TGR5 Expression Is Associated with Changes in the Heart and Urinary Bladder of Rats with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Blood Biomarkers
2.3. Cardiac Performance in Langendorff Apparatus
2.4. Cystometrogram of Urinary Bladder
2.5. Measurement of Intracellular cAMP Levels in Isolated Urinary Bladders
2.6. Real-Time Quantitative PCR
- TGR5
- F: 5′-TGGCTGCTGTGACTCTTTGA-3′
- R: 5′-TGTGACATCATGGGTCTTGG-3′
- BNP
- F: 5′-GTCAGTCGCTTGGGCTGT-3′;
- R: 5′-CCAGAGCTGGGGAAAGAAG-3′;
- β-MHC
- F: 5′-CATCCCCAATGAGACGAAGT-3′;
- R: 5′-GGGAAGCCCTTCCTACAGAT-3′;
- β-actin
- F: 5′-CTAAGGCCAACCGTGAAAAG-3′;
- R: 5′-GCCTGGATGGCTACGTACA-3′
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
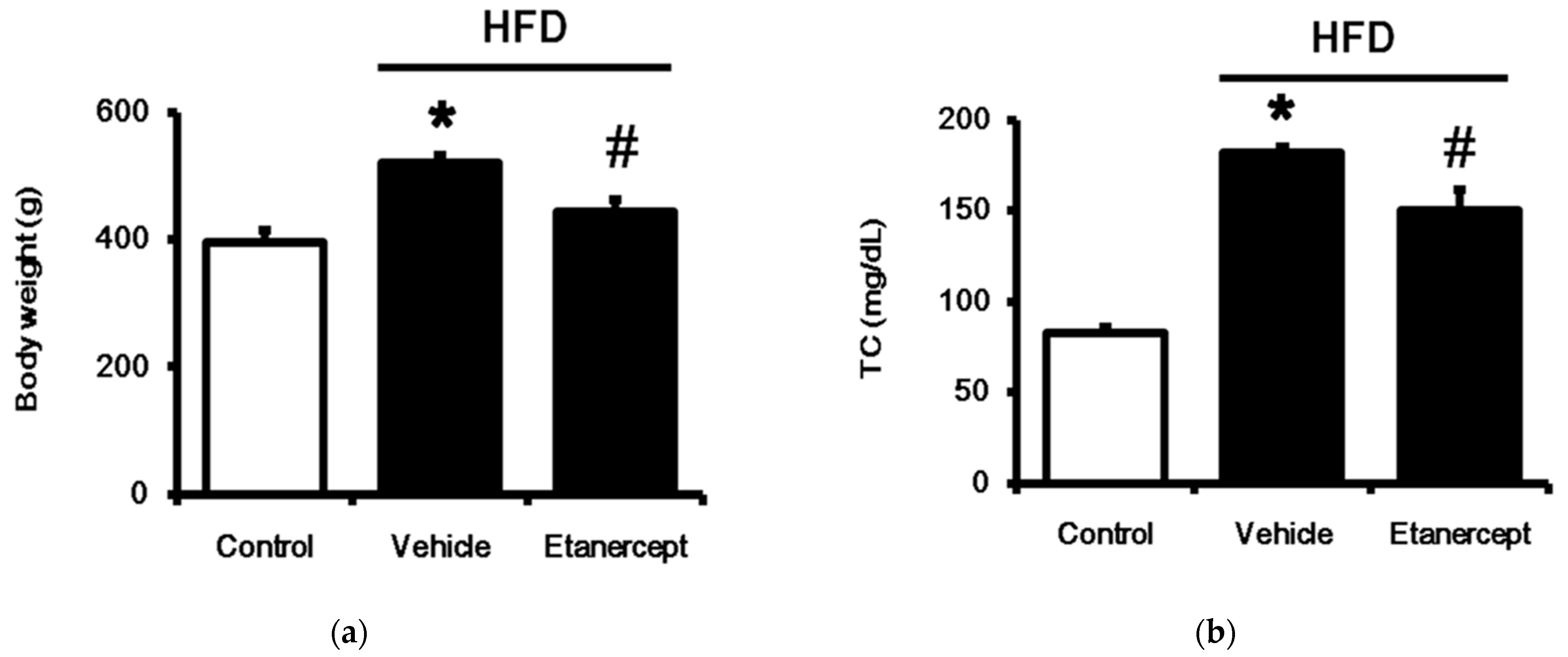
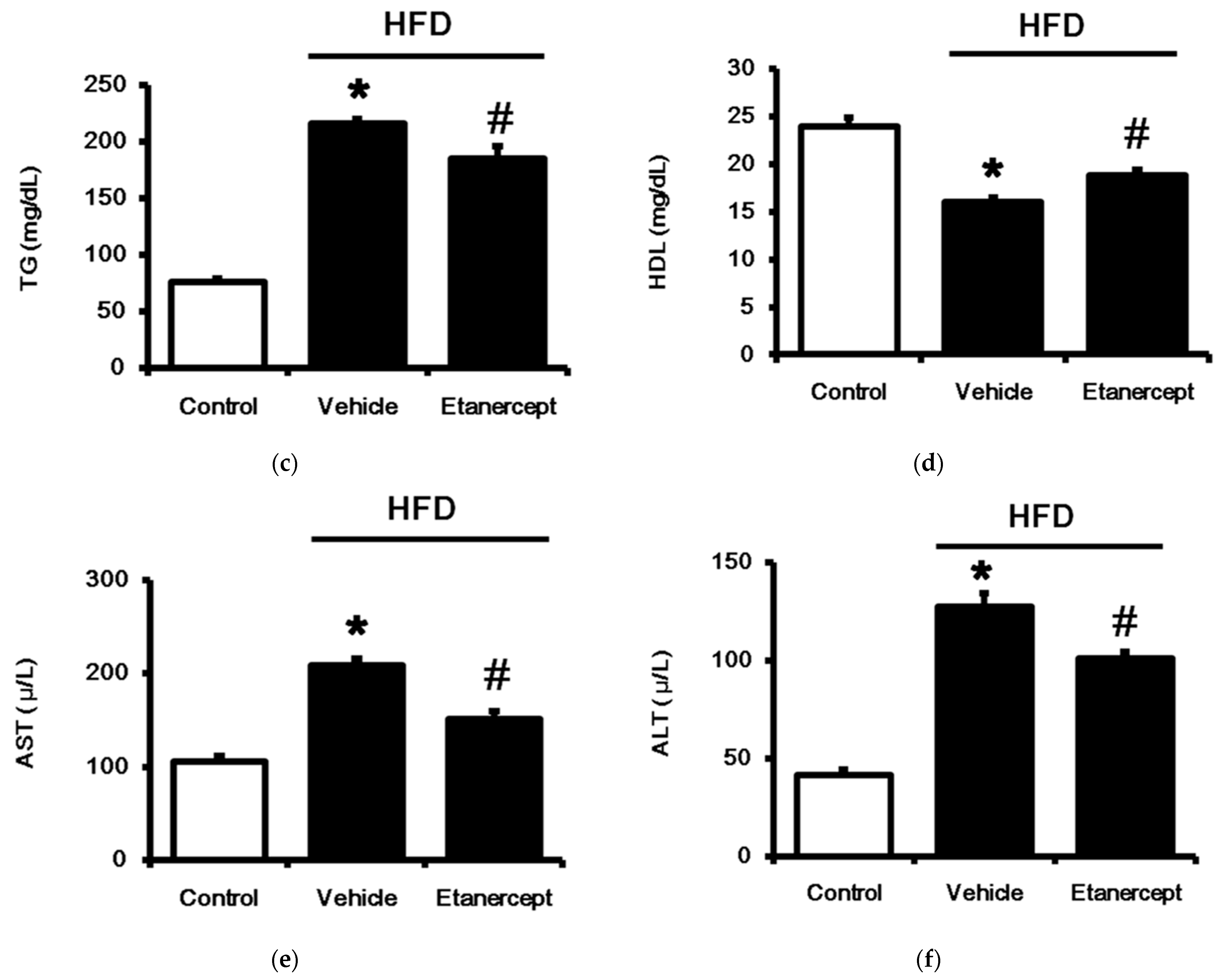
3.1. Role of TNFα in MetS Induced in HFD-Fed Rats
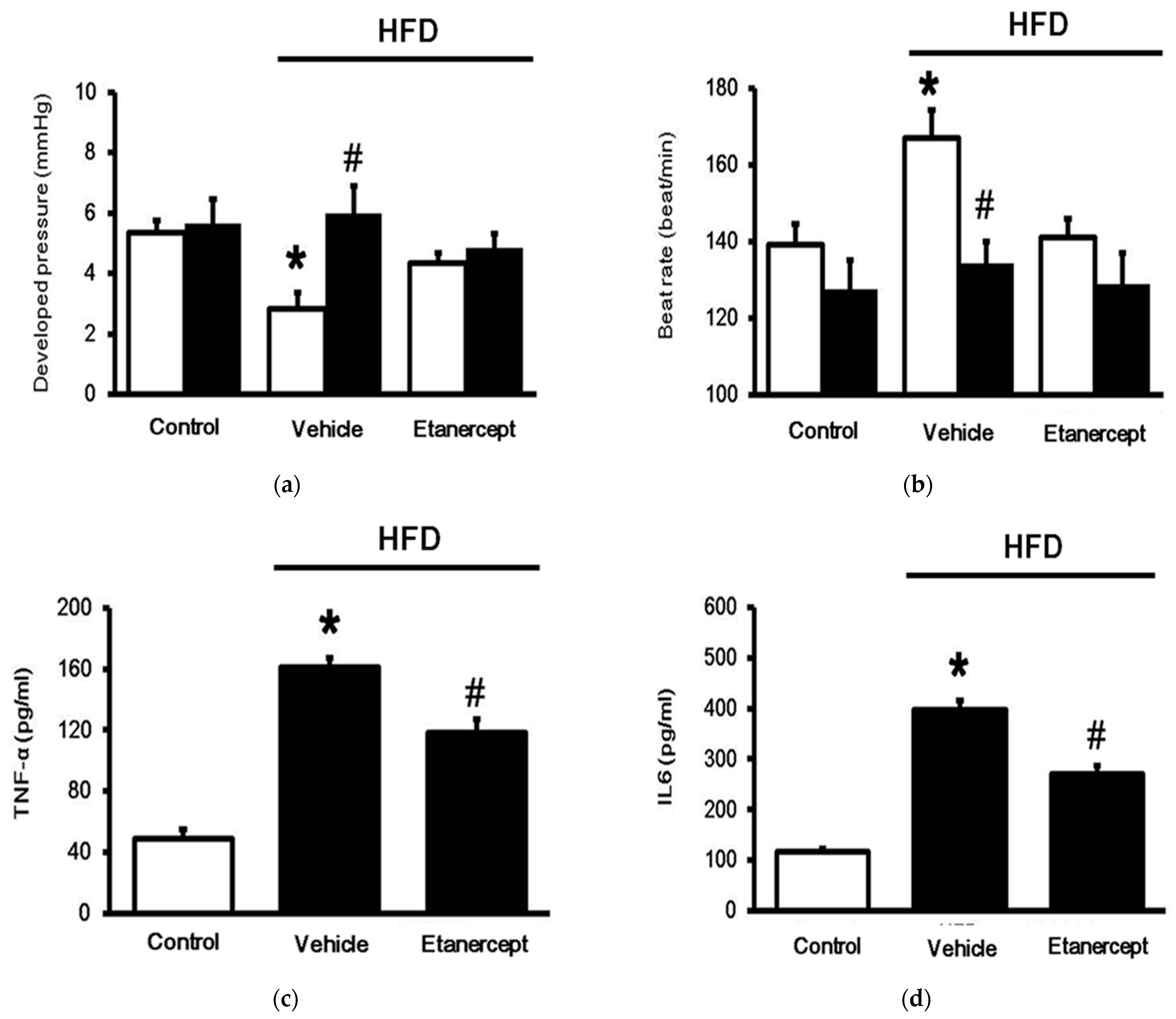
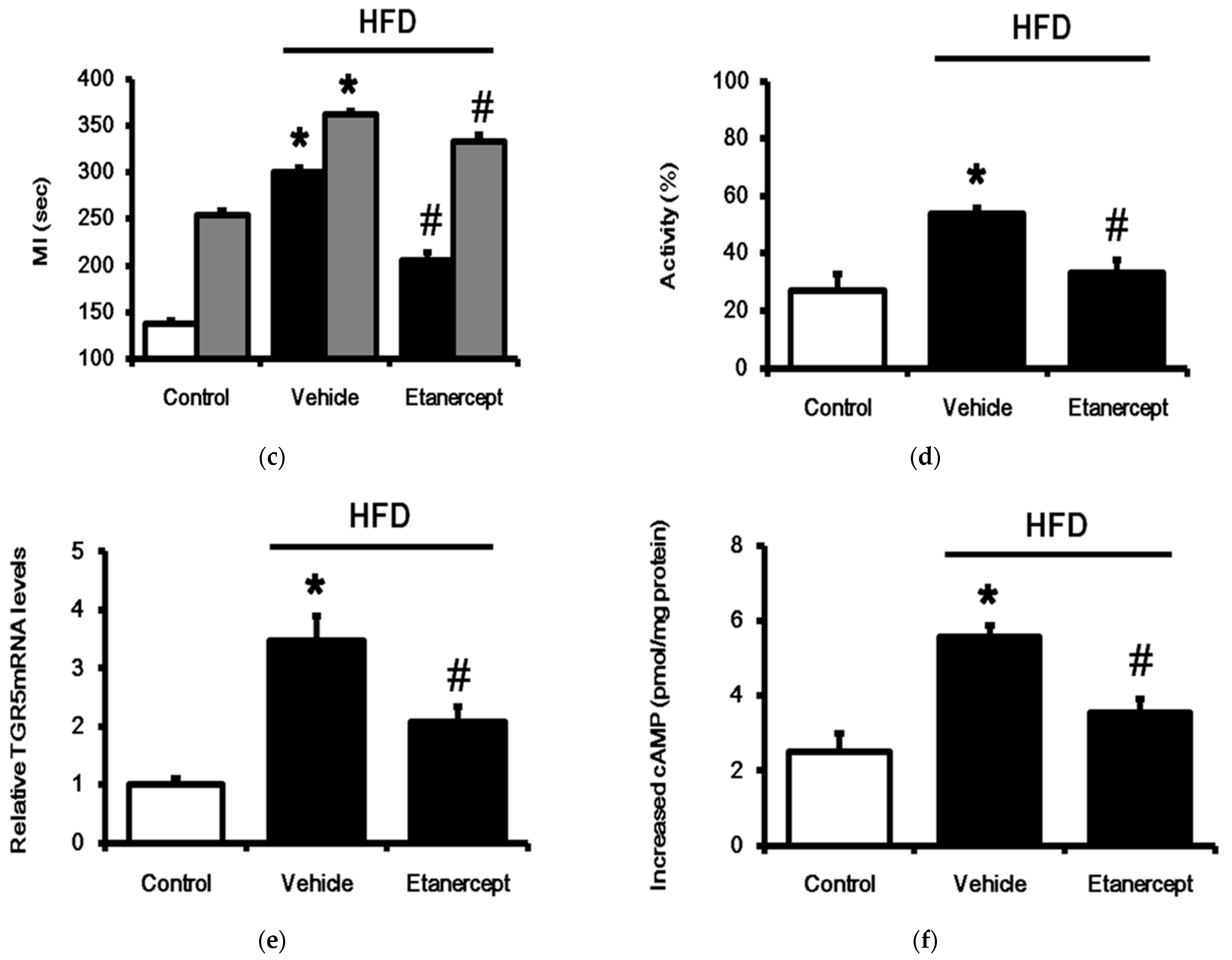
3.2. Changes in Cardiac Performance in HFD-Fed Rats
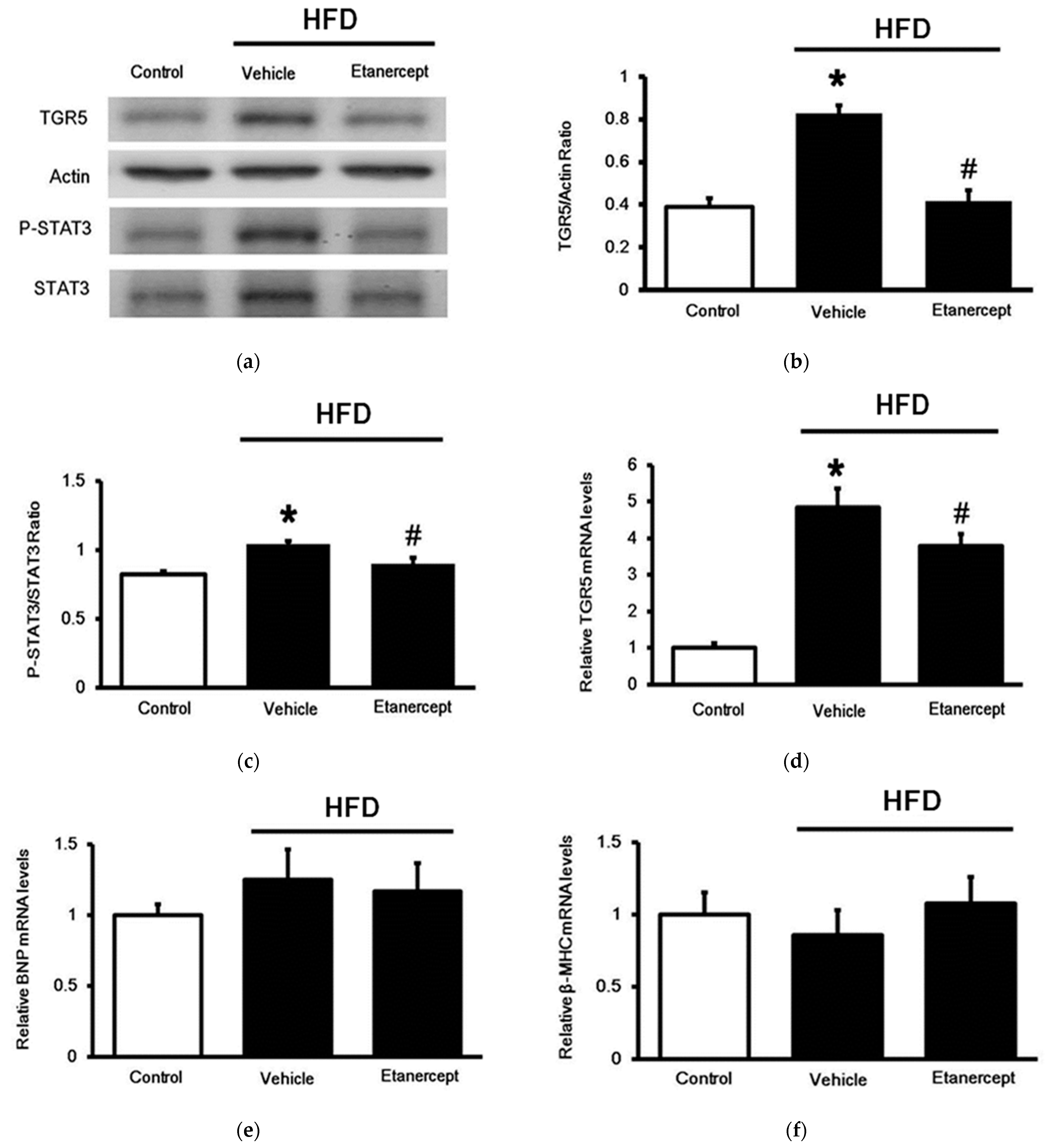
3.3. Changes in TGR5 Expression in the Hearts
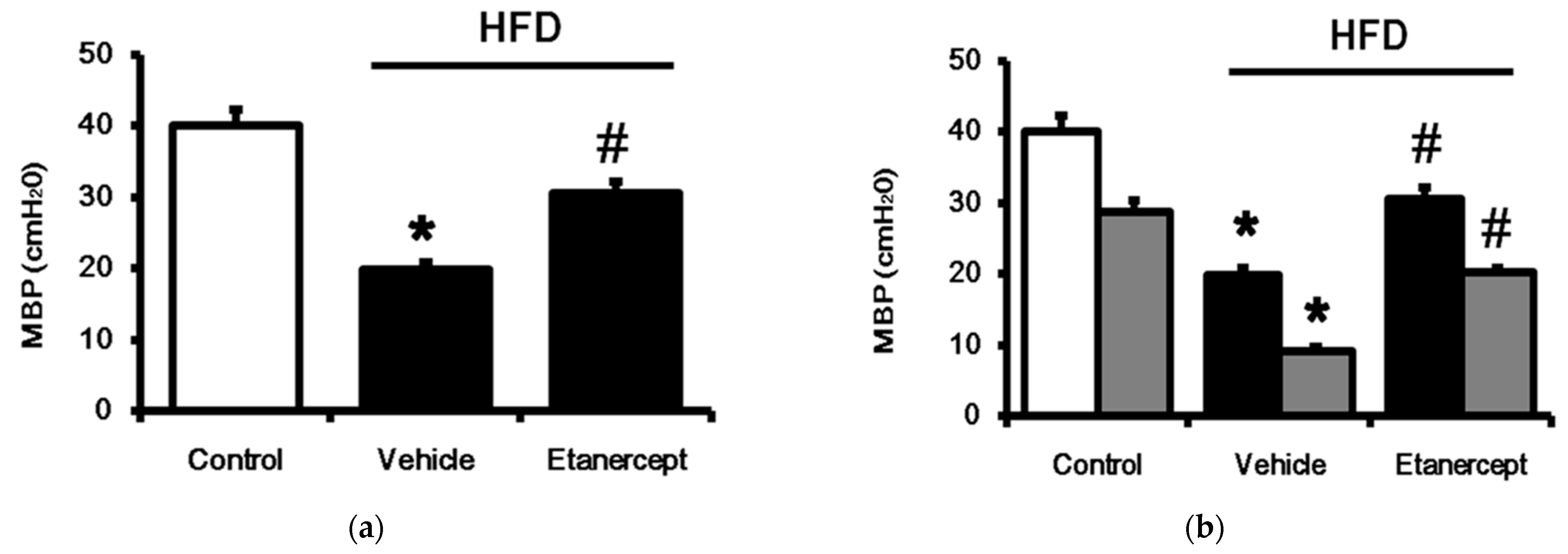
3.4. Changes of TGR5 Expression in Urinary Bladder of HFD-Fed Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, A.; Maiti, P. TGR5: An emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov. Today 2009, 14, 523–530. [Google Scholar] [CrossRef]
