Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review
Abstract
:1. Introduction
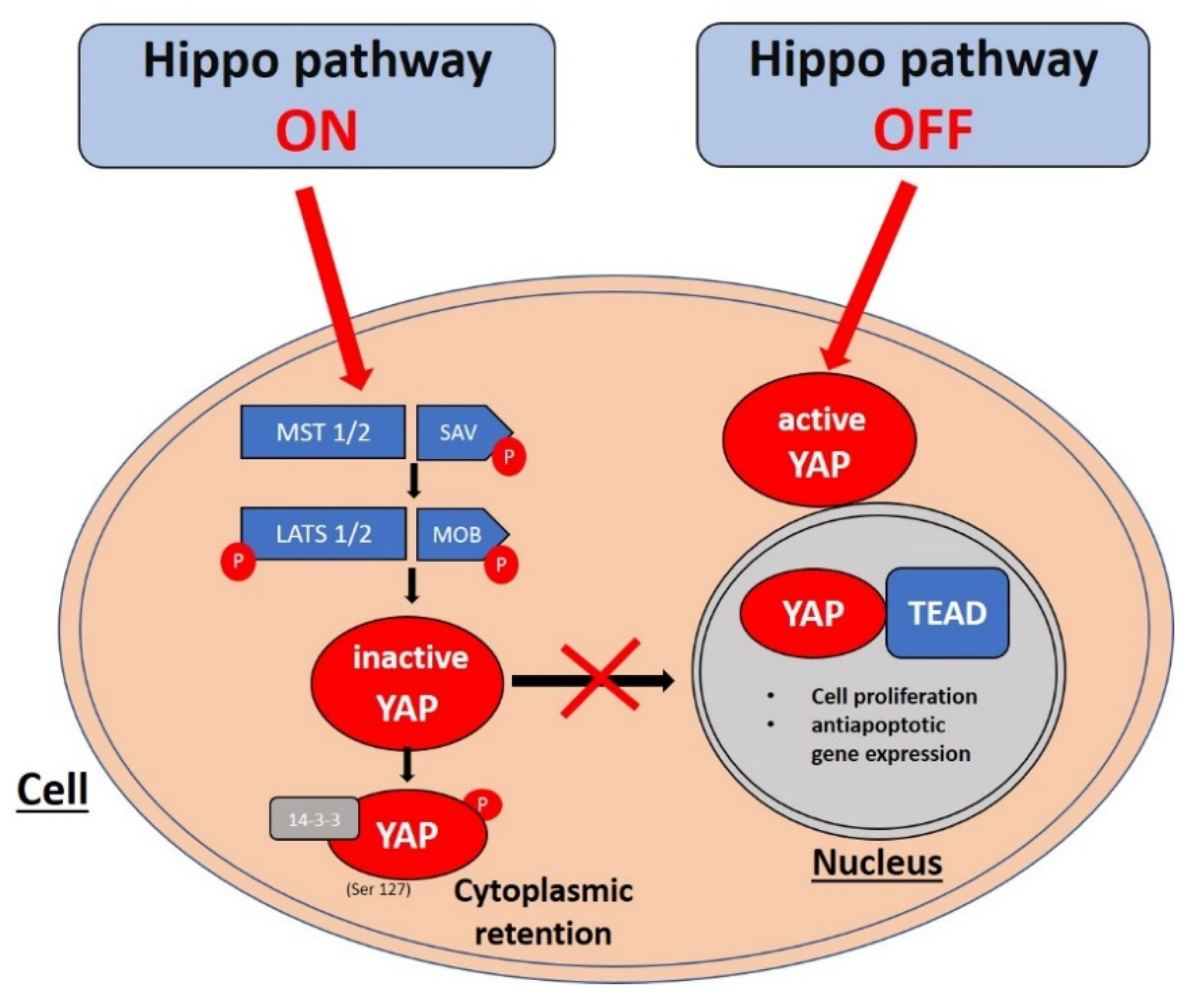
2. Hippo Pathway Signaling Cascade
3. Basal Cell Carcinoma
4. Squamous Cell Carcinoma
5. YAP/Hippo Signaling as a Therapeutic Target in NMSC
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non melanoma skin cancer pathogenesis overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-melanoma skin cancers: Biological and clinical features. Int. J. Mol. Sci. 2020, 21, 5934. [Google Scholar] [CrossRef]
- Justice, R.W.; Zilian, O.; Woods, D.F.; Noll, M.; Bryant, P.J. The Drosophila tumor suppressor gene warts encodes a homolog of hu-man myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995, 9, 534–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overholtzer, M.; Zhang, J.; Smolen, G.A.; Muir, B.; Li, W.; Sgroi, D.C.; Deng, C.-X.; Brugge, J.S.; Haber, D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 2006, 103, 12405–12410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Chen, Q.; Gutkind, J.S. Oncotargeting G proteins: The Hippo in the room. Oncotarget 2014, 5, 10997–10999. [Google Scholar] [CrossRef] [Green Version]
- Lamar, J.M.; Xiao, Y.; Norton, E.; Jiang, Z.-G.; Gerhard, G.M.; Kooner, S.; Warren, J.S.A.; Hynes, R.O. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J. Biol. Chem. 2019, 294, 2302–2317. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Q.; Zhang, Q.; Li, Z.; Wang, E.; Qiu, X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010, 101, 1279–1285. [Google Scholar] [CrossRef]
- Hall, C.A.; Wang, R.; Miao, J.; Oliva, E.; Shen, X.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo Pathway Effector Yap Is an Ovarian Cancer Oncogene. Cancer Res. 2010, 70, 8517–8525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.Z.; Yao, T.-J.; Lee, N.P.Y.; Ng, I.O.L.; Chan, Y.-T.; Zender, L.; Lowe, S.W.; Poon, R.T.P.; Luk, J.M. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009, 115, 4576–4585. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, L.; Purohit, V.; Shukla, S.K.; Chen, X.; Yu, F.; Fu, K.; Chen, Y.; Solheim, J.; Singh, P.K.; et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget 2015, 6, 36019–36031. [Google Scholar] [CrossRef] [Green Version]
- Nallet-Staub, F.; Marsaud, V.; Li, L.; Gilbert, C.; Dodier, S.; Bataille, V.; Sudol, M.; Herlyn, M.; Mauviel, A. Pro-invasive activity of the hippo pathway effectors YAP and TAZ in cutaneous melanoma. J. Investig. Dermatol. 2014, 134, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Steinhardt, A.A.; Gayyed, M.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; AOCS Study Group; George, J.M.; Deb, S.; Degoutin, J.L.; Takano, E.A.; Fox, S.B.; Bowtell, D.; Harvey, K.F. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011, 30, 2810–2822. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Chun, A.; Cheung, K.; Rashidi, B.; Yang, X. Tumor Suppressor LATS1 Is a Negative Regulator of Oncogene YAP. J. Biol. Chem. 2008, 283, 5496–5509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Xia, W.; Fisher, G.J.; Voorhees, J.J.; Quan, T. YAP/TAZ regulates TGF-β/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Commun. Signal. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nat. Cell Biol. 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Strano, S.; Monti, O.; Pediconi, N.; Baccarini, A.; Fontemaggi, G.; Lapi, E.; Mantovani, F.; Damalas, A.; Citro, G.; Sacchi, A.; et al. The transcriptional coactivator yes-associated pro-tein drives p73 gene-target specificity in response to DNA damage. Mol. Cell 2005, 18, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-K.; Yonehara, S. Identification of Mechanism That Couples Multisite Phosphorylation of Yes-associated Protein (YAP) with Transcriptional Coactivation and Regulation of Apoptosis. J. Biol. Chem. 2012, 287, 9568–9578. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Adamovich, Y.; Reuven, N.; Shaul, Y. Yap1 Phosphorylation by c-Abl Is a Critical Step in Selective Activation of Proapoptotic Genes in Response to DNA Damage. Mol. Cell 2008, 29, 350–361. [Google Scholar] [CrossRef]
- Tomlinson, V.; Gudmundsdottir, K.; Luong, P.; Leung, K.-Y.; Knebel, A.; Basu, S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010, 1, e29. [Google Scholar] [CrossRef] [Green Version]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Maturo, M.G.; Di Nardo, L.; Ciciarelli, V.; García-Rodrigo, C.G.; Fargnoli, M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 2485. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, H.J.; Pau, G.; Dijkgraaf, G.J.; Basset-Seguin, N.; Modrusan, Z.; Januario, T.; Tsui, V.; Durham, A.B.; Dlugosz, A.A.; Haverty, P.M.; et al. Genomic Analysis of Smoothened Inhibitor Resistance in Basal Cell Carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Atwood, S.; Chang, A.L.S.; Oro, A.E. Hedgehog pathway inhibition and the race against tumor evolution. J. Cell Biol. 2012, 199, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Xu, Y.; Qin, Z.; Robichaud, P.; Betcher, S.; Calderone, K.; He, T.; Johnson, T.M.; Voorhees, J.J. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: Impact on keratinocyte proliferation and stromal cell activation. Am. J. Pathol. 2014, 184, 937–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debaugnies, M.; Sánchez-Danés, A.; Rorive, S.; Raphaël, M.; Liagre, M.; Parent, M.A.; Brisebarre, A.; Salmon, I.; Blanpain, C. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 2018, 19, e45809. [Google Scholar] [CrossRef]
