Molecular Components of Nitrogen Fixation Gene Cluster and Associated Enzymatic Activities of Non-Heterocystous Thermophilic Cyanobacterium Thermoleptolyngbya sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genomic Sequence Information
2.2. Nitrogen Fixation Gene Cluster, ANI and AAI Analysis
2.3. Phylogenetic Analysis and KEGG Pathway
2.4. B121 Cultivation and Maintenance
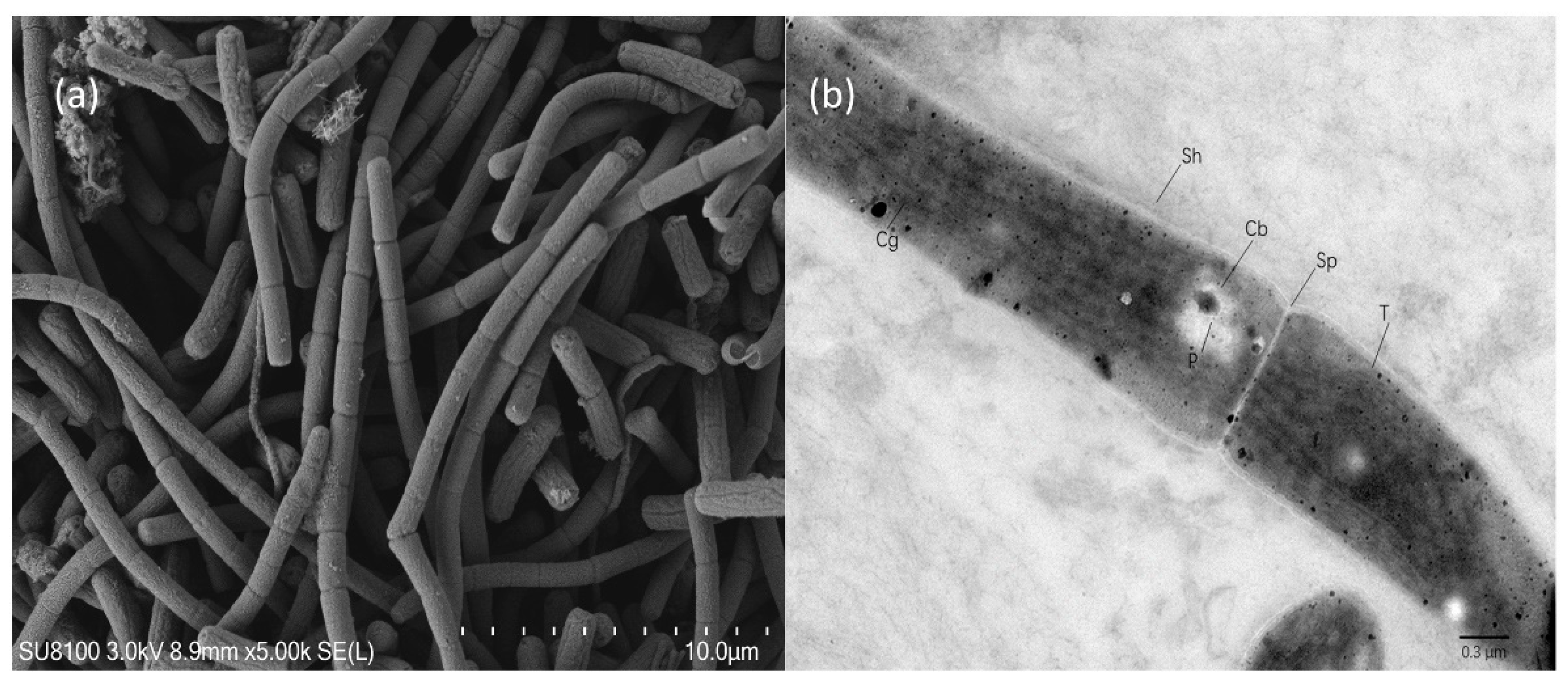
2.5. B121 Electron Microscopy
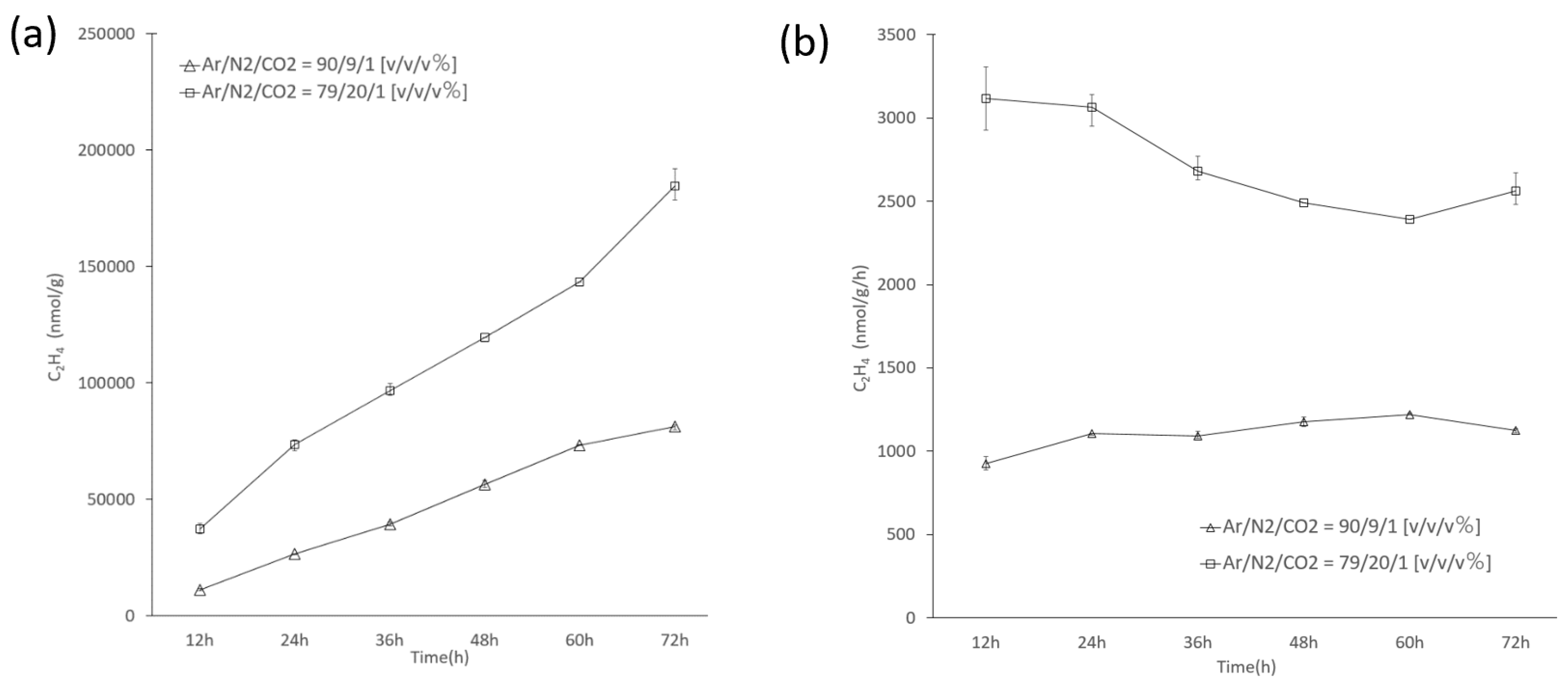
2.6. B121 Nitrogenase Assay
3. Results
3.1. Nitrogen Fixation Gene Cluster, ANI and AAI Analysis
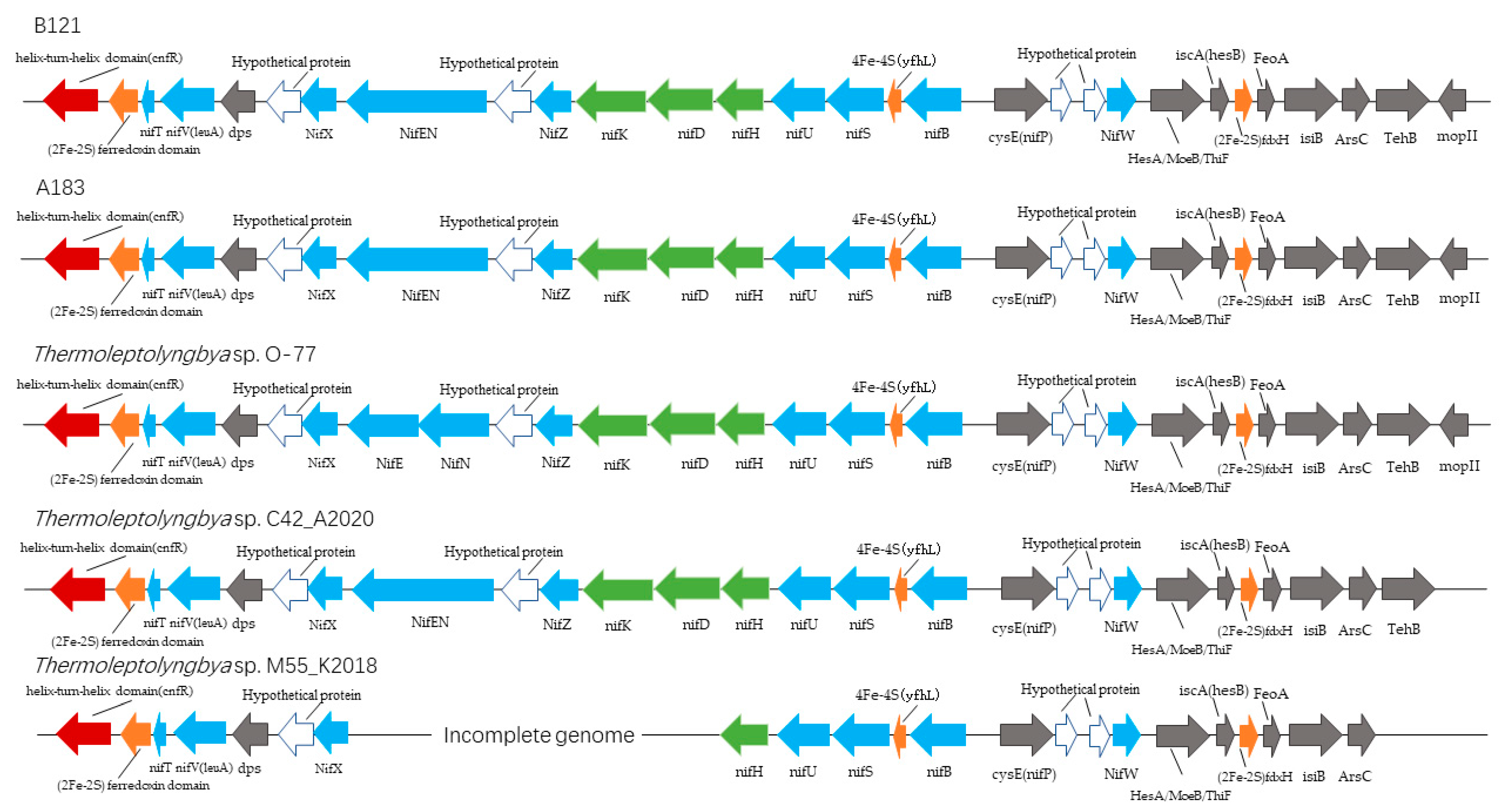
3.1.1. Nitrogen Fixation Gene Cluster
3.1.2. ANI and AAI Analysis
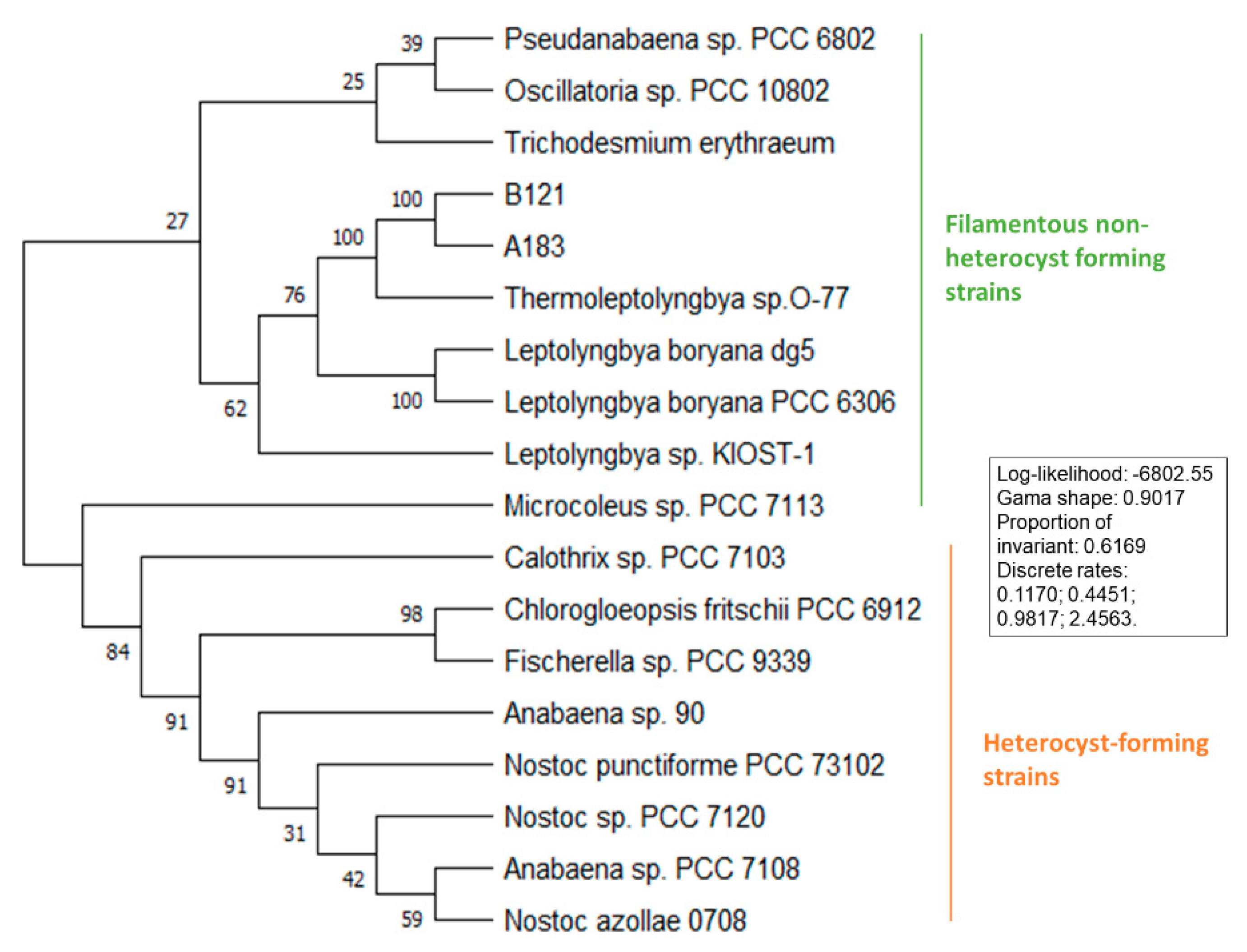
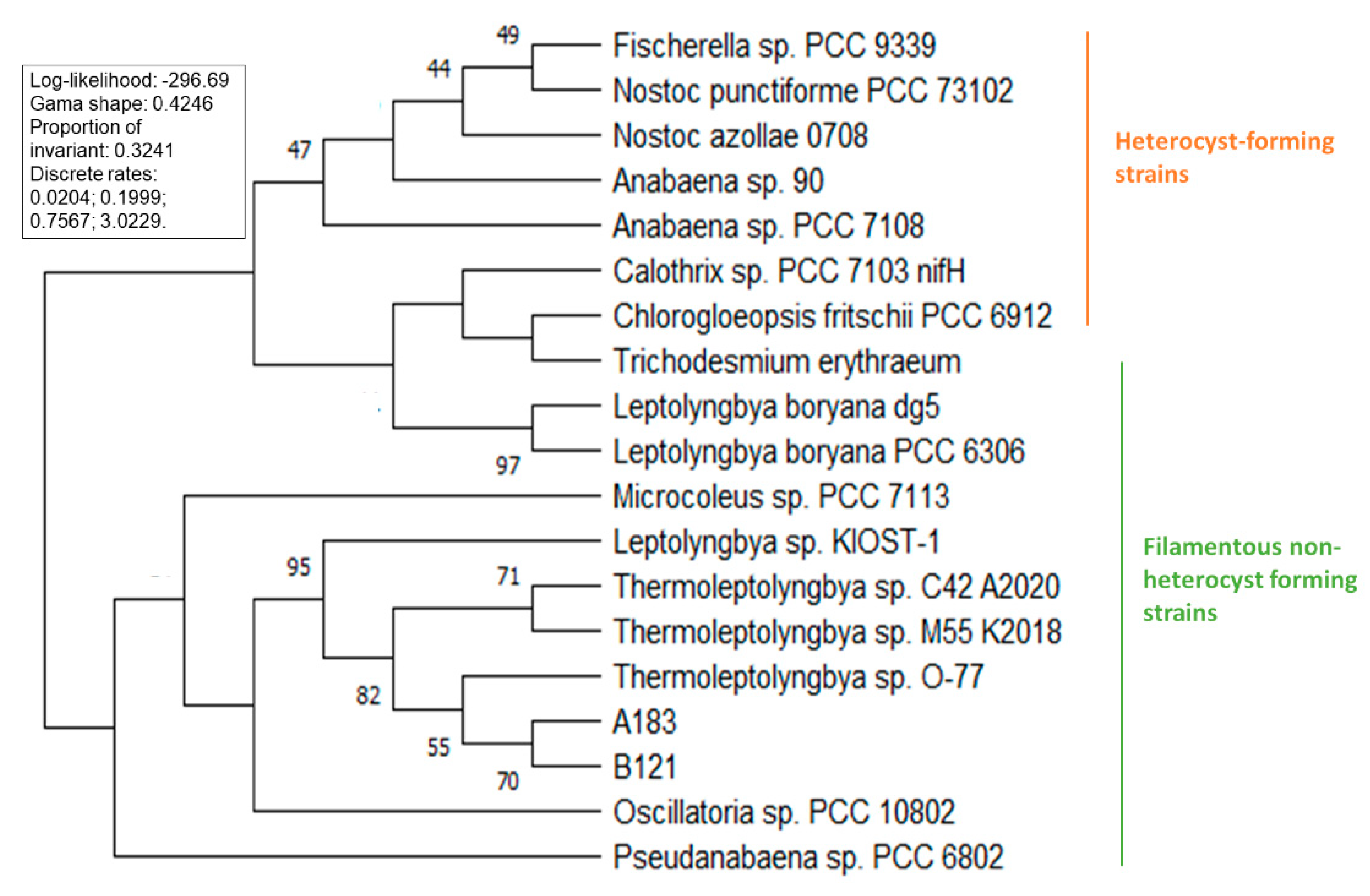
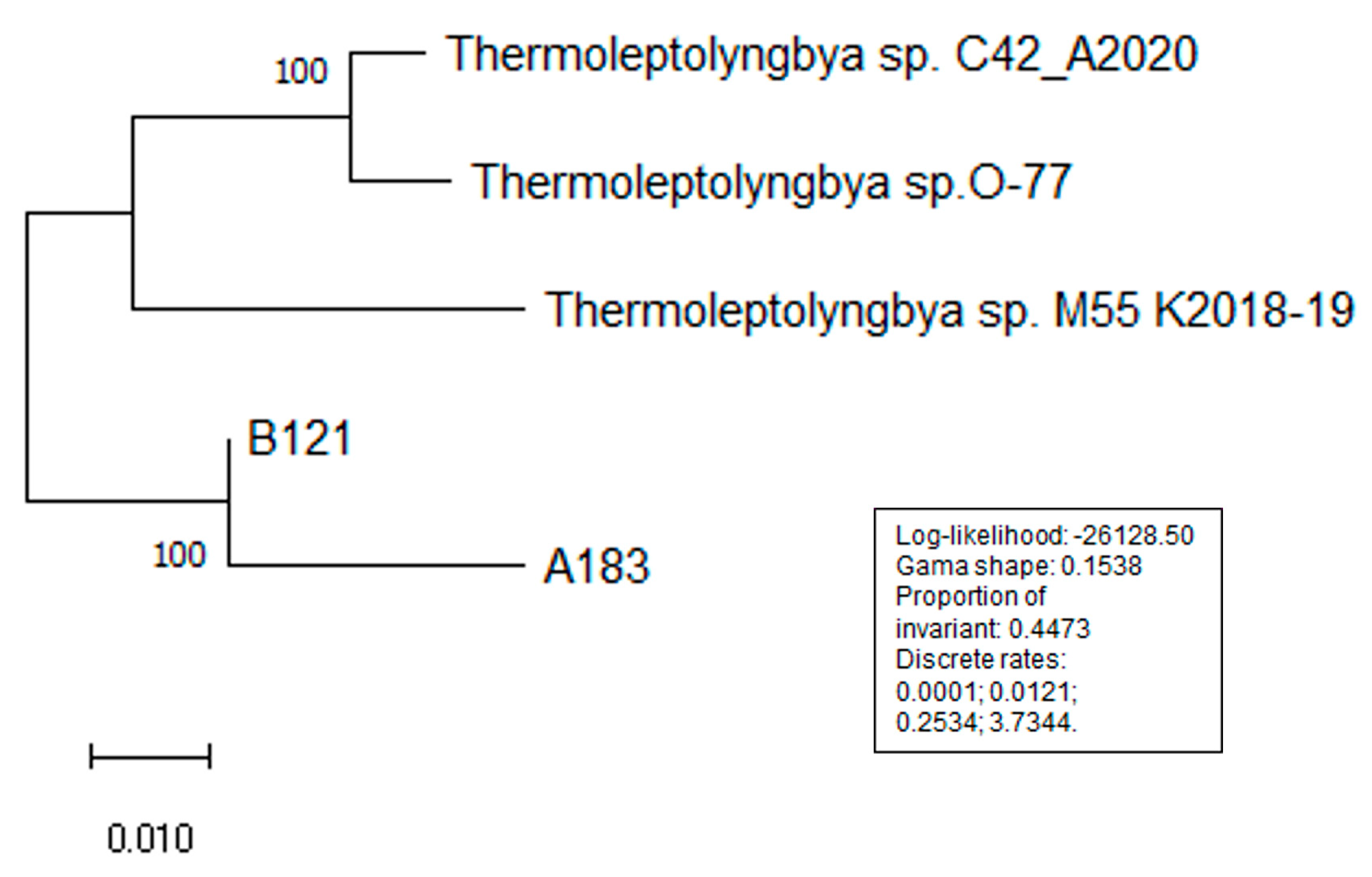
3.2. Phylogenetic Analysis
3.3. Morphological Investigation of B121
3.4. Nitrogenase Activity of Cell Suspensions of Thermoleptolyngbya sp. B121 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiel, T. Organization and regulation of cyanobacterial nif gene clusters: Implications for nitrogenase expression in plant cells. FEMS Microbiol. Lett. 2019, 366, 1–8. [Google Scholar] [CrossRef]
- Fay, P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 1992, 56, 340–373. [Google Scholar] [CrossRef] [PubMed]