- Desai, M.S.; Shabier, Z.; Taylor, M.; Lam, F.; Thevananther, S.; Kosters, A.; Karpen, S.J. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology 2010, 51, 2097–2107. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Penny, D.J. Bile acids induce arrhythmias: Old metabolite, new tricks. Heart 2013, 99, 1629–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Chen, W.D.; Wang, Y.D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Eblimit, Z.; Thevananther, S.; Karpen, S.J.; Taegtmeyer, H.; Moore, D.D.; Adorini, L.; Penny, D.J.; Desai, M.S. TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice. Cardiovasc. Ther. 2018, 36, e12462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, R.; Wan, H. Overexpression of TGR5 alleviates myocardial ischemia/reperfusion injury via AKT/GSK-3beta mediated inflammation and mitochondrial pathway. Biosci. Rep. 2020, 40, BSR20193482. [Google Scholar] [CrossRef]
- Sheikh Abdul Kadir, S.H.; Miragoli, M.; Abu-Hayyeh, S.; Moshkov, A.V.; Xie, Q.; Keitel, V.; Nikolaev, V.O.; Williamson, C.; Gorelik, J. Bile acid-induced arrhythmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes. PLoS ONE 2010, 5, e9689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Chang, W.T.; Kuo, F.Y.; Chen, Z.C.; Li, Y.; Cheng, J.T. TGR5 activation ameliorates hyperglycemia-induced cardiac hypertrophy in H9c2 cells. Sci. Rep. 2019, 9, 3633. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.F.; Lee, S.P.; Cheng, J.T.; Wu, M.C. Cardiac TGR5 expression enhanced by hyperglycaemia in diabetic rats: A preclinical warning for disorders with excess bile acids. Integr. Mol. Med. 2019, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Axen, K.V.; Dikeakos, A.; Sclafani, A. High dietary fat promotes syndrome X in nonobese rats. J. Nutr. 2003, 133, 2244–2249. [Google Scholar] [CrossRef]
- Chan, R.S.; Woo, J. Prevention of overweight and obesity: How effective is the current public health approach. Int. J. Environ. Res. Public Health 2010, 7, 765–783. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Huang, B.; Zou, Y.; Liu, Y. Associations of dipping and non-dipping hypertension with cardiovascular diseases in patients with dyslipidemia. Arch. Med. Sci. 2019, 15, 337–342. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Chen, X.; Yu, W.; Li, W.; Zhang, H.; Huang, W.; Wang, J.; Zhu, W.; Fang, Q.; Chen, C.; Li, X.; et al. An anti-inflammatory chalcone derivative prevents heart and kidney from hyperlipidemia-induced injuries by attenuating inflammation. Toxicol. Appl. Pharmacol. 2018, 338, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mukhopadhyay, S. The evil axis of obesity, inflammation and type-2 diabetes. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, B.; Yuan, Y.; Hu, J.; Zhao, W.; Jiang, H.; Li, W.; Fan, Z.; Du, Z. Aloe Emodin Reduces Cardiac Inflammation Induced by a High-Fat Diet through the TLR4 Signaling Pathway. Mediat. Inflamm. 2020, 2020, 6318520. [Google Scholar] [CrossRef] [PubMed]
