh, M.; Babu, M.S.; Devananda, D. Chronic sub-clinical inflammation in the abdominal adipose tissue—Evaluation of inflammatory cytokines and their link with insulin resistance in metabolically obese South Indians: Across-sectional observational study. Indian J. Endocrinol. Metab. 2016, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wener, M.H.; Daum, P.R.; McQuillan, G.M. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J. Rheumatol. 2000, 27, 2351–2359. [Google Scholar] [PubMed]
- McConnell, J.P.; Branum, E.L.; Ballman, K.V.; Lagerstedt, S.A.; Katzmann, J.A.; Jaffe, A.S. Gender Differences in C-Reactive Protein Concentrations—Confirmation with Two Sensitive Methods. Clin. Chem. Lab. Med. 2002, 40, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Krzesiński, P.; Hałas, K.; Gielerak, G.; Piotrowicz, K.; Stańczyk, A.; Piechota, W.; Jannasz, I.; Niedolaz, K.; Wojdat, M.; Skrobowski, A. Cardiovascular risk and inflammatory markers in patients with hypertension. Polski Merkur. Lek. Organ. Polskiego Towar. Lek. 2015, 38, 70–76. [Google Scholar]
- Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Bahari, T.; Ishizu, M.; Fujioka, M.; Arisawa, K. Relationships of serum high-sensitivity C-reactive protein and body size with insulin resistance in a Japanese cohort. PLoS ONE 2017, 12, e0178672. [Google Scholar] [CrossRef] [PubMed]
- Jeemon, P.; Prabhakaran, D.; Ramakrishnan, L.; Gupta, R.; Ahmed, F.; Thankappan, K.; Kartha, C.; Chaturvedi, V.; Reddy, K.; the Sentinel Surveillance in Industrial Populations Study Group. Association of high sensitive C-reactive protein (hsCRP) with established cardiovascular risk factors in the Indian population. Nutr. Metab. 2011, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Yuan, J.-Q.; Zhu, L.; Zhang, Y. High high-sensitivity C-reactive protein/BMI ratio predicts future adverse outcomes in patients with acute coronary syndrome. Coron. Artery Dis. 2019, 30, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Subasinghe, A.K.; on behalf of the YFHI and Safe-D Study Groups; Wark, J.D.; Gorelik, A.; Callegari, E.T.; Garland, S.M. The association between inflammation, obesity and elevated blood pressure in 16–25-year-old females. J. Hum. Hypertens. 2017, 31, 580–584. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Wang, P.-W.; Chen, T.-Y. The relationship between regional abdominal fat distribution and both insulin resistance and subclinical chronic inflammation in non-diabetic adults. Diabetol. Metab. Syndr. 