1. Introduction
Morphine is an opioid drug used as an effective analgesic for the treatment of postoperative and cancer pain. However, medication with morphine can create harmful side effects. Prolonged administration of this drug brings about a high risk of abuse and addiction, which manifests as physical dependence and/or psychological addiction [
1]. Physical dependence stems from neuroadaptive changes which occur at the molecular and cellular levels in the central nervous system (CNS) and are associated with the appearance of withdrawal symptoms following the discontinuation of drug use [
1,
2,
3]. In experimental conditions, the cessation of morphine use is accomplished by abruptly stopping chronic treatment with the drug or by administration of an opioid receptor antagonist which acts as a potent competitive inhibitor and blocks opioid receptors. In animals, this intervention evokes withdrawal symptoms such as jumping, paw tremors, teeth chattering, and diarrhea [
1].
During drug administration and withdrawal, neuroadaptations occurring in the brain are associated with alterations in synapses, ion channels and structural compartments. In this respect, the greatest attention has been focused on neurotransmitter glutamatergic, dopaminergic and GABAergic systems [
2,
3,
4]. However, it is highly likely that drug administration and withdrawal may induce changes in the expression and function of other proteins involved in the regulation of synaptic plasticity. The synapses, specialized sites capable of mediating communication between neurons in the CNS, are composed of pre- and postsynaptic compartments [
5]. The presynaptic terminal contains the active zone (AZ) with proteins involved in the recruitment and exocytosis of synaptic vesicles (SVs), which release neurotransmitters into the synaptic cleft. The postsynaptic site contains the postsynaptic density (PSD) with receptors and signaling components that respond to neurotransmitters released from the presynaptic terminal [
5]. Alterations in the expression and posttranslational modifications of synaptic proteins may cause synaptic dysfunction and subsequently lead to neurological disorders [
5,
6,
7].
The long-lasting brain adaptations connected with compulsive drug use and craving are not limited to only one brain region. Although the ventral tegmental area (VTA) and the nucleus accumbens (NA) are widely recognized as brain regions critical for the development of drug addiction and reward [
8,
9], it was found that dopaminergic neurons from the VTA project not only to the NA but also to the dorsal striatum, prefrontal cortex and hippocampus and, conversely, the NA receives glutamatergic inputs from the cortex and hippocampus [
10]. Dysregulated homeostasis in glutamatergic synapses is implicated in cognitive impairments and cravings associated with opioid use disorder [
9]. The dopamine system is also involved in opioid use disorder because low dopamine D2/3 receptor availability and low presynaptic dopamine were found in the striatum of opioid dependent patients [
11]. Addictive drugs also modify cerebellar glutamate and endocannabinoid interactions, norepinephrine and dopamine levels, and intracellular signaling transduction pathways [
12]. The reduced release of dopamine in the NA may also be driven by increased activity of dynorphin and the κ-opioid receptor system in the ventral striatum, thereby contributing to the negative emotional state associated with withdrawal and protracted abstinence [
13]. The hippocampus is critical for the formation of addictive memory and for triggering a relapse [
14]. Drug-induced dysregulation of neuronal networks in the cortex contributes to the functional abnormalities of brain reward systems and causes compulsive drug use [
13]. The cerebellum has been shown to be involved in emotional memory and experience, language, temporal perception, automatization of rules or decision making, which have all been found to be altered in addicted patients [
12,
15].
Morphine acts through opioid receptors (ORs). It has a high affinity for μ-OR and a somewhat lower affinity for κ- and δ-ORs [
16]. The distribution of ORs differs between different regions of rat brain. The highest number of μ-OR is found in the striatum, a lower number in the hippocampus, and the lowest number in the cortex [
17]. Although rat cerebellum was originally believed to be devoid of μ- and κ-ORs [
18], some later studies reported opposite results; not only μ- and δ-ORs [
19], but also κ-ORs [
20] were found there. ORs are apparently less abundant in rat cerebellum when compared to other brain regions. Anyways, there are some indications that morphine can have a huge impact on cerebellar Purkinje cells [
21]. Hence, the cerebellum should also be involved in dealing with opioid use disorder.