- Borst, S.E.; Conover, C.F. High-fat diet induces increased tissue expression of TNF-alpha. Life Sci. 2005, 77, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Chytilova, A.; Borchert, G.H.; Mandikova-Alanova, P.; Hlavackova, M.; Kopkan, L.; Khan, M.A.; Imig, J.D.; Kolar, F.; Neckar, J. Tumour necrosis factor-alpha contributes to improved cardiac ischaemic tolerance in rats adapted to chronic continuous hypoxia. Acta Physiol. 2015, 214, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Murdaca, G.; Spano, F.; Contatore, M.; Guastalla, A.; Magnani, O.; Puppo, F. Pharmacogenetics of etanercept: Role of TNF-alpha gene polymorphisms in improving its efficacy. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, J.; Szalai, G.D.; Wang, W.; Ma, X.; Diwan, A.; Mann, D.L. Impaired Protein Quality Control During Left Ventricular Remodeling in Mice With Cardiac Restricted Overexpression of Tumor Necrosis Factor. Circ. Heart Fail. 2017, 10, e004252. [Google Scholar] [CrossRef]
- Li, Q.; Yu, Q.; Na, R.; Liu, B. Etanercept protects rat cardiomyocytes against hypertrophy by regulating inflammatory cytokines secretion and cell apoptosis. Braz. J. Med. Biol. Res. 2017, 50, e5868. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Dong, X.; Liu, Q.; Wu, C.; Wang, Q.; Long, Z.; Li, L. Hydrophobic bile acids relax rat detrusor contraction via inhibiting the opening of the Na+/Ca2+ exchanger. Sci. Rep. 2016, 6, 