2014, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Leiva, E.; Mujica, V.; Brito, K.; Palomo, I.; Orrego, R.; Vásquez, M.; Guzman, L.; Núñez, S.; Moore-Carrasco, R.; Díaz, N.; et al. High levels of hsCRP are associated with carbohydrate metabolism disorder. J. Clin. Lab. Anal. 2011, 25, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Koenig, W.; Baumert, J.; Döring, A. Uric Acid Levels Are Associated With All-Cause and Cardiovascular Disease Mortality Independent of Systemic Inflammation in Men From the General Population. Arter. Thromb. Vasc. Biol. 2008, 28, 1186–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.Y.; Liu, B.; Ji, Y.; Zhuang, X.J.; Shen, Y.D.; Tian, H.R.; Li, L.X.; Liu, F. Association between serum uric acid levels and high sensitive C-reactive protein in patients with type 2 diabetes. Zhonghua Yi Xue Za Zhi 2017, 97, 2181–2185. [Google Scholar] [PubMed]
- Fizelova, M.; Jauhiainen, R.; Kangas, A.; Soininen, P.; Ala-Korpela, M.; Kuusisto, J.; Laakso, M.; Stančáková, A. Differential Associations of Inflammatory Markers With Insulin Sensitivity and Secretion: The Prospective METSIM Study. J. Clin. Endocrinol. Metab. 2017, 102, 3600–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagherniya, M.; Khayyatzadeh, S.S.; Bakavoli, A.R.H.; Ferns, G.A.; Ebrahimi, M.; Safarian, M.; Nematy, M.; Ghayour-Mobarhan, M. Serum high-sensitive C-reactive protein is associated with dietary intakes in diabetic patients with and without hypertension: A cross-sectional study. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 55, 422–429. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Ebrahimi, M.; Karimian, M.S.; Avan, A.; Tayefi, M.; Heidari-Bakavoli, A.; Parizadeh, M.R.; Moohebati, M.; Azarpazhooh, M.R.; Esmaily, H.; et al. Serum high-sensitivity C-reactive protein as a biomarker in patients with metabolic syndrome: Evidence-based study with 7284 subjects. Eur. J. Clin. Nutr. 2016, 70, 1298–1304. [Google Scholar] [CrossRef]
- Cannell, J.; Grant, W.B.; Holick, M.F. Vitamin D and inflammation. Derm. Endocrinol. 2014, 6, e983401. [Google Scholar] [CrossRef] [Green Version]
- Amer, M.; Qayyum, R. Relation Between Serum 25-Hydroxyvitamin D and C-Reactive Protein in Asymptomatic Adults (From the Continuous National Health and Nutrition Examination Survey 2001 to 2006). Am. J. Cardiol. 2012, 109, 226–230. [Google Scholar] [CrossRef]
- Van Der Velde, M.; Bello, A.K.; Brantsma, A.H.; El Nahas, M.; Bakker, S.J.; De Jong, P.E.; Gansevoort, R.T. Do albuminuria and hs-CRP add to the International Diabetes Federation definition of the metabolic syndrome in predicting outcome? Nephrol. Dial. Transplant. 2011, 27, 2275–2283. [Google Scholar] [CrossRef]
- Qi, Y.; Rathinasabapathy, A.; Huo, T.; Zhang, J.; Shang, H.; Katz, A.; Katovich, M.; Raizada, M.; Pepine, C. 7A.04. J. Hypertens. 2015, 33, e90. [Google Scholar] [CrossRef]
- Bustos, P.; Rosas, B.; Román, P.; Villagrán, J.; Amigo, H. Síndrome metabólico e inflamación en adultos: Un estudio poblacional. Rev. Médica Chile 2016, 144, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
