Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Plant Material
2.2. Preliminary Phytochemical Analysis
2.3. Antimicrobial Assays
2.4. DPPH Free Radical Scavenging Assay
2.5. Anticancer Assays
3. Results and Discussion
3.1. Phytochemical Screening
3.2. Antimicrobial Assay
3.3. Antioxidant Activity
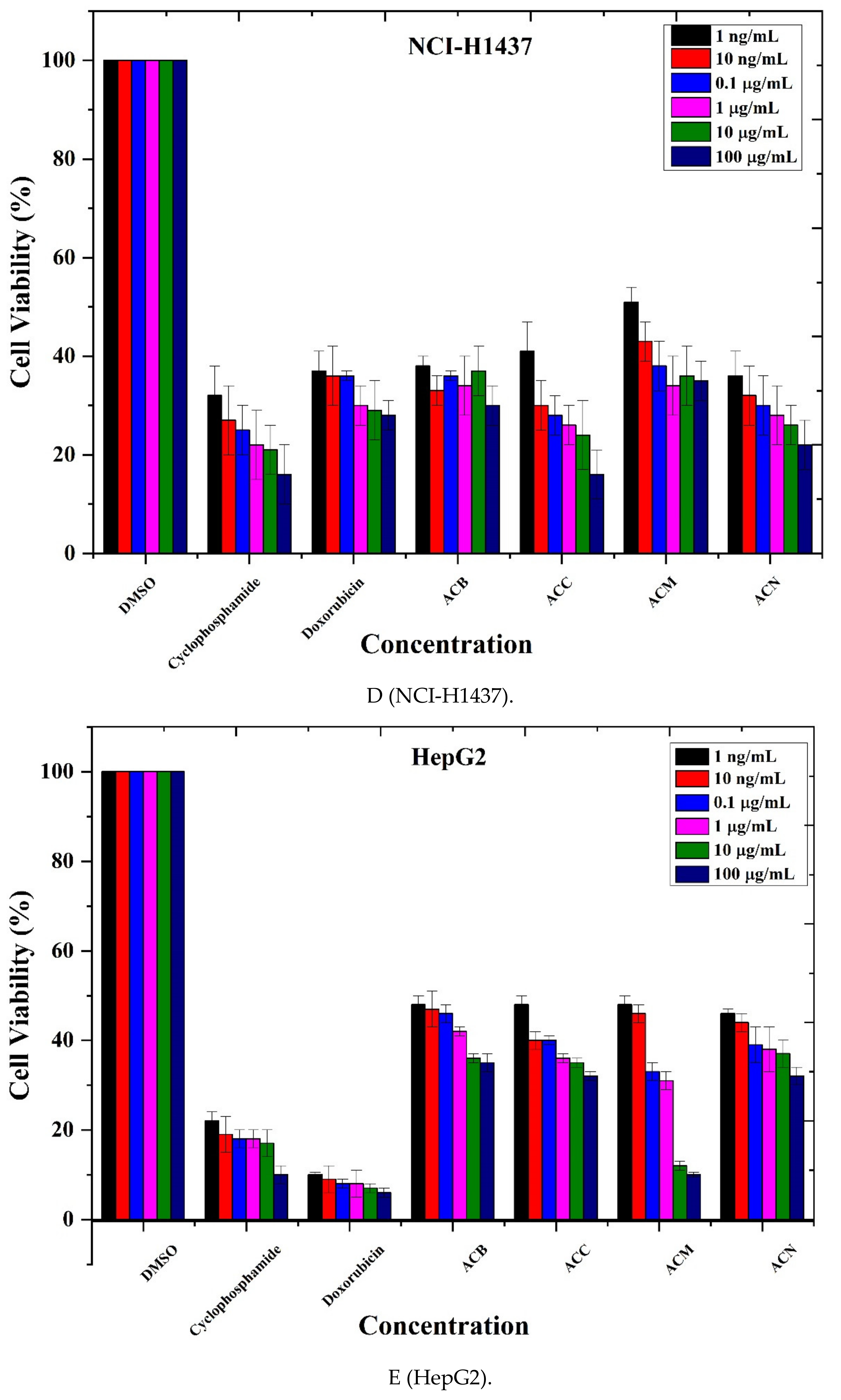
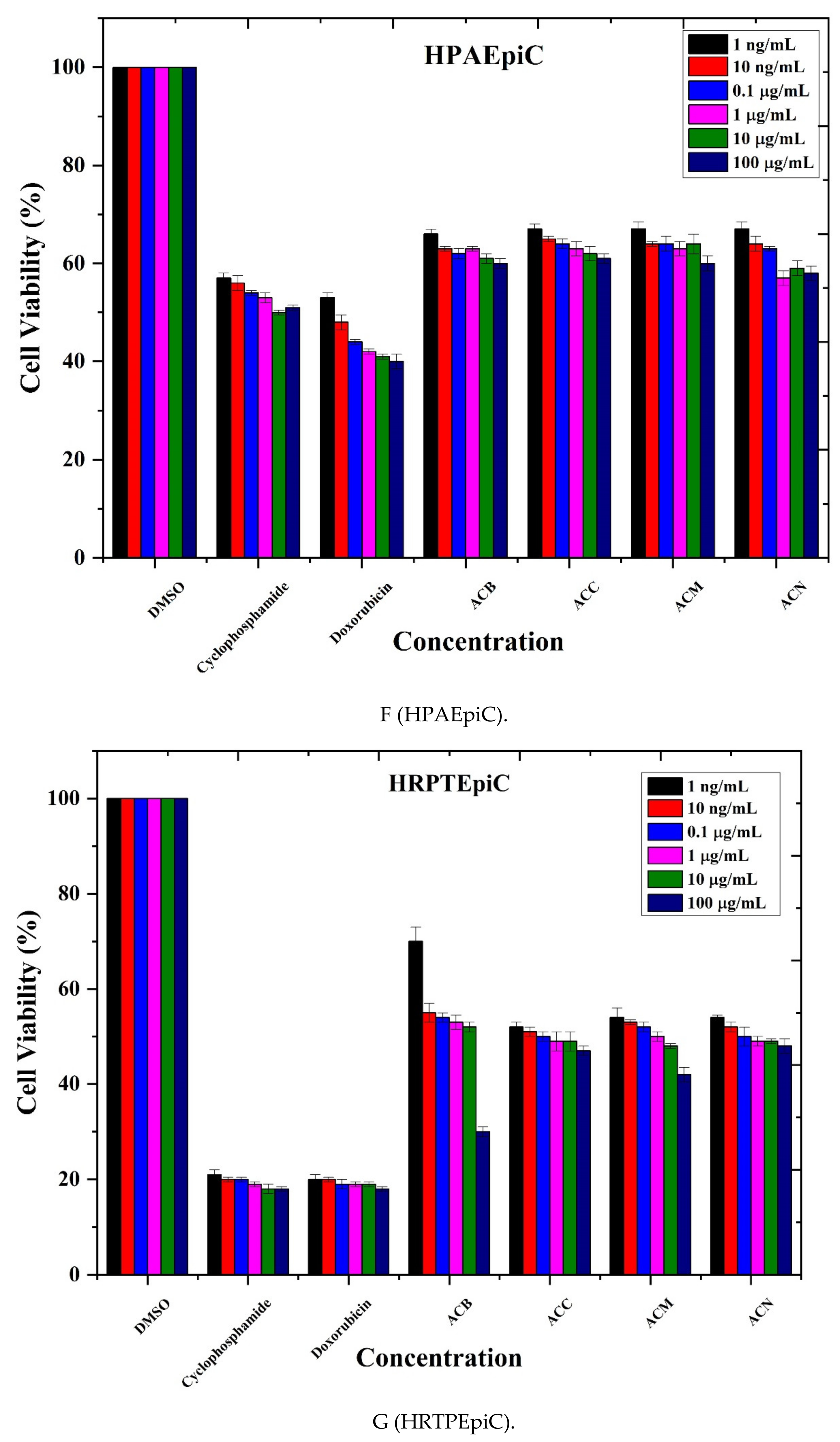
3.4. Anticancer Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef]
- Baldé, M.A.; Tuenter, E.; Traoré, M.S.; Matheeussen, A.; Cos, P.; Maes, L.; Camara, A.; Haba, N.L.; Gomou, K.; Diallo, M.S.T.; et al. Antimicrobial investigation of ethnobotanically selected guinean plant species. J. Ethnopharmacol. 2020, 263, 113232. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Couture, J.J.; Singh, A.; Rubert-Nason, K.F.; Serbin, S.P.; Lindroth, R.L.; Townsend, P.A. Spectroscopic determination of ecologically relevant plant secondary metabolites. Methods Ecol. Evol. 2016, 7, 1402–1412. [Google Scholar] [CrossRef]
- Lee, S.; Oh, D.-G.; Singh, D.; Lee, J.S.; Lee, S.; Lee, C.H. Exploring the metabolomic diversity of plant species across spatial (leaf and stem) components and phylogenic groups. BMC Plant Biol. 2020, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Tlili, H.; Hanen, N.; Ben Arfa, A.; Neffati, M.; Boubakri, A.; Buonocore, D.; Dossena, M.; Verri, M.; Doria, E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 2019, 14, e0213049. [Google Scholar] [CrossRef]
- Murugan, T.; Wins, J.A.; Murugan, M. Antimicrobial activity and phytochemical constituents of leaf extracts of Cassia auriculata. Indian J. Pharm. Sci. 2013, 75, 122–125. [Google Scholar] [CrossRef]
- Bhaskarachary, K.; Naveena, N.; Polasa, K. Potential benefits of plant metabolites for human health. IJFND 2015, 52, 213–225. [Google Scholar]
- Seca, A.M.; Pinto, D.C. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef]
- Hamayun, M. Ethnobotanical studies of some useful shrubs and trees of District Buner, NWFP, Pakistan. Ethnobot. Leafl. 2003, 2003, 12. [Google Scholar]