21358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayramgurler, D.; Karson, A.; Yazir, Y.; Celikyurt, I.K.; Kurnaz, S.; Utkan, T. The effect of etanercept on aortic nitric oxide-dependent vasorelaxation in an unpredictable chronic, mild stress model of depression in rats. Eur. J. Pharmacol. 2013, 710, 67–72. [Google Scholar] [CrossRef]
- Liao, P.C.; Chao, L.K.; Chou, J.C.; Dong, W.C.; Lin, C.N.; Lin, C.Y.; Chen, A.; Ka, S.M.; Ho, C.L.; Hua, K.F. Lipopolysaccharide/adenosine triphosphate-mediated signal transduction in the regulation of NLRP3 protein expression and caspase-1-mediated interleukin-1beta secretion. Inflamm. Res. 2013, 62, 89–96. [Google Scholar] [CrossRef]
- Liu, K.M.; Chuang, S.M.; Long, C.Y.; Lee, Y.L.; Wang, C.C.; Lu, M.C.; Lin, R.J.; Lu, J.H.; Jang, M.Y.; Wu, W.J.; et al. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am. J. Physiol. Renal. Physiol. 2015, 309, F318–F331. [Google Scholar] [CrossRef] [Green Version]
- Juan, Y.S.; Chuang, S.M.; Lee, Y.L.; Long, C.Y.; Wu, T.H.; Chang, W.C.; Levin, R.M.; Liu, K.M.; Huang, C.H. Green tea catechins decrease oxidative stress in surgical menopause-induced overactive bladder in a rat model. BJU Int. 2012, 110, E236–E244. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Hsu, C.T.; Niu, H.S.; Chang, C.H.; Cheng, J.T.; Shieh, J.M. Lung damage induced by hyperglycemia in diabetic rats: The role of signal transducer and activator of transcription 3 (STAT3). J. Diabetes Complicat. 