Effect of the Membrane Composition of Giant Unilamellar Vesicles on Their Budding Probability: A Trade-Off between Elasticity and Preferred Area Difference
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
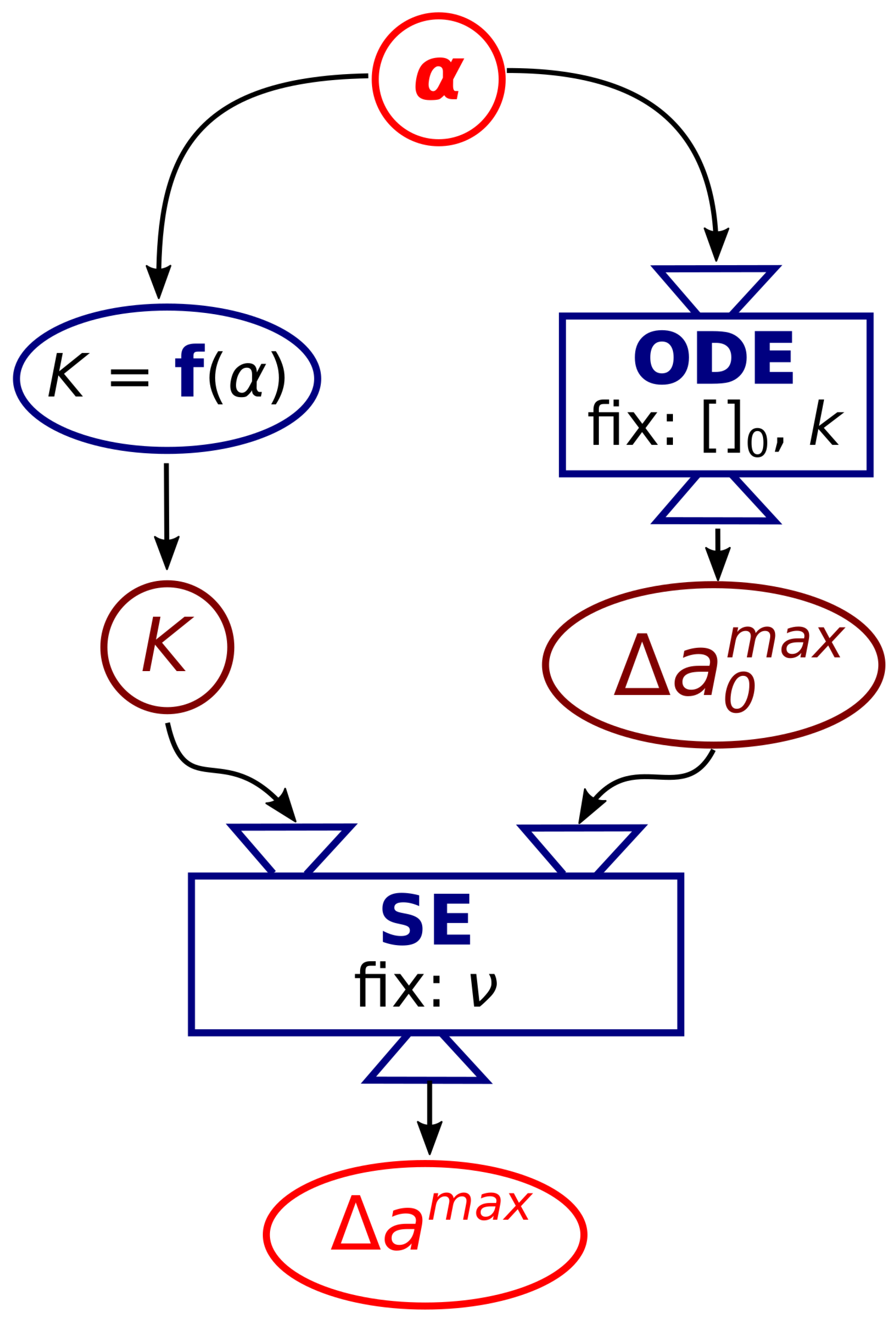
2.2. Numerical Calculation
2.2.1. Surface Evolver
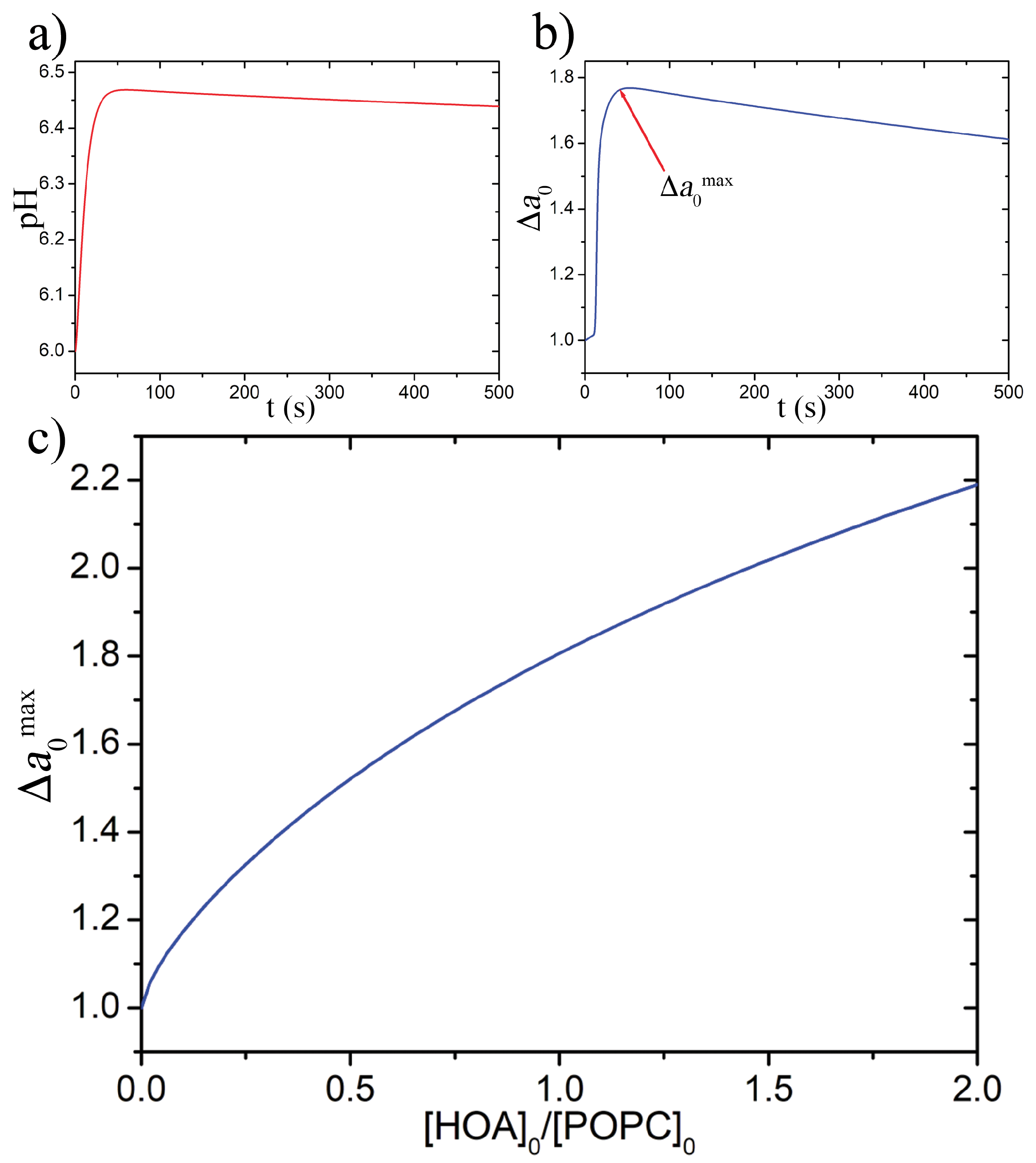
2.2.2. ODE Model
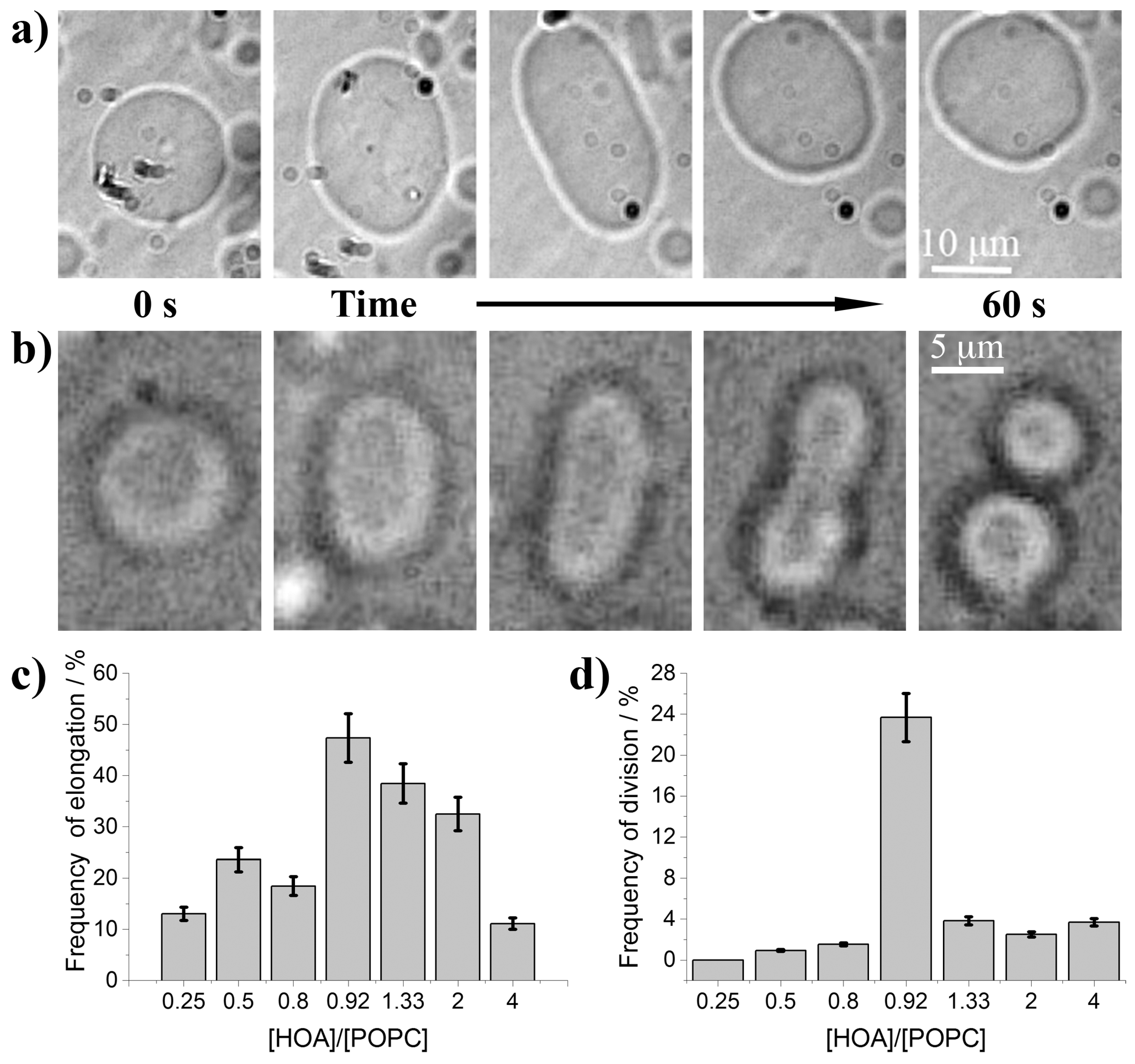
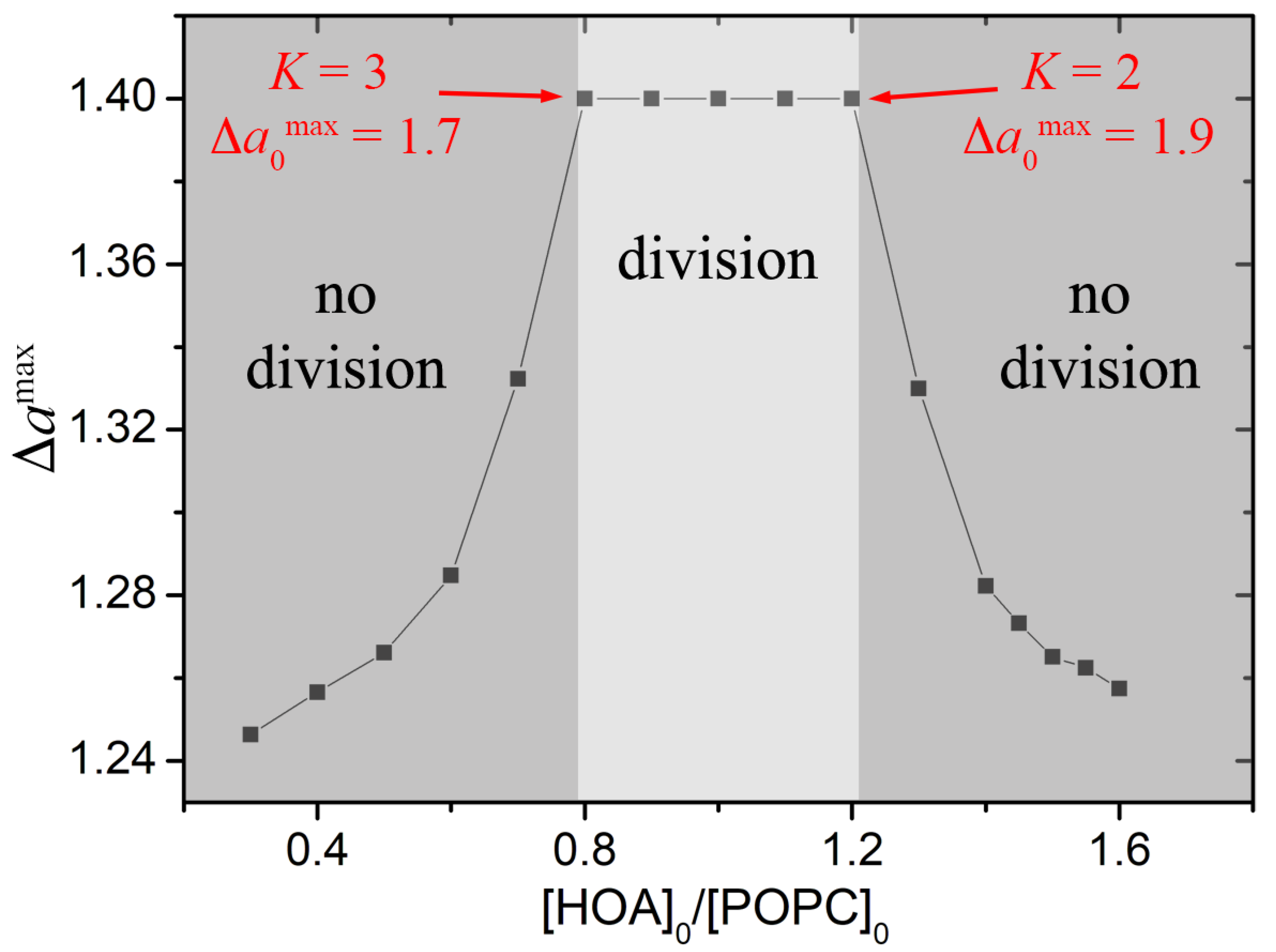
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ludlow, R.F.; Otto, S. Systems Chemistry. Chem. Soc. Rev. 2008, 37, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Ashkenasy, G.; Hermans, T.M.; Otto, S.; Taylor, A.F. Systems Chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. [Google Scholar] [CrossRef]
- Stano, P.; Rampioni, G.; D’Angelo, F.; Altamura, E.; Mavelli, F.; Marangoni, R.; Rossi, F.; Damiano, L. Current Directions in Synthetic Cell Research. In Advances in Bionanomaterials; Lecture Notes in Bioengineering; Springer: Cham, Switzerland, 2018; pp. 141–154. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Giuseppone, N. Toward Self-Constructing Materials: A Systems Chemistry Approach. Acc. Chem. Res. 2012, 45, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Fiore, M. Investigating Prebiotic Protocells for a Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef]
- Blain, J.C.; Szostak, J.W. Progress Toward Synthetic Cells. Annu. Rev. Biochem. 2014, 83, 615–640. [Google Scholar] [CrossRef] [PubMed]
- Buddingh’, B.C.; van Hest, J.C.M. Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity. Acc. Chem. Res. 2017, 50, 769–777. [Google Scholar] [CrossRef]
- Rampioni, G.; Damiano, L.; Messina, M.; D’Angelo, F.; Leoni, L.; Stano, P. Chemical Communication between Synthetic and Natural Cells: A Possible Experimental Design. Electron. Proc. Theor. Comput. Sci. 2013, 130, 14–26. [Google Scholar] [CrossRef][Green Version]