It has been observed that cytoskeletal dynamics may contribute to synaptic plasticity in addicted animals and the actin filaments were studied in the greatest detail in this respect [
22]. The organization and remodeling of the microtubule network are also essential for synapse formation and stability. This network is affected by microtubule dynamic instability, branching, orientation, posttranslational modifications and interactions with microtubule-associated proteins (MAPs) [
23,
24]. Microtubules are composed of α- and β-tubulin heterodimers, whose posttranslational modifications might facilitate the recruitment of distinct MAPs for local regulation of microtubule function and are critical for neuronal health at all stages of life [
24,
25,
26]. There are different types of MAPs with various functions and posttranslational modifications which affect their interaction with microtubules, microtubule dynamics and rearrangements of the microtubule network when neuroplasticity occurs [
26]. Phosphorylation mediated by Ca
2+/calmodulin-dependent kinase II (CaMKII) [
26,
27], glycogen synthase kinase III β (GSKIIIβ), cdc2 kinase, c-Jun N-terminal kinase 1 (JNK1), cAMP-dependent protein kinase (PKA), protein kinase C (PKC), and serine/threonine-protein kinase MARK [
26] is an essential posttranslational modification of tubulins and MAPs. Tubulins and MAPs bind to many other interacting partners. Tubulin directly interacts with synapsin-1, a presynaptic vesicle protein, which is a neuronal phosphoprotein that contributes to the clustering of synaptic vesicles with cytoskeletal elements at the presynaptic terminals and thereby regulates SV cycling and neurotransmitter release [
28]. Synapsin-1 can be phosphorylated at different phosphorylation sites by different kinases, and phosphorylation patterns coordinate the interaction of synapsin-1 with actin filaments or microtubules [
28]. The other interacting partners of tubulins comprise tubulin polymerization-promoting protein (Tppp) [
29] and stathmin [
30]. The interacting partners of MAPs include calcium and potassium channels, neurotransmitter receptors, and spectrin [
26,
31]. Microtubules contribute to the formation, maintenance and function of axons and dendrites. Interestingly, modifications in dendrite branching as well as changed expression of tubulin, tau, stathmin and other cytoskeletal components were observed following chronic morphine treatment and spontaneous withdrawal [
32,
33].
Small GTPases of the Ras superfamily, which can be divided into several subfamilies such as Ras, Rho, Rab, Arf, and Ran, are important regulators of cytoskeletal reorganization. These GTP-binding proteins are involved in the regulation of cytoskeletal dynamics, vesicle trafficking and synaptic connectivity and plasticity [
34,
35,
36,
37,
38,
39]. Rab and Arf GTPases mediate coupling between microtubule motor proteins and vesicles, as well as transport of the cargo vesicles along microtubules and actin filaments to specific locations [
35]. Rho GTPases play an essential role in changes of intracellular cytoskeleton dynamics [
34,
37]. Rab GTPases are known to modulate Rho activity to mediate cytoskeleton remodeling [
35]. The activity of small GTPases is regulated by guanine nucleotide exchange factors (GEFs) and GTPase activator proteins (GAPs). While GEFs stimulate the exchange of GDP for GTP to activate small GTPases, GAPs promote GTP hydrolysis to inactivate them [
34]. Most of the 150 GEFs and GAPs that have been identified so far are expressed in the brain with a specific spatial and temporal distribution pattern, but their function has not yet been elucidated [
38]. Small GTPases and their regulators are modified by phosphorylation regulating their stability and activity, subcellular localization, and interactions with binding partners [
40,
41,
42,
43]. RhoA/Rho kinase signaling was shown to be downregulated in the NA of cocaine-dependent rats and may thus contribute to synaptic changes leading to drug addiction [
44]. Rho signaling might also be related to synaptic plasticity in the amygdala and prefrontal cortex, which were identified as regulators of the reward circuitry as well [
34].
The present study aimed to explore the long-term impact of morphine withdrawal on proteomic profiling of selected rat brain regions. It is known that drug addiction is associated with dysregulation of the dopamine and glutamate systems [
9]. However, it would be too simplistic to assume that this process is mediated only by changes in neurotransmitters and their receptors. It is imaginable that dysregulation of neurotransmitter systems is associated with changes in the synaptic vesicle cycle that is comprised of several individual processes. It is also imaginable that postsynaptic signal transmission depends not only on the number of receptors on the postsynaptic membrane but also on the composition of the postsynaptic density. Likewise, changes in neurotransmitter signaling, which may affect cognitive functions (e.g., memory and learning, addiction, reward, motivation, and habits), are presumably related to changes in protein expression and protein posttranslational modifications determining the protein functions. The comprehensive analysis of the proteome and phosphoproteome can help us determine which of the components of synaptic vesicles, proteins of the presynaptic active zone and the postsynaptic density are affected by morphine, and reveal which synaptic processes could potentially be modulated by pharmacotherapeutic interventions during the withdrawal period.