2016, 30, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fang, Q.; Zhong, P.; Chen, L.; Wang, L.; Zhang, Y.; Wang, J.; Li, X.; Wang, Y.; Wang, J.; et al. EGFR Inhibition Blocks Palmitic Acid-induced inflammation in cardiomyocytes and Prevents Hyperlipidemia-induced Cardiac Injury in Mice. Sci. Rep. 2016, 6, 24580. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.; Cheng, K.C.; Li, Y.X.; Chang, C.H.; Cheng, J.T.; Lee, K.S. Development of betulinic acid as an agonist of TGR5 receptor using a new in vitro assay. Drug Des. Devel. Ther. 2016, 10, 2669–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cheng, K.C.; Niu, C.S.; Lo, S.H.; Cheng, J.T.; Niu, H.S. Investigation of triamterene as an inhibitor of the TGR5 receptor: Identification in cells and animals. Drug Des. Devel. Ther. 2017, 11, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zhou, S.Y.; Gillilland, M., 3rd; Li, J.Y.; Lee, A.; Gao, J.; Zhang, G.; Xu, X.; Owyang, C. Bile acid toxicity in Paneth cells contributes to gut dysbiosis induced by high-fat feeding. JCI Insight 2020, 5, e138881. [Google Scholar] [CrossRef]
- Huang, S.; Ma, S.; Ning, M.; Yang, W.; Ye, Y.; Zhang, L.; Shen, J.; Leng, Y. TGR5 agonist ameliorates insulin resistance in the skeletal muscles and improves glucose homeostasis in diabetic mice. Metabolism 2019, 99, 45–56. [Google Scholar] [CrossRef]