- Steunou, A.S.; Bhaya, D.; Bateson, M.M.; Melendrez, M.C.; Ward, D.M.; Brecht, E.; Peters, J.W.; Kühl, M.; Grossman, A.R. In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc. Natl. Acad. Sci. USA 2006, 103, 2398–2403. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, T.L.; Lange, R.K.; Boyd, E.S.; Peters, J.W. Biological nitrogen fixation in acidic high-temperature geothermal springs in Yellowstone National Park, Wyoming. Environ. Microbiol. 2011, 13, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liang, Y.; Jiang, D.; Li, L.; Luo, Y.; Shah, M.R.; Daroch, M. Temperature-controlled thermophilic bacterial communities in hot springs of western Sichuan, China. BMC Microbiol. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Kaczmarek, M.B.; Kasprzak, A.K.; Tang, J.; Shah, M.M.R.; Jin, P.; Klepacz-Smółka, A.; Cheng, J.J.; Daroch, M. Thermosynechococcaceae as a source of thermostable C-phycocyanins: Properties and molecular insights. Algal Res. 2018, 35, 223–235. [Google Scholar] [CrossRef]
- Alcorta, J.; Vergara-Barros, P.; Antonaru, L.A.; Alcamán-Arias, M.E.; Nürnberg, D.J.; Díez, B. Fischerella thermalis: A model organism to study thermophilic diazotrophy, photosynthesis and multicellularity in cyanobacteria. Extremophiles 2019, 23, 635–647. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hallenbeck, P.C.; Benemann, J.R. Nitrogen Fixation by Thermophilic Blue-Green Algae (Cyanobacteria): Temperature Characteristics and Potential Use in Biophotolysis. Appl. Environ. Microbiol. 