4. Discussion
The present study followed from previous work in which alterations in protein expression and phosphorylation were assessed by 2-D electrophoresis and label-free quantification in selected brain regions of rats three months after cessation of chronic morphine treatment [
46]. Here, we observed that cytoskeletal proteins (actin and tubulin) and their binding partners (Tppp, Dpysl2, F-actin capping protein β) were differentially expressed or phosphorylated mainly in the cortex and hippocampus. The tubulin polymerization promoting protein is specifically expressed in oligodendrocytes [
29], and has two cellular functions. It promotes microtubule polymerization and regulates HDAC6 activity [
93]. Tppp dynamically colocalizes with microtubules and induces microtubule bundling and stabilization followed by increased acetylation of microtubules [
97]. The phosphorylation of human Tppp at Ser32, corresponding to Ser31 in rat Tppp, mediated by Rock kinase contributes to inhibition of its binding to HDAC6, subsequent increase in HDAC6 activity and tubulin deacetylation [
93]. While the level of Tppp protein was downregulated following a three-month morphine withdrawal in the cortex and hippocampus and hypophosphorylated in the striatum [
46], a six-month morphine withdrawal resulted in hyperphosphorylation of Tppp at Ser34 in the cortex and hypophosphorylation at Ser31 in the hippocampus, suggesting that, at least in rat hippocampus, regulation of tubulin acetylation via Tppp expression or phosphorylation at Ser31 might occur over the course of several months after morphine withdrawal.
Dihydropyrimidinase-related protein 2 (Dpysl2) is another protein which was found to be differentially phosphorylated after a three- as well as six-month morphine withdrawal. Whereas this protein was hyperphosphorylated in the cortex and hypophosphorylated in the hippocampus after a three-month morphine withdrawal [
46], it was hyperphosphorylated at Ser537 in the striatum and at Ser542 in the cerebellum after a six-month morphine withdrawal. The other dihydropyrimidinase-related proteins, Crmp1 (collapsin response mediator protein 1) and Dpysl3, were also differentially phosphorylated after a six-month morphine withdrawal. The majority of differentially phosphorylated sites on dihydropyrimidinase-related proteins were located in the C-terminal region, and only one differentially phosphorylated site in Crmp1 was located in the N-terminal region. Whereas the N-terminal dihydropyrimidinase-like domain appears to promote microtubule assembly, the C-terminal region of Crmp1 and Crmp2 (Dpysl2) is sufficient to stabilize the microtubules [
76]. The phosphorylation of Crmps at its C-terminal domains causes microtubule destabilization, while inhibition of the C-terminal phosphorylation has a stabilizing effect [
98]. The residues Thr509, Thr514 and Ser518 at the C-terminus of collapsin response mediator proteins are phosphorylated by GSK-3β [
76]. In the present study, differential phosphorylation patterns at these residues in Dpysl3 (Crmp4) were observed in all four brain regions under scrutiny. Hyperphosphorylation at Thr509 and hypophosphorylation at Ser518 in Dpysl3 were found in the hippocampus and striatum, respectively. The residues Thr509 and Thr514 were simultaneously hyperphosphorylated in the cortex and hypophosphorylated in the cerebellum, indicating an opposing trend in the binding of Dpysl3 to microtubules in the cortex and cerebellum. Taken together, collapsin response mediator proteins are other microtubule-binding phosphoproteins that were affected by protracted morphine withdrawal.
Morphine belongs to addictive opioid pain relievers that are often used in medical care and have a high potential for abuse. Drug abuse is a relapsing brain disease characterized by the adaptations within the mesolimbic reward system and associated neural circuits that may persist a long time after cessation of drug intake [
99]. It was demonstrated that morphine-abstinent mice develop low sociability and despair-like behavior detectable up to four weeks after discontinuation of chronic drug exposure [
100]. In the cocaine self-administration model of relapse liability, many protein alterations occurring during cocaine self-administration returned to normal levels between 1 and 100 d of abstinence, but some remained altered even after 100 d. On the other hand, some proteins which were not affected during cocaine self-administration altered during the abstinence period. Differentially expressed proteins during the abstinence period may contribute to specific functions related to relapse liability [
101]. In that report, proteins associated with synaptic plasticity were altered in the prefrontal cortex, suggesting the importance of synaptic communication in withdrawal-associated behavior. Cocaine administration induced changes in the level of Dpysl2 and the following drug withdrawal was associated with changed expression of SNAP-25, a component of the SNARE complex, and dynamin-1 located in the postsynaptic density [
101]. This is consistent with our current results suggesting that alterations in the proteome profiles of synaptic proteins may contribute to molecular neuroadaptations associated with chronic drug exposure and long-term drug abstinence.