- Cole, M.A.; Murray, A.J.; Cochlin, L.E.; Heather, L.C.; McAleese, S.; Knight, N.S.; Sutton, E.; Jamil, A.A.; Parassol, N.; Clarke, K. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res. Cardiol. 2011, 106, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Ideris, A.; Abdullah, M.A. High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats. J. Biomed. Sci. 2015, 22, 96. [Google Scholar] [CrossRef]
- Speyer, C.L.; Ward, P.A. Role of endothelial chemokines and their receptors during inflammation. J. Investig. Surg. 2011, 24, 18–27. [Google Scholar] [CrossRef]
- Prabhu, S.D. Cytokine-induced modulation of cardiac function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.; Kennedy, R.H.; Liu, S.J. TNF-alpha-induced impairment of mitochondrial integrity and apoptosis mediated by caspase-8 in adult ventricular myocytes. Cytokine 2006, 34, 96–105. [Google Scholar] [CrossRef]
- Miller, A.M.; Wang, H.; Bertola, A.; Park, O.; Horiguchi, N.; Ki, S.H.; Yin, S.; Lafdil, F.; Gao, B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology 2011, 54, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.E.; Lee, H.G.; Cho, I.H.; Chung, D.H.; Yoon, S.H.; Yang, Y.M.; Lee, J.W.; Choi, S.; Park, J.W.; Ye, S.K.; et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005, 19, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, C.; Zouein, F.A.; Kurdi, M.; Booz, G.W. Differential STAT3 signaling in the heart: Impact of concurrent signals and oxidative stress. JAKSTAT 2012, 1, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting Janus Kinases and Signal Transducer and