- Bandar, H.; Hijazi, A.; Rammal, H.; Hachem, A.; Saad, Z.; Badran, B. Techniques for the extraction of bioactive compounds from Lebanese Urtica Dioica. Am. J. Phytomedicine Clin. Ther. 2013, 1, 507–513. [Google Scholar]
- NarasingaRao, V.; Dowluru, K. Phytochemical and biochemical studies of medicinal plant Globba bulbifera. Int. J. Phytother. 2014, 4, 50–53. [Google Scholar]
- Weli, A.M.; AL-Hinai, J.R.; Al-Mjrafi, J.M.; Alnaaimi, J.R.; Hossain, M.A.; Saeed, S.; Aktar, M.S. Effect of different polarities leaves crude extracts of Omani juniperus excels on antioxidant, antimicrobial and cytotoxic activities and their biochemical screening. Asian Pac. J. Reprod. 2014, 3, 218–223. [Google Scholar] [CrossRef]
- Ventura, J.; Fernandes, P.; Meira, D.; Sales, M.; Costa, H. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar]
- Arulmozhi, P.; Vijayakumar, S.; Kumar, T. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected pathogenic microorganisms. Microb. Pathog. 2018, 123, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Qureshi, R.A.; Khan, J.; Ullah, F.; Fahad, S.; Ullah, F.; Khan, A.M.; Hussain, I.; Khan, N. Pharmacological screening of Morchella esculenta (L.) Pers. Calvatia gigantea (Batsch ex Pers.) Lloyd and Astraeus hygrometricus Pers. mushroom collected from South Waziristan (FATA.). J. Med. Plants Res. 2012, 6, 1853–1859. [Google Scholar]
- Ahmad, S.; AbdEl-Salam, N.M.; Ullah, R. In vitro antimicrobial bioassays, DPPH radical scavenging activity, and FTIR spectroscopy analysis of Heliotropium bacciferum. BioMed Res. Int. 2016, 2016, 3818945. [Google Scholar] [CrossRef]
- Malki, F.; Touati, A.; Moulay, S. Extraction and recrystallization of mesoionic pyrimidinium betaines. Int. J. Chem. Eng. Appl. 2014, 5, 151. [Google Scholar] [CrossRef][Green Version]
- Ogbole, O.; Segun, P.; Akinleye, T.; Fasinu, P. Antiprotozoal, antiviral and cytotoxic properties of the Nigerian Mushroom, Hypoxylon fuscum Pers. Fr.(Xylariaceae). ACTA Pharm. Sci. 2018, 56, 43. [Google Scholar] [CrossRef]