1979, 37, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Bergman, B.; Gallon, J.; Rai, A.; Stal, L.J. N2 Fixation by non-heterocystous cyanobacteria. FEMS Microbiol. Rev. 1996, 19, 139–185. [Google Scholar] [CrossRef]
- Esteves-Ferreira, A.A.; Cavalcanti, J.H.F.; Vaz, M.G.M.V.; Alvarenga, L.V.; Nunes-Nesi, A.; Araújo, W.L. Cyanobacterial nitrogenases: Phylogenetic diversity, regulation and functional predictions. Genet. Mol. Biol. 2017, 40, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Janson, S.; Carpenter, E.J.; Bergman, B. Compartmentalisation of nitrogenase in a non-heterocystous cyanobacterium: Trichodesmium contortum. FEMS Microbiol. Lett. 1994, 118, 9–14. [Google Scholar] [CrossRef]
- Fredriksson, C.; Bergman, B. Nitrogenase quantity varies diurnally in a subset of cells within colonies of the non-heterocystous cyanobacteria Trichodesmium spp. Microbiology 1995, 141, 2471–2478. [Google Scholar] [CrossRef] [Green Version]
- Sciuto, K.; Moro, I. Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, Leptolyngbyaceae) using the 16S rRNA gene and 16S–23S ITS region. Mol. Phylogenet. Evol. 2016, 105, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, L.; Li, M.; Du, L.; Waleron, M.; Waleron, M.; Waleron, K.; Daroch, M. Description, taxonomy, and comparative genomics of a novel Thermoleptolyngby strain isolated from hot springs of Ganzi, Sichuan China. BioRxiv 2021. [Google Scholar] [CrossRef]