Activator of Transcription 3 to Treat Inflammation, Fibrosis, and Cancer: Rationale, Progress, and Caution. Pharmacol. Rev. 2020, 72, 486–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Miyamoto, T.; Yoshida, H.; Asakawa, M.; Kawasumi, M.; Kobayashi, T.; Morioka, H.; Chiba, K.; Toyama, Y.; Yoshimura, A. IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int. Immunol. 2011, 23, 701–712. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-C.; Cheng, K.-C.; Li, Y.; Hsu, P.-H.; Cheng, J.-T.; Niu, H.-S. TGR5 Expression Is Associated with Changes in the Heart and Urinary Bladder of Rats with Metabolic Syndrome. Life 2021, 11, 695. https://doi.org/10.3390/life11070695
Hsu C-C, Cheng K-C, Li Y, Hsu P-H, Cheng J-T, Niu H-S. TGR5 Expression Is Associated with Changes in the Heart and Urinary Bladder of Rats with Metabolic Syndrome. Life. 2021; 11(7):695. https://doi.org/10.3390/life11070695
Chicago/Turabian StyleHsu, Chia-Chen, Kai-Chun Cheng, Yingxiao Li, Ping-Hao Hsu, Juei-Tang Cheng, and Ho-Shan Niu. 2021. "TGR5 Expression Is Associated with Changes in the Heart and Urinary Bladder of Rats with Metabolic Syndrome" Life 11, no. 7: 695. https://doi.org/10.3390/life11070695
APA StyleHsu, C.-C., Cheng, K.-C., Li, Y., Hsu, P.-H., Cheng, J.-T., & Niu, H.-S. (2021). TGR5 Expression Is Associated with Changes in the Heart and Urinary Bladder of Rats with Metabolic Syndrome. Life, 11(7), 695. https://doi.org/10.3390/life11070695