| Parameter | N | Mean | SD | Median | Min. | Max. | IQR | CV |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 180 | 60.8 | 17.0 | 61.5 | 23.0 | 95.0 | 22.5 | 27.9 |
| Weight (kg) | 180 | 77.4 | 16.7 | 74.9 | 41.5 | 150.0 | 20.0 | 21.5 |
| Height (cm) | 180 | 166.1 | 9.78 | 165.0 | 145.0 | 193.0 | 13.50 | 5.89 |
| BMI | 180 | 28.0 | 5.4 | 27.5 | 16.6 | 49.5 | 6.7 | 19.4 |
| Waist circumference (cm) | 180 | 91.6 | 13.00 | 90.5 | 60.0 | 141.0 | 16.50 | 14.18 |
| hsCRP (mg/L) | 180 | 0.223 | 0.202 | 0.159 | 0.010 | 0.995 | 0.214 | 90.587 |
| Total cholesterol (mg/dL) | 170 | 196.4 | 43.9 | 190.5 | 107.0 | 347.0 | 61.0 | 22.3 |
| LDL cholesterol (mg/dL) | 170 | 111.3 | 38.4 | 108.5 | 29.0 | 244.0 | 52.0 | 34.5 |
| HDL cholesterol (mg/dL) | 170 | 60.5 | 16.7 | 59.0 | 28.0 | 108.0 | 26.0 | 27.5 |
| Triglycerides (mg/dL) | 170 | 120.7 | 50.9 | 105.5 | 46.0 | 267.0 | 63.0 | 42.1 |
| Glucose (mg/dL) | 178 | 98.0 | 19.6 | 93.5 | 61.0 | 225.0 | 17.0 | 19.9 |
| Uric acid (mg/dL) | 180 | 5.6 | 1.4 | 5.5 | 1.7 | 8.8 | 2.5 | 25.8 |
| Vitamin D (ng/mL) | 178 | 23.5 | 9.4 | 23.0 | 8.0 | 67.0 | 10.0 | 39.8 |
| Parameter | rs * | p Value |
|---|---|---|
| Age (years) | 0.304 | 0.0000 |
| BMI | 0.295 | 0.0001 |
| Waist circumference (cm) | 0.250 | 0.0007 |
| Total cholesterol (mg/dL) | −0.119 | 0.1226 |
| LDL cholesterol (mg/dL) | −0.149 | 0.0521 |
| HDL cholesterol (mg/dL) | 0.002 | 0.9805 |
| Triglycerides (mg/dL) | −0.084 | 0.2770 |
| Glucose (mg/dL) | 0.173 | 0.0207 |
| Uric acid (mg/dL) | 0.090 | 0.2300 |
| Vitamin D (ng/mL) | −0.203 | 0.0065 |
| Parameter | hsCRP (mg/L) | p Value | |||||
|---|---|---|---|---|---|---|---|
| N | Median | Min. | Max. | IQR | |||
| Gender | women | 121 | 0.172 | 0.010 | 0.995 | 0.224 | 0.0173 * |
| men | 59 | 0.107 | 0.020 | 0.978 | 0.143 | ||
| BMI | <25 | 55 | 0.103 | 0.010 | 0.818 | 0.156 | 0.0010 ** 0.0843 # 0.0007 ## 0.2245 ### |
| <25;30> | 73 | 0.159 | 0.020 | 0.995 | 0.166 | ||
| >30 | 52 | 0.203 | 0.036 | 0.767 | 0.281 | ||
| Waist circumference (cm) | M > 94, K > 80 | 114 | 0.183 | 0.030 | 0.995 | 0.241 | 0.0008 |
| M ≤ 94, K ≤ 80 | 66 | 0.109 | 0.010 | 0.818 | 0.150 | ||
| Total cholesterol (mg/dL) | ≥200 | 74 | 0.132 | 0.020 | 0.995 | 0.185 | 0.1290 * |
| <200 | 96 | 0.164 | 0.010 | 0.978 | 0.232 | ||
| LDL cholesterol (mg/dL) | ≥100 | 101 | 0.123 | 0.020 | 0.995 | 0.185 | 0.0849 * |
| <100 | 69 | 0.181 | 0.010 | 0.978 | 0.230 | ||
| HDL cholesterol (mg/dL) | M < 40, K < 50 | 33 | 0.175 | 0.020 | 0.765 | 0.279 | 0.0602 * |
| M ≥ 40, K ≥ 50 | 137 | 0.124 | 0.010 | 0.995 | 0.192 | ||
| Triglycerides (mg/dL) | ≥150 | 38 | 0.144 | 0.020 | 0.978 | 0.189 | 0.6849 * |