- Tomasi, R.; Noel, J.M.; Zenati, A.; Ristori, S.; Rossi, F.; Cabuil, V.; Kanoufi, F.; Abou-Hassan, A. Chemical Communication between Liposomes Encapsulating a Chemical Oscillatory Reaction. Chem. Sci. 2014, 5, 1854–1859. [Google Scholar] [CrossRef]
- Niederholtmeyer, H.; Chaggan, C.; Devaraj, N.K. Communication and Quorum Sensing in Non-Living Mimics of Eukaryotic Cells. Nat. Commun. 2018, 9, 5027. [Google Scholar] [CrossRef]
- Aufinger, L.; Simmel, F.C. Establishing Communication Between Artificial Cells. Chem. Eur. J. 2019, 25, 12659–12670. [Google Scholar] [CrossRef]
- Budroni, M.A.; Torbensen, K.; Ristori, S.; Abou-Hassan, A.; Rossi, F. Membrane Structure Drives Synchronization Patterns in Arrays of Diffusively Coupled Self-Oscillating Droplets. J. Phys. Chem. Lett. 2020, 11, 2014–2020. [Google Scholar] [CrossRef]
- Altamura, E.; Milano, F.; Tangorra, R.R.; Trotta, M.; Omar, O.H.; Stano, P.; Mavelli, F. Highly Oriented Photosynthetic Reaction Centers Generate a Proton Gradient in Synthetic Protocells. Proc. Natl. Acad. Sci. USA 2017, 114, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- Altamura, E.; Albanese, P.; Marotta, R.; Milano, F.; Fiore, M.; Trotta, M.; Stano, P.; Mavelli, F. Chromatophores Efficiently Promote Light-Driven ATP Synthesis and DNA Transcription inside Hybrid Multicompartment Artificial Cells. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.P.; Steiniger, F.; Stano, P.; Fahr, A.; Luisi, P.L. Spontaneous Crowding of Ribosomes and Proteins inside Vesicles: A Possible Mechanism for the Origin of Cell Metabolism. ChemBioChem 2011, 12, 2325–2330. [Google Scholar] [CrossRef]