- Yoon, K.S.; Nguyen, N.T.; Tran, K.T.; Tsuji, K.; Ogo, S. Nitrogen fixation genes and nitrogenase activity of the non-heterocystous cyanobacterium Thermoleptolyngbya sp. O-77. Microbes Environ. 2017, 32, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcorta, J.; Alarcón-Schumacher, T.; Salgado, O.; Díez, B. Taxonomic Novelty and Distinctive Genomic Features of Hot Spring Cyanobacteria. Front. Genet. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jiang, D.; Luo, Y.; Liang, Y.; Li, L.; Shah, M.M.R.; Daroch, M. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018, 31, 14–20. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, J.; Luo, Y.; Kaczmarek, M.B.; Li, X.; Daroch, M. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour. Technol. 2019, 278, 255–265. [Google Scholar] [CrossRef]
- Riaz, S.; Xiao, M.; Chen, P.; Li, M.; Cui, Y.; Darocha, M. The Genome Copy Number of the Thermophilic Cyanobacterium Thermosynechococcus elongatus E542 Is Controlled by Growth Phase and Nutrient Availability. Appl. Environ. Microbiol. 2021, 87, 1–13. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tang, J.; Bromfield, E.S.P.; Rodrigue, N.; Cloutier, S.; Tambong, J.T. Microevolution of symbiotic Bradyrhizobium populations associated with soybeans in east North America. Ecol. Evol. 2012, 2, 2943–2961. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Dominic, B.; Mellon, M.T.; Zehr, J.P. Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. strain IMS 101. J. Bacteriol. 1998, 180, 3598–3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves-Ferreira, A.A.; Inaba, M.; Fort, A.; Araújo, W.L.; Sulpice, R. Nitrogen metabolism in cyanobacteria: Metabolic and molecular control, growth consequences and biotechnological applications. Crit. Rev. Microbiol. 2018, 44, 541–560. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, C.; Malin, G.; Siddiqui, P.J.A.; Bergman, B. Aerobic nitrogen fixation is confined to a subset of cells in the non-heterocystous cyanobacterium Symploca PCC 8002. New Phytol. 1998, 140, 531–538. [Google Scholar] [CrossRef]
- Berrendero, E.; Valiente, E.F.; Perona, E.; Gómez, C.L.; Loza, V.; Munõz-Martín, M.Á.; Mateo, P. Nitrogen fixation in a non-heterocystous cyanobacterial mat from a mountain river. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Kim, K.R.; Brand, J. Nitrogen fixation by a marine non-heterocystous cyanobacterium requires a heterotrophic bacterial consort. Environ. Microbiol. 2010, 12, 1185–1193. [Google Scholar] [CrossRef]
- Ohki, K.; Fujita, Y. Aerobic nitrogenase activity measured as acetylene reduction in the marine non-heterocystous cyanobacterium Trichodesmium spp. grown under artificial conditions. Mar. Biol. 1988, 98, 111–114. [Google Scholar] [CrossRef]