- Thakkar, K.; Prasad, A.; Nayak, J.; Iyer, S.V.; Kumar, S. Antioxidant and in vitro cytotoxic activity of extracts of aerial parts of Cocculus hirsutus (L) using cell line cultures (breast cell line). J. Phytopharm. 2014, 3, 395–399. [Google Scholar]
- Chan, S.M.; Khoo, K.S.; Sit, N.W. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop. J. Pharm. Res. 2015, 14, 1991–1998. [Google Scholar] [CrossRef]
- Pareira, A.G.; Azevedo, E.; Pereira, F.; Ferreira, L.; Azevedo, R. Antimicrobial Potential of Plant Extracts from the Pitangui-Mg Region, Brazil. Arch. Biomed. Eng. Biotechnol. 2019, 1, 1. [Google Scholar]
- Cen, Y.-K.; Lin, J.-G.; Wang, Y.-L.; Wang, J.-Y.; Liu, Z.-Q.; Zheng, Y.-G. The gibberellin producer Fusarium fujikuroi: Methods and technologies in the current toolkit. Front. Bioeng. Biotechnol. 2020, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.; Robson, J. Complete resolution of pulmonary Rhizopus oryzae infection with itraconazole treatment: More evidence of the utility of azoles for zygomycosis. Mycoses 2004, 47, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Osburn, R.; Schroth, M.; Hancock, J.; Hendson, M. Dynamics of sugar beet seed colonization by Pythium ultimum and Pseudomonas species: Effects on seed rot and damping-off. Phytopathology 1989, 79, 709–716. [Google Scholar] [CrossRef]
- Nazir, N.; Khalil, A.A.K.; Nisar, M.; Zahoor, M.; Ahmad, S. HPLC-UV characterization, anticholinesterase, and free radical-scavenging activities of Rosa moschata Herrm. leaves and fruits methanolic extracts. Braz. J. Bot. 2020, 43, 523–530. [Google Scholar] [CrossRef]
- Rezaeizadeh, A.; Zuki, A.; Abdollahi, M.; Goh, Y.; Noordin, M.; Hamid, M.; Azmi, T. Determination of antioxidant activity in methanolic and chloroformic extracts of Momordica charantia. Afr. J. Biotechnol. 2011, 10, 4932–4940. [Google Scholar]
- Sowndhararajan, K.; Kang, S.C. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J. Biol. Sci. 2013, 20, 319–325. [Google Scholar] [PubMed]