- van Roekel, H.W.H.; Rosier, B.J.H.M.; Meijer, L.H.H.; Hilbers, P.A.J.; Markvoort, A.J.; Huck, W.T.S.; de Greef, T.F.A. Programmable Chemical Reaction Networks: Emulating Regulatory Functions in Living Cells Using a Bottom-up Approach. Chem. Soc. Rev. 2015, 44, 7465–7483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.F.; Szostak, J.W. Coupled growth and division of model protocell membranes. J. Am. Chem. Soc. 2009, 131, 5705–5713. [Google Scholar] [CrossRef]
- Peterlin, P.; Arrigler, V.; Kogej, K.; Svetina, S.; Walde, P. Growth and shape transformations of giant phospholipid vesicles upon interaction with an aqueous oleic acid suspension. Chem. Phys. Lipids 2009, 159, 67–76. [Google Scholar] [CrossRef]
- Kurihara, K.; Tamura, M.; Shohda, K.I.; Toyota, T.; Suzuki, K.; Sugawara, T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 2011, 3, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Imai, M. From Vesicles to Protocells: The Roles of Amphiphilic Molecules. Life 2015, 5, 651–675. [Google Scholar] [CrossRef]
- Jimbo, T.; Sakuma, Y.; Urakami, N.; Ziherl, P.; Imai, M. Role of Inverse-Cone-Shape Lipids in Temperature-Controlled Self-Reproduction of Binary Vesicles. Biophys. J. 2016, 110, 1551–1562. [Google Scholar] [CrossRef]
- Dervaux, J.; Noireaux, V.; Libchaber, A.J. Growth and instability of a phospholipid vesicle in a bath of fatty acids. Eur. Phys. J. Plus 2017, 132, 284. [Google Scholar] [CrossRef]
- Litschel, T.; Ramm, B.; Maas, R.; Heymann, M.; Schwille, P. Beating Vesicles: Encapsulated Protein Oscillations Cause Dynamic Membrane Deformations. Angew. Chem. Int. Ed. 2018, 57, 16286–16290. [Google Scholar] [CrossRef]
- Kurisu, M.; Aoki, H.; Jimbo, T.; Sakuma, Y.; Imai, M.; Serrano-Luginbühl, S.; Walde, P. Reproduction of vesicles coupled with a vesicle surface-confined enzymatic polymerisation. Commun. Chem. 2019, 2, 117. [Google Scholar] [CrossRef]