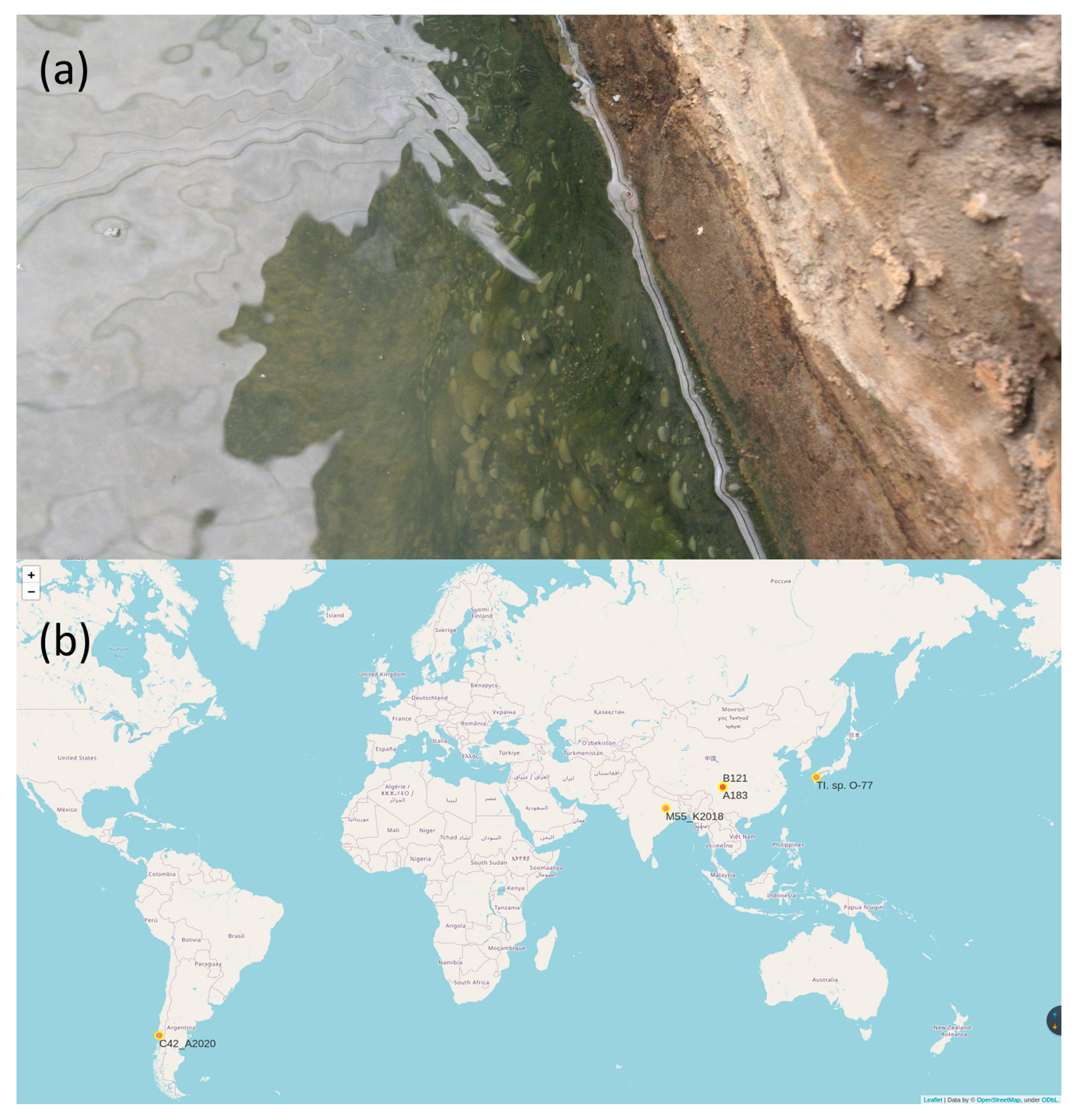
| Strain | Accession Number | Genome Description | Strain Origin |
|---|---|---|---|
| Thermoleptolyngbya sp. PKUAC-SCTB121 | CP070366.1 | Complete genome sequence | 30°15′57″ N 101°56′55″ E |
| Thermoleptolyngbya sp. PKUAC-SCTA83 | CP053661 | Complete genome sequence | 30°05′14″ N 101°52′24″ E |
| Thermoleptolyngbya sp. O-77 | AP017367 | Complete genome sequence | 32°48′11″N 130°42′28″E |
| Thermoleptolyngbya sp. C42_A2020 | JACYLP000000000.1 | Metagenomic bin | 29°69′00″N 83°68′00″E |
| Thermoleptolyngbya sp. M55_K2018 | DVEA00000000.1 | Metagenomic bin | 39°51′00″S 72°50′00″W |
| Gene Name (B121 Convention) | Length (bp) | Function or Putative Function |
|---|---|---|
| helix-turn-helix domain (cnfR) | 1599 | Transcriptional regulator |
| (2Fe-2S) ferredoxin domain | 654 | (2Fe-2S) ferredoxin domain-containing protein |
| nifT | 204 | putative nitrogen fixation protein |
| NifV(leuA) | 1122 | Homocitrate or 2-isopropylmalate synthase |
| dps | 543 | DNA starvation/stationary phase protection protein |
| Hypothetical protein | 477 | NifX-associated protein |
| NifX | 426 | Nitrogen fixation protein |
| NifEN | 2760 | bifunctional nitrogenase iron-molybdenum cofactor biosynthesis protein NifEN |
| Hypothetical protein | 306 | Nitrogenase C-terminal domain-containing protein |
| NifZ | 303 | nitrogen fixation protein |