- Maglic, D.; Schlegelmilch, K.; Dost, A.F.; Panero, R.; Dill, M.T.; Calogero, R.A.; Camargo, F.D. YAP-TEAD signaling promotes basal cell carci-noma development via a c-JUN/AP1 axis. EMBO J. 2018, 37, 98642. [Google Scholar] [CrossRef]
- Lai, D.; Ho, K.C.; Hao, Y.; Yang, X. Taxol Resistance in Breast Cancer Cells Is Mediated by the Hippo Pathway Component TAZ and Its Downstream Transcriptional Targets Cyr61 and CTGF. Cancer Res. 2011, 71, 2728–2738. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pasolli, H.A.; Fuchs, E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA 2011, 108, 2270–2275. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Lau, L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009, 41, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.M.; Gritli-Linde, A.; Smeyne, R.; Kottmann, A.; McMahon, A.P. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev. Biol. 2004, 270, 393–410. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-L, A.; Northcott, P.A.; Dalton, J.; Fraga, C.; Ellison, D.; Angers, S.; Taylor, M.D.; Kenney, A.M. YAP1 is amplified and up-regulated in hedge-hog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009, 23, 2729–2741. [Google Scholar] [CrossRef] [Green Version]
- Akladios, B.; Reinoso, L.M.; Cain, J.E.; Wang, T.; Lambie, D.; Watkins, D.N.; Beverdam, A. Positive regulatory interactions between YAP and Hedgehog signalling in skin homeostasis and BCC development in mouse skin in vivo. PLoS ONE 2017, 12, e0183178. [Google Scholar] [CrossRef] [Green Version]
- Tate, G.; Kishimoto, K.; Mitsuya, T. Biallelic alterations of the large tumor suppressor 1 (LATS1) gene in infiltrative, but not superficial, basal cell carcinomas in a Japanese patient with nevoid basal cell carcinoma syndrome. Med. Mol. Morphol. 2014, 48, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tate, G.; Kishimoto, K.; Mitsuya, T. Biallelic disruption of the PTCH1 gene in multiple basal cell carcinomas in Japanese patients with nevoid basal cell carcinoma syndrome. Acta Med. Okayama 2014, 68, 163–170. [Google Scholar] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldman, A.; Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol. Clin. N. Am. 2019, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, L.; Pellegrini, C.; Di Stefani, A.; Del Regno, L.; Sollena, P.; Piccerillo, A.; Longo, C.; Garbe, C.; Fargnoli, M.C.; Peris, K. Molecular genetics of cutaneous squamous cell carcinoma: Perspective for treatment strategies. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 932–941. [Google Scholar] [CrossRef]
- Sambandam, S.A.; Kasetti, R.B.; Xue, L.; Dean, D.C.; Lu, Q.; Li, Q. 14-3-3σ Regulates Keratinocyte Proliferation and Differentiation by Modulating Yap1 Cellular Localization. J. Investig. Dermatol. 2015, 135, 1621–1628. [Google Scholar] [CrossRef] [Green Version]