- Li, Y.; ten Wolde, P.R. Shape Transformations of Vesicles Induced by Swim Pressure. Phys. Rev. Lett. 2019, 123, 148003. [Google Scholar] [CrossRef] [PubMed]
- Vutukuri, H.R.; Hoore, M.; Abaurrea-Velasco, C.; van Buren, L.; Dutto, A.; Auth, T.; Fedosov, D.A.; Gompper, G.; Vermant, J. Active particles induce large shape deformations in giant lipid vesicles. Nature 2020, 586, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Dreher, Y.; Jahnke, K.; Bobkova, E.; Spatz, J.P.; Göpfrich, K. Division and Regrowth of Phase-Separated Giant Unilamellar Vesicles. Angew. Chem. Int. Ed. 2021, 133, 10756–10764. [Google Scholar] [CrossRef]
- Miele, Y.; Medveczky, Z.; Hollo, G.; Tegze, B.; Derenyi, I.; Horvolgyi, Z.; Altamura, E.; Lagzi, I.; Rossi, F. Self-Division of Giant Vesicles Driven by an Internal Enzymatic Reaction. Chem. Sci. 2020, 11, 3228–3235. [Google Scholar] [CrossRef]
- Holló, G.; Miele, Y.; Rossi, F.; Lagzi, I. Shape Changes and Budding of Giant Vesicles Induced by an Internal Chemical Trigger: An Interplay between Osmosis and pH Change. Phys. Chem. Chem. Phys. 2021, 23, 4262–4270. [Google Scholar] [CrossRef]
- Hu, G.; Pojman, J.A.; Scott, S.K.; Wrobel, M.M.; Taylor, A.F. Base-Catalyzed Feedback in the Urea-Urease Reaction. J. Phys. Chem. B 2010, 114, 14059–14063. [Google Scholar] [CrossRef] [PubMed]
- Miele, Y.; Bánsági, T.; Taylor, A.; Stano, P.; Rossi, F. Engineering Enzyme-Driven Dynamic Behaviour in Lipid Vesicles. In Advances in Artificial Life, Evolutionary Computation and Systems Chemistry; Rossi, F., Mavelli, F., Stano, P., Caivano, D., Eds.; Number 587 in Communications in Computer and Information Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 197–208. [Google Scholar] [CrossRef]