| <150 | 132 | 0.156 | 0.010 | 0.995 | 0.216 | ||
| Dyslipidemia | present | 145 | 0.172 | 0.020 | 0.995 | 0.229 | 0.0159 * |
| absent | 35 | 0.107 | 0.010 | 0.636 | 0.149 | ||
| Glucose (mg/dL) | >99 | 61 | 0.181 | 0.020 | 0.995 | 0.314 | 0.0889 * |
| ≤99 | 117 | 0.147 | 0.010 | 0.978 | 0.180 | ||
| Uric acid (mg/dL) | >7 | 31 | 0.180 | 0.032 | 0.700 | 0.301 | 0.5815 * |
| ≤7 | 149 | 0.159 | 0.010 | 0.995 | 0.198 | ||
| Vitamin D (ng/mL) | <30 | 149 | 0.170 | 0.010 | 0.995 | 0.236 | 0.0870 * |
| ≥30 | 29 | 0.109 | 0.020 | 0.450 | 0.119 | ||
| Parameter | N | Median | Min. | Max. | IQR | p Value | |
|---|---|---|---|---|---|---|---|
| Cardiovascular disease | present | 112 | 0.178 | 0.020 | 0.995 | 0.270 | 0.0023 * |
| absent | 68 | 0.113 | 0.010 | 0.765 | 0.156 | ||
| Hypertension | present | 134 | 0.177 | 0.010 | 0.995 | 0.274 | 0.0018 * |
| absent | 46 | 0.106 | 0.030 | 0.708 | 0.133 | ||
| Diabetes | present | 41 | 0.236 | 0.020 | 0.818 | 0.299 | 0.0270 * |
| absent | 137 | 0.147 | 0.010 | 0.995 | 0.175 | ||
| Visceral obesity | present | 129 | 0.180 | 0.010 | 0.995 | 0.249 | 0.0023 * |
| absent | 51 | 0.110 | 0.020 | 0.818 | 0.143 | ||
| Parameter | N | Median | Min. | Max. | IQR | p Value | |
|---|---|---|---|---|---|---|---|
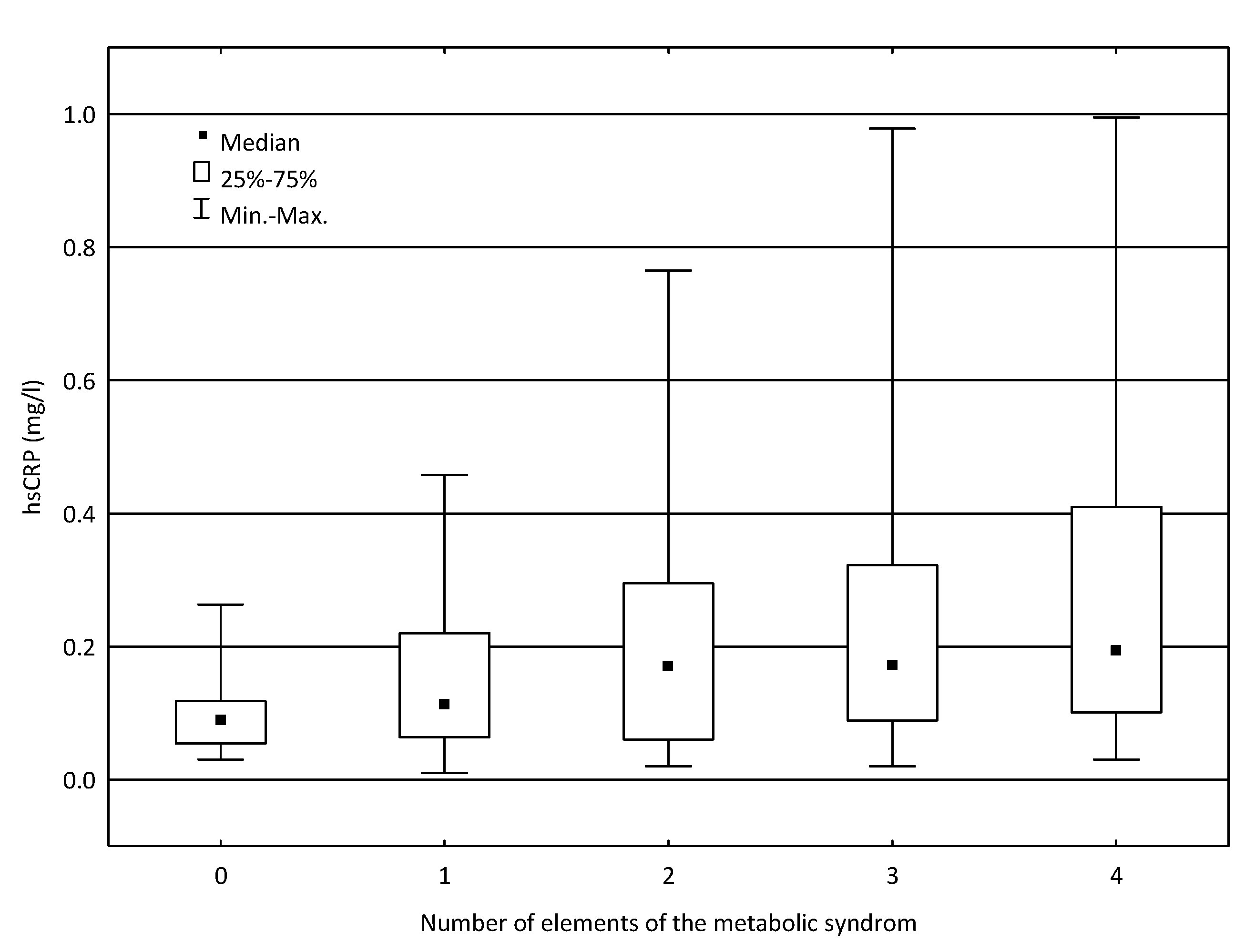
| Number of elements of the metabolic syndrome | 0 | 18 | 0.089 | 0.030 | 0.263 | 0.064 | 0.0053 ** |
| 1 | 20 | 0.113 | 0.010 | 0.458 | 0.156 | ||
| 2 | 33 | 0.170 | 0.020 | 0.765 | 0.235 | 0.0206 # | |
| 3 | 63 | 0.171 | 0.020 | 0.978 | 0.233 | 0.0036 ## | |
| 4 | 44 | 0.194 | 0.030 | 0.995 | 0.309 | >0.050 ### | |
| Number of risk factors of cardiovascular disease | 0 | 19 | 0.097 | 0.033 | 0.449 | 0.114 | 0.1451 ** |
| 1 | 36 | 0.137 | 0.010 | 0.708 | 0.167 | ||
| 2 | 50 | 0.176 | 0.022 | 0.995 | 0.264 | ||
| 3 | 39 | 0.175 | 0.032 | 0.978 | 0.221 | ||
| 4 | 19 | 0.170 | 0.020 | 0.767 | 0.261 | ||
| 5 | 6 | 0.261 | 0.020 | 0.700 | 0.371 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koziarska-Rościszewska, M.; Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors. Life 2021, 11, 742. https://doi.org/10.3390/life11080742
Koziarska-Rościszewska M, Gluba-Brzózka A, Franczyk B, Rysz J. High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors. Life. 2021; 11(8):742. https://doi.org/10.3390/life11080742
Chicago/Turabian StyleKoziarska-Rościszewska, Małgorzata, Anna Gluba-Brzózka, Beata Franczyk, and Jacek Rysz. 2021. "High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors" Life 11, no. 8: 742. https://doi.org/10.3390/life11080742
APA StyleKoziarska-Rościszewska, M., Gluba-Brzózka, A., Franczyk, B., & Rysz, J. (2021). High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors. Life, 11(8), 742. https://doi.org/10.3390/life11080742