- Rognoni, E.; Walko, G. The Roles of YAP/TAZ and the Hippo Pathway in Healthy and Diseased Skin. Cells 2019, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous squamous cell carcinoma: From pathophysiology to novel therapeutic approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Neubert, T.; Lehmann, P. Bowen’s disease—A review of newer treatment options. In Therapeutics and Clinical Risk Management; Dove Press: Macclesfield, UK, 2008; Volume 4, pp. 1085–1095. [Google Scholar]
- Venna, S.S.; Lee, D.; Stadecker, M.J.; Rogers, G.S. Clinical recognition of actinic keratoses in a high-risk population: How good are we? Arch. Dermatol. 2005, 141, 507–509. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Li, C.; Luo, S.; Liu-Smith, F.; Yang, J.; Wang, X.; Wang, N.; Lai, B.; Lei, T.; Wang, Q.; et al. Yes-Associated Protein Contributes to the Development of Human Cutaneous Squamous Cell Carcinoma via Activation of RAS. J. Investig. Dermatol. 2016, 136, 1267–1277. [Google Scholar] [CrossRef] [Green Version]
- Al-Busani, H.; Al-Sobaihi, S.; Nojima, K.; Tanemura, A.; Yaguchi, T.; Kawakami, Y.; Matsumura, H.; Nishimura, E.K.; Yokozeki, H.; Namiki, T. NUAK2 localization in normal skin and its expression in a variety of skin tumors with YAP. J. Dermatol. Sci. 2020, 97, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 Acts Downstream of α-Catenin to Control Epidermal Proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2018, 17, 1218–1227. [Google Scholar] [CrossRef]
- Piva de Freitas, P.; Senna, C.G.; Tabai, M.; Chone, C.T.; Altemani, A. Metastatic Basal Cell Carcinoma: A Rare Manifestation of a Common Disease. Case Rep. Med. 2017, 2017, 8929745. [Google Scholar] [CrossRef] [Green Version]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Hollmig, S.T.; Sachdev, R.; Cockerell, C.J.; Posten, W.; Chiang, M.; Kim, J. Spindle cell neoplasms encountered in dermatologic sur-gery: A review. Dermatol. Surg. 2012, 38, 825–850. [Google Scholar] [CrossRef]
- Toll, A.; Masferrer, E.; Hernández-Ruiz, M.; Ferrandiz-Pulido, C.; Yébenes, M.; Jaka, A.; Tuneu, A.; Jucglà, A.; Gimeno, J.; Baró, T.; et al. Epithelial to mesenchymal transition markers are associated with an increased metastatic risk in primary cutaneous squamous cell carcinomas but are attenuated in lymph node metastases. J. Dermatol. Sci. 2013, 72, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Mistiaen, Z.; Elbediwy, A.; Vanyai, H.; Cotton, J.; Stamp, G.; Nye, E.; Spencer-Dene, B.; Thomas, G.J.; Mao, J.; Thompson, B. YAP drives cutaneous squamous cell carcinoma formation and progression. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Kai, F.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef]
- cBioPortal for Cancer Genomics. Available online: http://www.cbioportal.org/ (accessed on 30 May 2021).