- Nemati, F.; Dehpouri, A.A.; Eslami, B.; Mahdavi, V.; Mirzanejad, S. Cytotoxic properties of some medicinal plant extracts from Mazandaran, Iran. Iran. Red Crescent Med. J. 2013, 15, e8871. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N. Cytotoxicity evaluation of methanol extracts of some medicinal plants on P19 embryonal carcinoma cells. J. Appl. Pharm. Sci. 2017, 7, 142–149. [Google Scholar]
- Lombardi, V.R.; Carrera, I.; Cacabelos, R. In vitro screening for cytotoxic activity of herbal extracts. Evid. Based Complement. Altern. Med. 2017, 2017, 2675631. [Google Scholar] [CrossRef] [PubMed]
- Nelson, V.K.; Sahoo, N.K.; Sahu, M.; Sudhan, H.H.; Pullaiah, C.P.; Muralikrishna, K.S. In vitro anticancer activity of Eclipta alba whole plant extract on colon cancer cell HCT-116. BMC Complement. Med. Ther. 2020, 20, 2–8. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Li, L.; Seeram, N.P. Anticancer effects of maple syrup phenolics and extracts on proliferation, apoptosis, and cell cycle arrest of human colon cells. J. Funct. Foods 2012, 4, 185–196. [Google Scholar] [CrossRef]
- Eo, H.J.; Park, G.H.; Kim, D.S.; Kang, Y.; Park, Y. Antioxidant and Anticancer Activities of Leaves Extracts from Acer tegmentosum. Korean J. Plant Resour. 2020, 33, 551–557. [Google Scholar]




| Constituents | Tests | ACB | ACC | ACE | ACM | ACN |
|---|---|---|---|---|---|---|
| Alkaloids | Mayer’s reagent test | + | + | + | + | + |
| Tannins | FeCl3 test | + | − | − | + | − |
| Alkaline reagent test | + | − | − | + | − | |
| Sterol | − | − | − | − | − | |
| Steroids | − | − | − | + | − | |
| Phytosteroids | + | − | + | − | − | |
| Glycosides | + | + | + | + | + | |
| Quinones | + | + | + | + | − | |
| Protein detection | xanthoprotiec test | + | + | + | + | + |
| Isolates | B. subtilus | E. coli | K. pneumonia | S. entrica | A. baumanni |
|---|---|---|---|---|---|
| Extracts | Zone of Inhibition (mm) | ||||
| ACB | 15 | 14.5 | 15 | 11.5 | 16 |
| ACC | 9.5 | 10.5 | 10 | 0 | 14 |