- Miele, Y.; Bánsági, T.; Taylor, A.; Rossi, F. Modelling Approach to Enzymatic pH Oscillators in Giant Lipid Vesicles. In Advances in Bionanomaterials I; Piotto, S., Rossi, F., Concilio, S., Reverchon, E., Cattaneo, G., Eds.; Lecture Notes in Bioengineering; Springer International Publishing: Cham, Switzerland, 2018; pp. 63–74. [Google Scholar] [CrossRef]
- Svetina, S.; Žekš, B. Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur. Biophys. J. 1989, 17, 101–111. [Google Scholar] [CrossRef]
- Käs, J.; Sackmann, E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys. J. 1991, 60, 825–844. [Google Scholar] [CrossRef]
- Wiese, W.; Harbich, W.; Helfrich, W. Budding of lipid bilayer vesicles and flat membranes. J. Phys. Condens. Matter 1992, 4, 1647. [Google Scholar] [CrossRef]
- Miao, L.; Seifert, U.; Wortis, M.; Döbereiner, H.G. Budding transitions of fluid-bilayer vesicles: The effect of area-difference elasticity. Phys. Rev. E 1994, 49, 5389. [Google Scholar] [CrossRef]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Svetina, S.; Žekš, B. Shape behavior of lipid vesicles as the basis of some cellular processes. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2002, 268, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, V.; Svetina, S.; Žekš, B. Nonaxisymmetric vesicle shapes in a generalized bilayer-couple model and the transition between oblate and prolate axisymmetric shapes. Phys. Rev. E 1993, 48, 3112. [Google Scholar] [CrossRef] [PubMed]
- Ikari, K.; Sakuma, Y.; Jimbo, T.; Kodama, A.; Imai, M.; Monnard, P.A.; Rasmussen, S. Dynamics of fatty acid vesicles in response to pH stimuli. Soft Matter 2015, 11, 6327–6334. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Litvinov, S.; Koumoutsakos, P. Bending Models of Lipid Bilayer Membranes: Spontaneous Curvature and Area-Difference Elasticity. Comput. Methods Appl. Mech. Eng. 2020, 359, 112758. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of Unilamellar Vesicles Using an Inverted Emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Carrara, P.; Stano, P.; Luisi, P.L. Giant Vesicles Colonies: A Model for Primitive Cell Communities. ChemBioChem 2012, 13, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Wodlei, F.; Carrara, P.; Ristori, S.; Marchettini, N.; Rossi, F. Approaches to Molecular Communication Between Synthetic Compartments Based on Encapsulated Chemical Oscillators. In Advances in Artificial Life and Evolutionary Computation; Pizzuti, C., Spezzano, G., Eds.; Number 445 in Communications in Computer and Information Science; Springer International Publishing: Cham, Switzerland, 2014; pp. 58–74. [Google Scholar] [CrossRef]
- Fiore, M.; Maniti, O.; Girard-Egrot, A.; Monnard, P.A.; Strazewski, P. Glass Microsphere-Supported Giant Vesicles for the Observation of Self-Reproduction of Lipid Boundaries. Angew. Chem. Int. Ed. 2018, 57, 282–286. [Google Scholar] [CrossRef]
- Sakashita, A.; Urakami, N.; Ziherl, P.; Imai, M. Three-dimensional analysis of lipid vesicle transformations. Soft Matter 2012, 8, 8569–8581. [Google Scholar] [CrossRef]
- Helfrich, W. The size of bilayer vesicles generated by sonication. Phys. Lett. A 1974, 50, 115–116. [Google Scholar] [CrossRef]
- Brakke, K.A. The Surface Evolver. Exp. Math. 1992, 1, 141–165. [Google Scholar] [CrossRef]
- Carrara, P. Constructing a Minimal Cell. Ph.D. Thesis, University of Roma Tre, Rome, Italy, 2011. [Google Scholar]
- Tyler, A.I.I.; Greenfield, J.L.; Seddon, J.M.; Brooks, N.J.; Purushothaman, S. Coupling Phase Behavior of Fatty Acid Containing Membranes to Membrane Bio-Mechanics. Front. Cell Dev. Biol. 2019, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.; Suga, K.; Kuhl, T.L. Interaction Forces and Membrane Charge Tunability: Oleic Acid Containing Membranes in Different pH Conditions. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 211–217. [Google Scholar] [CrossRef]