| nifK | 1578 | Nitrogenase molybdenum-iron protein beta chain |
| nifD | 1455 | nitrogenase molybdenum-iron protein alpha chain |
| nifH | 876 | Nitrogenase iron protein |
| nifU | 906 | Fe-S cluster assembly protein |
| nifS | 1194 | Cysteine desulfurase |
| 4Fe-4S (yfhL) | 375 | 4Fe-4S binding protein |
| nifB | 1491 | nitrogenase cofactor biosynthesis protein NifB |
| cysE (nifP) | 726 | serine O-acetyltransferase |
| Hypothetical protein | 267 | unknown |
| Hypothetical protein | 219 | unknown |
| NifW | 318 | nitrogenase-stabilizing/protective protein |
| HesA/MoeB/ThiF | 783 | Molybdopterin-synthase adenylyltransferase |
| iscA (hesB) | 369 | iron-sulfur cluster assembly accessory protein |
| (2Fe-2S) fdxH | 303 | 2Fe-2S iron-sulfur cluster binding domain-containing protein |
| FeoA | 312 | ferrous iron transport protein A |
| isiB | 531 | Flavodoxin |
| ArsC | 414 | nitrogenase-associated protein |
| TehB | 603 | tellurite resistance protein |
| mopII | 210 | Molybdenum-pterin-binding protein 2 |
| ANI Identity | B121 | A183 | O-77 | C42_A2020 | M55_K2018 |
|---|---|---|---|---|---|
| B121 | 99.95 | 93.97 | 93.84 | 95.54 | |
| A183 | 99.95 | 93.87 | 94.02 | 95.23 | |
| O-77 | 93.97 | 93.87 | 98.10 | 94.96 | |
| C42_A2020 | 93.84 | 94.02 | 98.10 | 95.23 | |
| M55_K2018 | 95.54 | 95.23 | 94.96 | 95.23 |
| AAI Identity | B121 | A183 | O-77 | C42_A2020 | M55_K2018 |