Synapsin-1 is one of crucial proteins whose phosphorylation occurs commonly during withdrawal from different drugs. This protein was differentially phosphorylated at Ser9, Ser62 and Ser67 in the NA two hours after cocaine self-administration and phosphorylation at Ser9 was still elevated after 22 h [
102]. Phosphorylation at Ser603 of synapsin-1 was increased in the mouse NA after chronic nicotine administration and decreased 24 h after drug cessation [
103]. Synapsin acts as a key protein for maintaining SVs within the reserve pool, which is a large SV cluster distal to the active zone. The reserve pool serves as a store that replenishes SVs into a readily-releasable pool following exocytosis of neurotransmitters [
104]. The mechanism of maintaining SVs within the reserve pool by synapsin is unclear. The first hypothesis relies on the involvement of synapsin in cross-linking of SVs, thereby anchoring SVs to each other. The cross-linking of SVs follows dimerization and tetramerization of synapsin mediated by its conserved domain C. The second hypothesis relies on creating a liquid phase that allows SVs to float within a synapsin droplet. Such formation of liquid condensate is mediated by the variable IDR domains at the C-terminal end of the molecule [
104]. It is hard to imagine that such process depends on the level of expression of these IDR domains and it should be mediated by posttranslational modifications, including phosphorylation. Interestingly, phosphorylation of synapsin-1 by CaMKII caused a disassembling of the liquid phase of synapsin [