- Majhenc, J.; Božič, B.; Svetina, S.; Žekš, B. Phospholipid Membrane Bending as Assessed by the Shape Sequence of Giant Oblate Phospholipid Vesicles. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1664, 257–266. [Google Scholar] [CrossRef]
- Novák, B.; Tyson, J.J. Design principles of biochemical oscillators. Nat. Rev. Mol. Cell Biol. 2008, 9, 981–991. [Google Scholar] [CrossRef]
- Murtas, G. Early self-reproduction, the emergence of division mechanisms in protocells. Mol. Biosyst. 2013, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Halevy, I.; Bachan, A. The Geologic History of Seawater pH. Science 2017, 355, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miele, Y.; Holló, G.; Lagzi, I.; Rossi, F. Effect of the Membrane Composition of Giant Unilamellar Vesicles on Their Budding Probability: A Trade-Off between Elasticity and Preferred Area Difference. Life 2021, 11, 634. https://doi.org/10.3390/life11070634
Miele Y, Holló G, Lagzi I, Rossi F. Effect of the Membrane Composition of Giant Unilamellar Vesicles on Their Budding Probability: A Trade-Off between Elasticity and Preferred Area Difference. Life. 2021; 11(7):634. https://doi.org/10.3390/life11070634
Chicago/Turabian StyleMiele, Ylenia, Gábor Holló, István Lagzi, and Federico Rossi. 2021. "Effect of the Membrane Composition of Giant Unilamellar Vesicles on Their Budding Probability: A Trade-Off between Elasticity and Preferred Area Difference" Life 11, no. 7: 634. https://doi.org/10.3390/life11070634
APA StyleMiele, Y., Holló, G., Lagzi, I., & Rossi, F. (2021). Effect of the Membrane Composition of Giant Unilamellar Vesicles on Their Budding Probability: A Trade-Off between Elasticity and Preferred Area Difference. Life, 11(7), 634. https://doi.org/10.3390/life11070634