- Juan, W.C.; Hong, W. Targeting the Hippo signaling pathway for tissue regeneration and cancer therapy. Genes 2016, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Pobbati, A.V.; Hong, W. A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy. Theranostics 2020, 10, 3622–3635. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.-J.; Anders, R.A.; Liu, Y.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pobbati, A.V.; Han, X.; Hung, A.W.; Weiguang, S.; Huda, N.; Chen, G.Y.; Kang, C.; Chia, C.S.B.; Luo, X.; Hong, W.; et al. Targeting the Central Pocket in Human Transcrip-tion Factor TEAD as a Potential Cancer Therapeutic Strategy. Structure 2015, 23, 2076–2086. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, G.; Ruggeri, N.; Specchia, V.; Cordenonsi, M.; Mano, M.; Dupont, S.; Manfrin, A.; Ingallina, E.; Sommaggio, R.; Piazza, S.; et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014, 16, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.W.; Xiao, S.; Ogomori, K.; Hammarstedt, J.; Little, E.C.; Lang, D. The Efficiency of Verteporfin as a Therapeutic Option in Pre-Clinical Models of Melanoma. J. Cancer 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halgren, T.A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Deng, X.; Fang, L. VGLL4 is a transcriptional cofactor acting as a novel tumor suppressor via interacting with TEADs. Am. J. Cancer Res. 2018, 8, 932–943. [Google Scholar] [PubMed]
- Jiao, S.; Wang, H.; Shi, Z.; Dong, A.; Zhang, W.; Song, X.; He, F.; Wang, Y.; Zhang, Z.; Wang, W.; et al. A Peptide Mimicking VGLL4 Function Acts as a YAP Antagonist Therapy against Gastric Cancer. Cancer Cell 2014, 25, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Neoadjuvant Zoledronate and Atorvastatin in Triple Negative Breast Cancer (YAPPETIZER). Available online: https://clinicaltrials.gov/ct2/show/NCT03358017 (accessed on 31 May 2021).
| Gene Name | Mutation Frequency (%) | |
|---|---|---|
| BCC (n = 293) | cSCC (n = 151) | |
| YAP | 1.7 | 2.6 |
| TEAD1 | 0.7 | 0.7 |
| TEAD2 | 1.4 | 7.3 |
| TEAD3 | - | 2.0 |
| TEAD4 | 0.3 | 2.6 |
| LATS1 | 8.5 | 11.9 |
| LATS2 | 9.2 | 11.3 |
| MST1 | 1.7 | 3.3 |
| MST2 | - | - |
| Compound | Mechanism of Action | Clinical Relevance | References |
|---|---|---|---|
| Verteporfin | Directly binds YAP | No effect on melanoma initiation/progression | [61,64] |
| Flufenamic acid | Disrupts YAP–TEAD interaction | None reported | [62] |
| VGLL-4-mimicking peptide | Disrupts YAP–TEAD interaction | Inhibits in vitro growth of primary human gastric cancer | [66] |
| Zoledronic acid | Inhibits YAP phosphorylation | In clinical trial in triple-negative breast cancer | [63,68] |
| Statins | Inhibits YAP phosphorylation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarski, I.A.; Ciążyńska, M.; Wódz, K.; Dróżdż, I.; Skibińska, M.; Narbutt, J.; Lesiak, A. Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review. Life 2021, 11, 680. https://doi.org/10.3390/life11070680
Bednarski IA, Ciążyńska M, Wódz K, Dróżdż I, Skibińska M, Narbutt J, Lesiak A. Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review. Life. 2021; 11(7):680. https://doi.org/10.3390/life11070680
Chicago/Turabian StyleBednarski, Igor Aleksander, Magdalena Ciążyńska, Karolina Wódz, Izabela Dróżdż, Małgorzata Skibińska, Joanna Narbutt, and Aleksandra Lesiak. 2021. "Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review" Life 11, no. 7: 680. https://doi.org/10.3390/life11070680
APA StyleBednarski, I. A., Ciążyńska, M., Wódz, K., Dróżdż, I., Skibińska, M., Narbutt, J., & Lesiak, A. (2021). Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review. Life, 11(7), 680. https://doi.org/10.3390/life11070680