|---|---|---|---|---|---|
| B121 | 99.90 | 95.95 | 96.87 | 96.64 | |
| A183 | 99.90 | 96.18 | 96.69 | 97.36 | |
| O-77 | 95.95 | 96.18 | 98.77 | 95.98 | |
| C42_A2020 | 96.87 | 96.69 | 98.77 | 96.92 | |
| M55_K2018 | 96.64 | 97.36 | 95.98 | 96.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Cheng, L.; Tang, J.; Daroch, M. Molecular Components of Nitrogen Fixation Gene Cluster and Associated Enzymatic Activities of Non-Heterocystous Thermophilic Cyanobacterium Thermoleptolyngbya sp. Life 2021, 11, 640. https://doi.org/10.3390/life11070640
Li M, Cheng L, Tang J, Daroch M. Molecular Components of Nitrogen Fixation Gene Cluster and Associated Enzymatic Activities of Non-Heterocystous Thermophilic Cyanobacterium Thermoleptolyngbya sp. Life. 2021; 11(7):640. https://doi.org/10.3390/life11070640
Chicago/Turabian StyleLi, Meijin, Lei Cheng, Jie Tang, and Maurycy Daroch. 2021. "Molecular Components of Nitrogen Fixation Gene Cluster and Associated Enzymatic Activities of Non-Heterocystous Thermophilic Cyanobacterium Thermoleptolyngbya sp." Life 11, no. 7: 640. https://doi.org/10.3390/life11070640
APA StyleLi, M., Cheng, L., Tang, J., & Daroch, M. (2021). Molecular Components of Nitrogen Fixation Gene Cluster and Associated Enzymatic Activities of Non-Heterocystous Thermophilic Cyanobacterium Thermoleptolyngbya sp. Life, 11(7), 640. https://doi.org/10.3390/life11070640