105]. In our present study, the phosphorylation of synapsin induced by a six-month morphine withdrawal occurred in phosphorylation sites in domains D and E, which are located in IDR domains at the C-terminal end of synapsin (
Table 2,
Table 3,
Table 4 and
Table 5). Although these phosphorylation sites are of unknown functions and the protein kinase phosphorylating them is not known as well, they might be proposed as phosphorylation sites of CaMKII in the formation and disassembly of the liquid phase of synapsin.
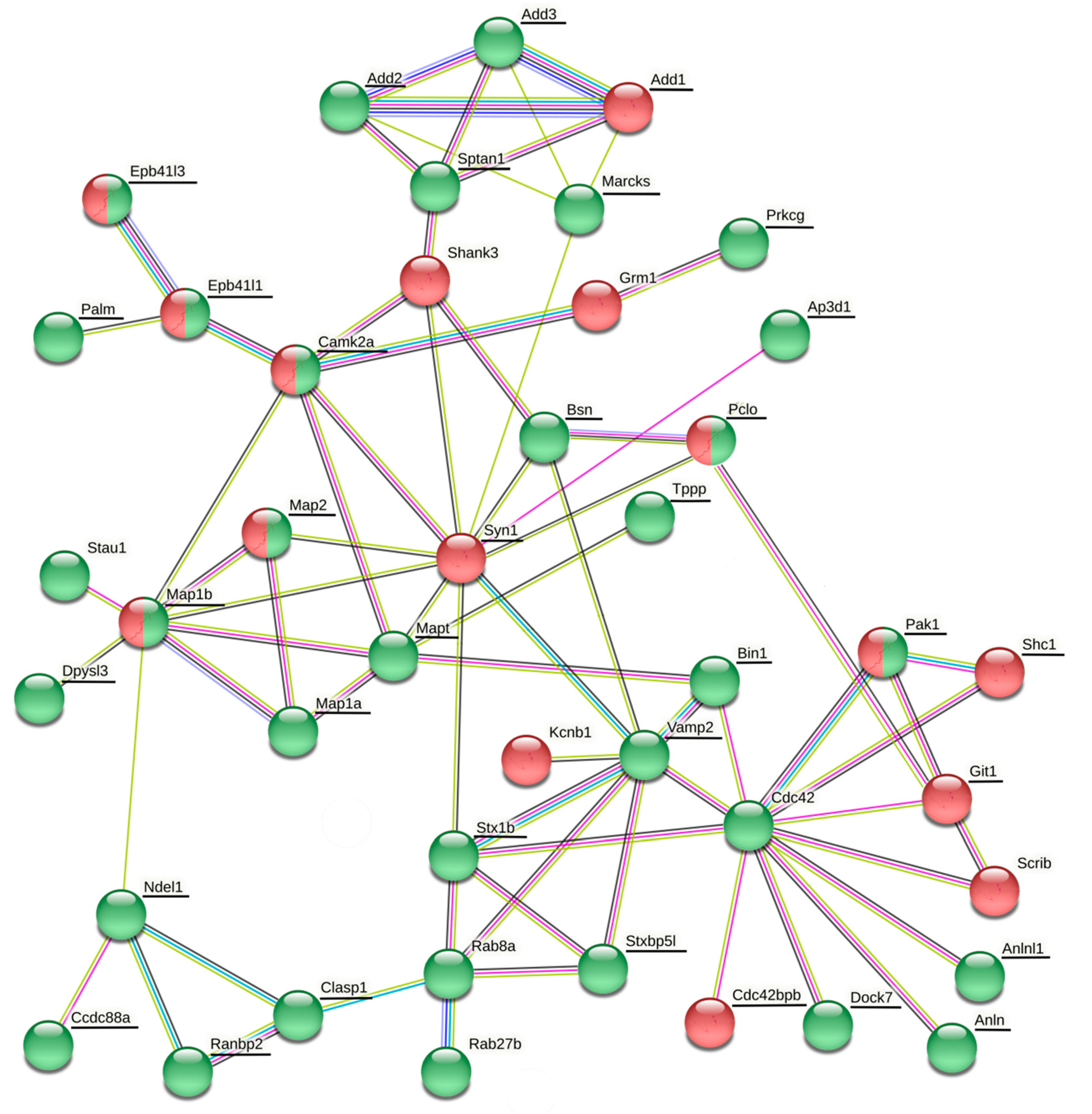
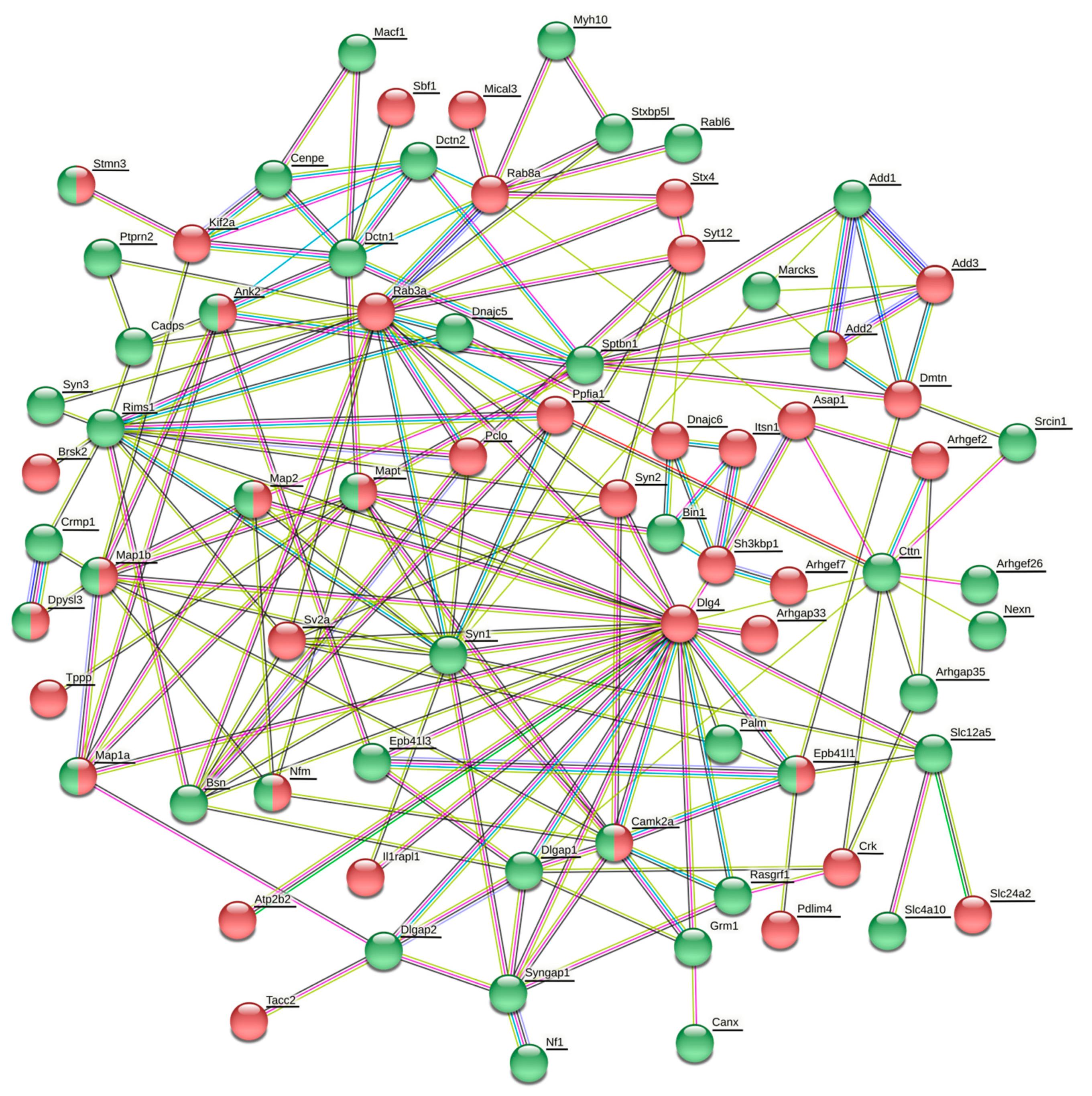
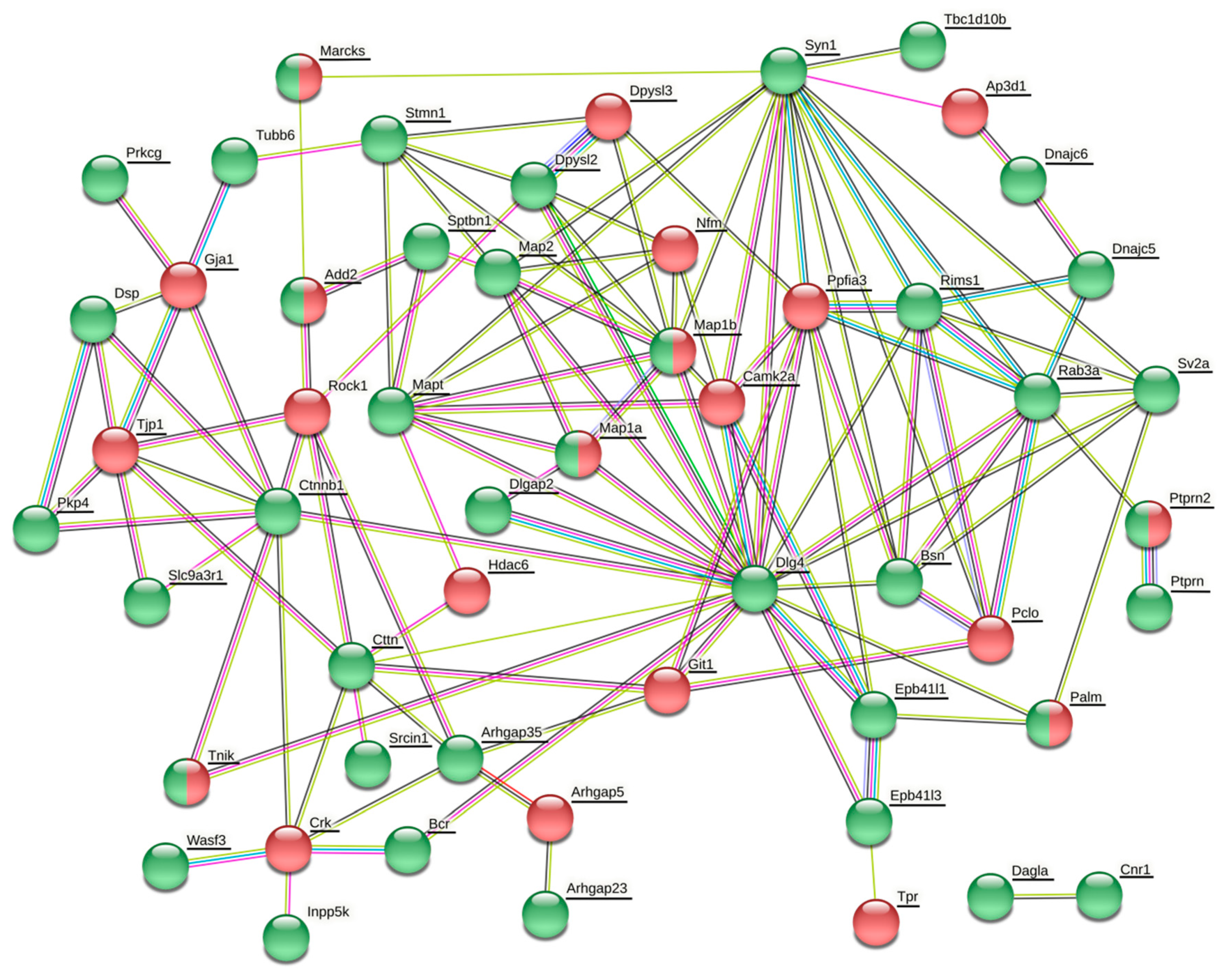
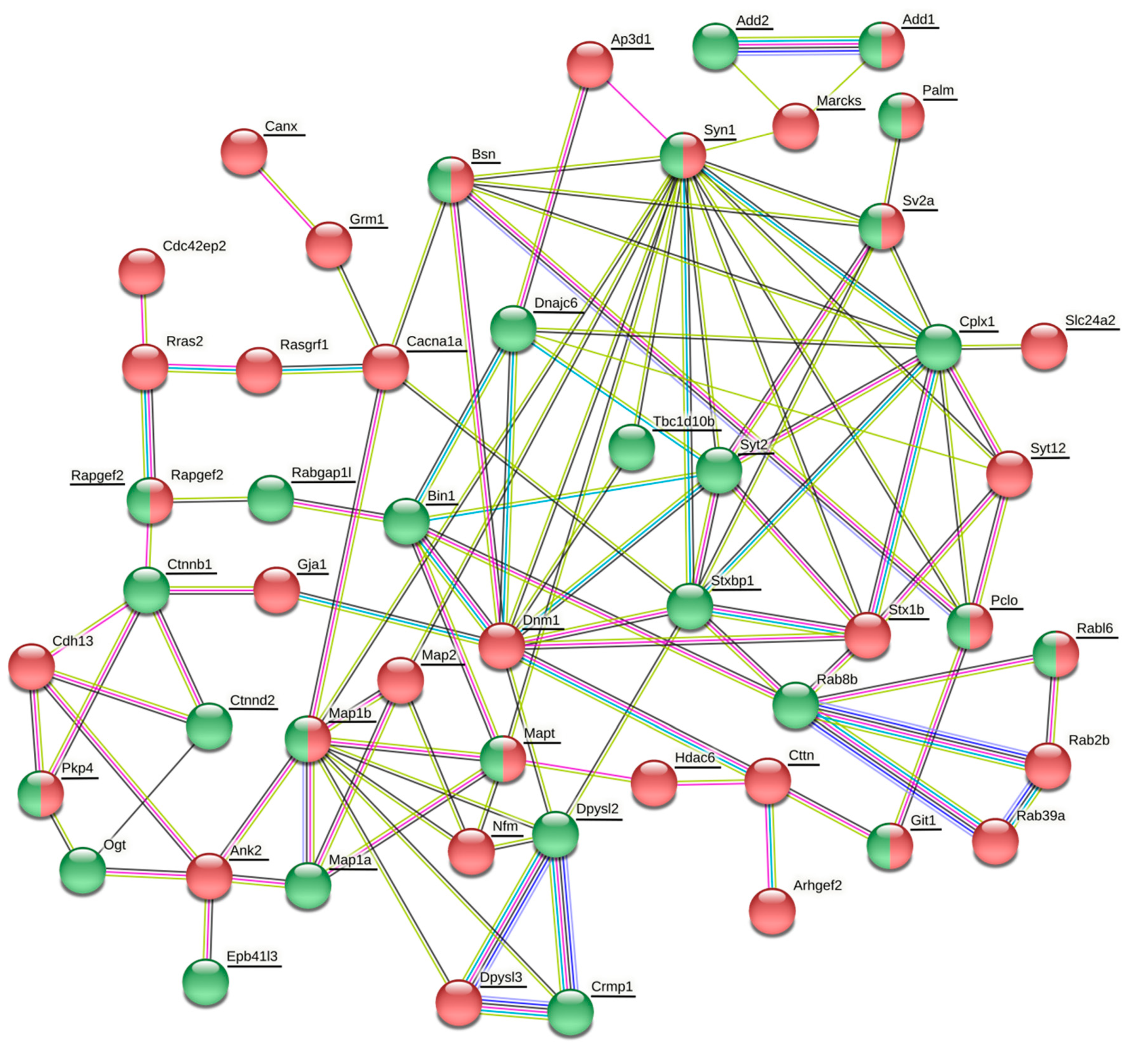
The vesicle cluster near the active zone has been suggested to be the main source for many other proteins, including Rab3, complexin, synaptobrevin (Vamp2), amphiphysin (Bin1), Rim2 (similar to Rims1), bassoon, cortactin, and tubulin [
106]. In our present study, many of these proteins or their isoforms were differentially phosphorylated or expressed (
Table 2,
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7). The vesicle cluster has been proposed to serve as a buffer for soluble accessory proteins involved in vesicle recycling and to ensure that the soluble recycling proteins are delivered upon demand during synaptic activity and thereby to support neurotransmission indirectly [
106]. However, there is yet another possibile way the phosphorylation of synaptic proteins may be employed in the synaptic vesicle cycle. It might contribute to the interaction between vesicles and soluble proteins.