| ACE | 12 | 13.5 | 14.5 | 12 | 13 |
| ACM | 16.5 | 16.25 | 16.5 | 15 | 11 |
| ACN | 13 | 11 | 11.5 | 0 | 12 |
| Extracts | F. fujikuroi | R. oryzae | P. ultimum |
|---|---|---|---|
| ACB | 47 | 47 | 53 |
| ACC | 34 | 45 | 53 |
| ACE | 50 | 51 | 52 |
| ACM | 48 | 52 | 49 |
| ACN | 40 | 49 | 56 |
| Positive control | 56 | 79 | 62 |
| Sample | IC50 (μg/mL) |
|---|---|
| ACB | 0.5233 |
| ACC | 0.3597 |
| ACE | 0.5146 |
| ACM | 0.5987 |
| ACN | 0.5108 |
| Ascorbic acid | 15.67 |
| Cell Lines | % Age Cell Viability | IC50 Value | |
|---|---|---|---|
| Cancer Cell Lines | A549 | 20% | 5.88 |
| Caco 2 | 10–20% | 6.16 | |
| MDA MB-321 | 20% | 7.11 | |
| NCI-H1437 | 30–35% | 8.97 | |
| HepG2 | 40–50% | 9.11 | |
| Normal Cell Lines | HPAEpiC | 57–68% | 13.44 |
| HRPTEpiC | 50–60% | 14.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, F.; Farooqi, M.-A.; Farooqi, H.-M.-U.; Salih, A.-R.-C.; Khalil, A.-A.-K.; Kang, C.-w.; Mahmoud, M.H.; Batiha, G.-E.-S.; Choi, K.-h.; Mumtaz, A.-S. Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled. Life 2021, 11, 656. https://doi.org/10.3390/life11070656
Kausar F, Farooqi M-A, Farooqi H-M-U, Salih A-R-C, Khalil A-A-K, Kang C-w, Mahmoud MH, Batiha G-E-S, Choi K-h, Mumtaz A-S. Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled. Life. 2021; 11(7):656. https://doi.org/10.3390/life11070656
Chicago/Turabian StyleKausar, Farzana, Muhammad-Awais Farooqi, Hafiz-Muhammad-Umer Farooqi, Abdul-Rahim-Chethikkattuveli Salih, Atif-Ali-Khan Khalil, Chul-woong Kang, Mohamed H. Mahmoud, Gaber-El-Saber Batiha, Kyung-hyun Choi, and Abdul-Samad Mumtaz. 2021. "Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled" Life 11, no. 7: 656. https://doi.org/10.3390/life11070656
APA StyleKausar, F., Farooqi, M.-A., Farooqi, H.-M.-U., Salih, A.-R.-C., Khalil, A.-A.-K., Kang, C.-w., Mahmoud, M. H., Batiha, G.-E.-S., Choi, K.-h., & Mumtaz, A.-S. (2021). Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled. Life, 11(7), 656. https://doi.org/10.3390/life11070656