Some proteins from presynaptic and postsynaptic compartments engaged in cell adhesion, scaffolding, exocytosis and neurotransmitter transport may be implicated in several synaptopathies, causing neurological disorders [
5]. Not only mutations and deletions in genes producing synaptic proteins, but also aberrant phosphorylation of proteins related to synaptic plasticity and cytoskeleton organization may play an important role in the pathogenesis of neurological diseases. One of the most studied phosphoprotein whose hyperphosphorylation is associated with the pathology of Alzheimer’s disease is tau [
6,
83,
84]. Tau hyperphosphorylation alters the ability of tau to stabilize microtubules and subsequently impairs axonal transport [
6]. The stabilization of microtubules was shown to improve cognitive function and axonal transport [
107,
108]. The other neurotoxic effects of tau hyperphosphorylation in Alzheimer’s disease include the impairment of long-term depression, NMDA receptor hypofunction, impaired neuronal hyperexcitability and reduced Fyn-induced Src family kinase activity [
6]. In our study, tau was hypophosphorylated only in the cerebellum, but hyperphoshorylated in cortex, hippocampus and striatum. The greatest degree of hyperphosphorylation was detected in the hippocampus and most of the hyperphosphorylated phosphosites were located in proline-rich domains involving tubulin-binding site, the motif contributing to the regulation of tau interaction with microtubules and promoting microtubule polymerization [
90], suggesting that hyperphosphorylation of tau in the hippocampus induced by long-term morphine withdrawal might affect the stabilization of microtubules associated with alterations in cognitive functions.
MAP1A, MAP1B, MAP2, collapsin response mediator proteins, α- and β-adducins, ankyrin 2, Akap12, Stxbp1, Marcks, and stathmin represent another group of differentially phosphorylated phosphoproteins whose aberrant phosphorylation is associated with neurological diseases [
6]. Besides MAPs and collapsing response mediator proteins, stathmins possess microtubule-destabilizing activity, which is mediated by protein phosphorylation [
109,
110]. This suggests that alterations in microtubule stability can be one of the neuroadaptive mechanisms induced by long-term drug withdrawal which might be associated with changes in cognitive functions. A 30-day withdrawal from cocaine self-administration resulted in changes of Src kinase/Srcin1 signaling together with microtubule and actin remodeling followed by increased dendritic spine density and morphological restructuring of dendritic spines in NA [
111]. Our results suggest the Src kinase/Srcin1 signaling as well as microtubule and actin dynamics are affected during morphine withdrawal also in the cortex, hippocampus and striatum by differential phosphorylation of Srcin1 and microtubule- and actin-associated proteins. Repeated morphine treatment elicits changes in the density of dendrites and dendritic spines in the cortex and hippocampus [
112,
113,
114], suggesting that Src kinase/Srcin1 signaling and microtubule/actin dynamics should be a common mechanism affecting the morphology of dendrites and dendritic spines during drug withdrawal. The morphology of dendrites and dendritic spines are also regulated by Rho and Ras family of GTPases [
115], as well as by some regulators of GTPases such as SynGAP, ArhGEF7, ArhGAP35 [
115,
116]. Dendrites and dendritic spines have been recognized to be critical for synaptic plasticity related to cognitive processes such as learning and memory. Reward learning is encoded by dendritic spine changes from the first drug exposure to relapse even long into the withdrawal period [
111].
Phosphoproteomic analysis revealed the phosphosite Ser331 in CaMKII whose phosphorylation was associated with inhibition of CaMKII activity and memory extinction in the amygdala from rats self-administered with cocaine for ten days [
117]. In our study, the phosphosite Ser331 in CaMKII was hyperphoshorylated in the cortex after morphine withdrawal, suggesting memory extinction in cortex associated with opioid-related reward memories [
118]. Because the consequences of morphine use are long-lasting, even many months after the cessation of drug administration, it is highly desirable to find novel strategies that could reverse cellular processes leading to drug relapse. Our results suggest that therapeutic agents affecting the phosphorylation state of synaptic proteins and improving the formation of the reserve pool and morphology of dendritic spines might be considered as potential candidates for restoring synaptic function and thus reversing drug seeking and relapse during protracted withdrawal.