Herbal Extract from Codonopsis pilosula (Franch.) Nannf. Enhances Cardiogenic Differentiation and Improves the Function of Infarcted Rat Hearts
Abstract
1. Introduction
2. Materials and Methods
2.1. Vector
2.2. Embryonic Stem Cell Lines
2.3. Embryonic Stem Cell Differentiation
2.4. Preparation of Herbal Extracts
2.5. Immunocytochemical Staining
2.6. Effects of Herbal Extracts on Differentiating EMG8 Cells
2.7. Experimental Animals
2.8. The Rat Myocardial Infarction Model
2.9. Effects of Herbal Extracts in the Rat Myocardial Infarction Model and Cardiac Function Assessment
3. Results
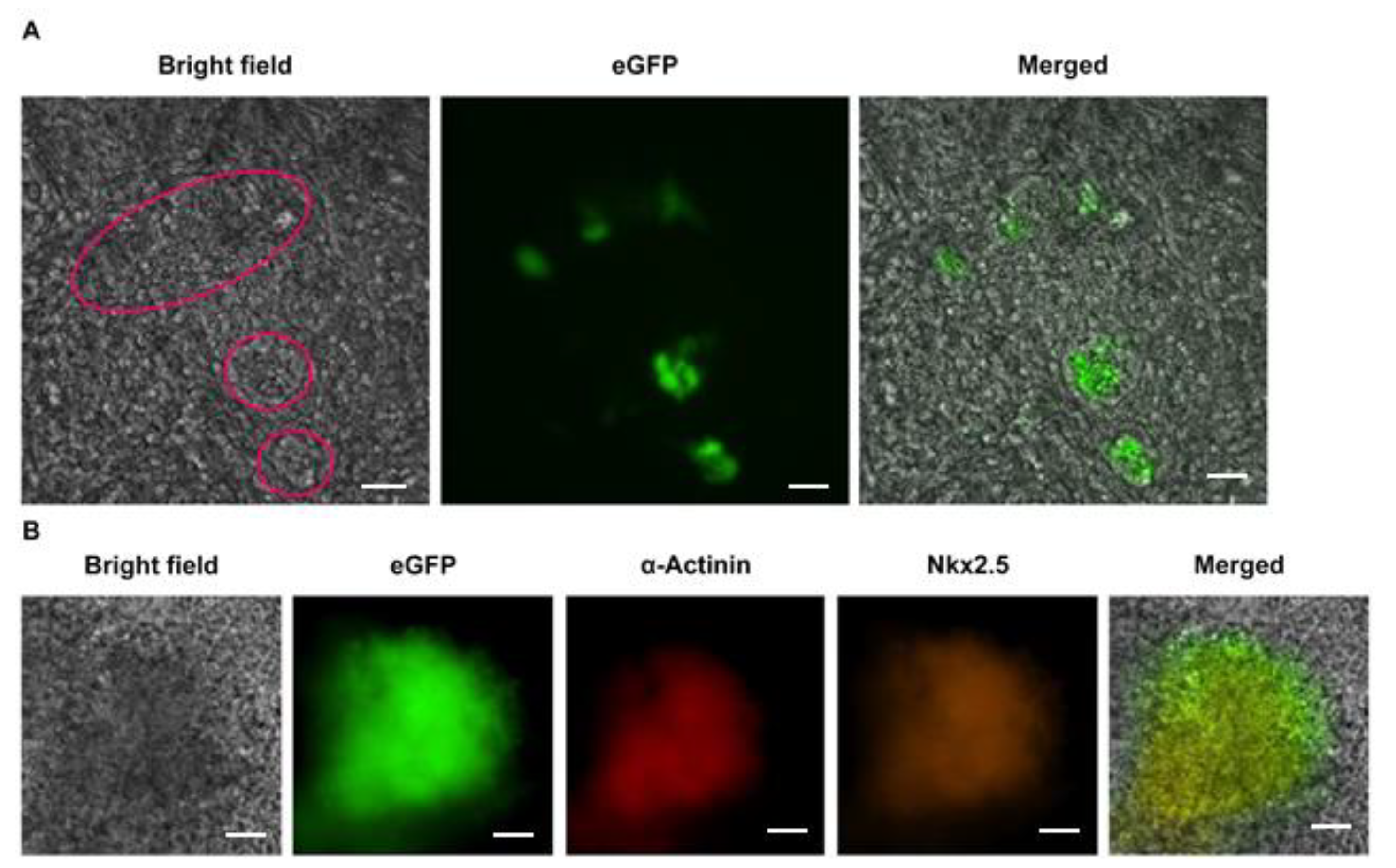
3.1. Cardiomyocyte Identity of Enhanced Green Fluorescent Protein (eGFP)-Expressing EB Outgrowths
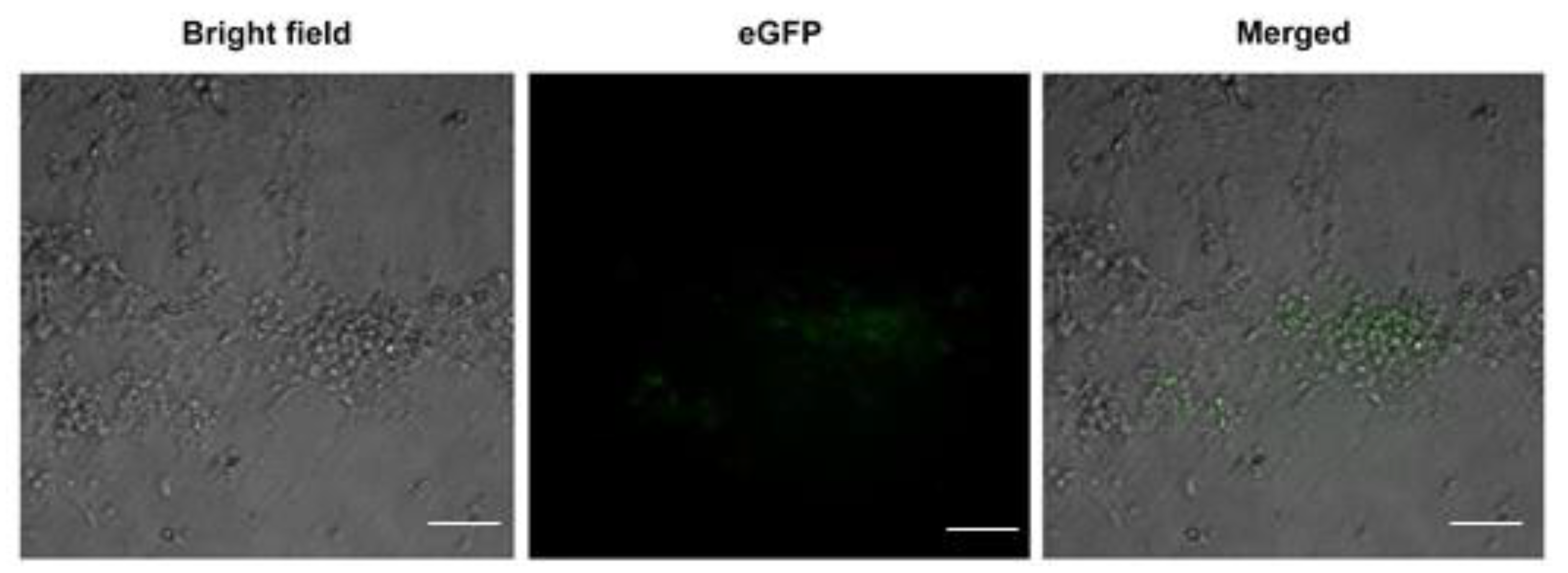
3.2. eGFP Expression on Spontaneous Differentiating EMG8 Cells
3.3. Resul ts of Herbal Extracts on Differentiating EMG8 Cells
3.4. Results of 417W in the Rat Myocardial Infarction Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC. Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gabriel-Costa, D. The pathophysiology of myocardial infarction-induced heart failure. Pathophysiology 2018, 25, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Hasan, A.; Faizy, A.F.; Mateen, S.; Fatima, N.; Moin, S. Elevated DNA damage, oxidative stress, and impaired response defense system inflicted in patients with myocardial infarction. Clin. Appl. Thromb. Hemost. 2018, 24, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanism, incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407–415. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V. Stem cells for the treatment of heart failure. Curr. Res. Transl. Med. 2016, 64, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Uriel, N.; Burkhoff, D. Reverse remodelling and myocardial recovery in heart failure. Nat. Rev. Cardiol. 2018, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yamanaka, S. Induced pluripotent stem cells 10 years later: For cardiac applications. Circ. Res. 2017, 120, 1958–1968. [Google Scholar] [CrossRef]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem cell therapy: A new therapeutic option for cardiovascular diseases. J. Cell. Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Morris, M.W., Jr.; Liechty, K.W. Cardiac progenitor cells in myocardial infarction wound healing: A critical review. Adv. Wound Care (New Rochelle) 2013, 2, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-W.; Lee, J.B.; Kim, B.; Jeon, S.; Kim, M.-K.; Nam, K.-H.; Ha, J.-R.; Bhatia, M.; Oh, G.T.; Kim, D.-Y. Peptidomimetic small-molecule compounds promoting cardiogenesis of stem cells. Arch. Pharmacal Res. 2012, 35, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Lord, B.; Schulze, P.C.; Fryer, R.M.; Sarang, S.S.; Gullans, S.R.; Lee, R.T. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 2003, 107, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Lanier, M.; Forte, E.; Lo, F.; Cashman, J.; Mercola, M. A chemical biology approach to myocardial regeneration. J. Cardiovasc. Transl. Res. 2011, 4, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Qu, Z.-Q.; Wang, S.-S.; Hao, X.-W.; Zhang, X.-Q.; Guan, J.; Han, F. Effects of Suxiao Jiuxin pill on oxidative stress and inflammatory response in rats with experimental atherosclerosis. J. Tradit. Chin. Med. 2011, 31, 107–111. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, H.; Li, S.; Xu, H. The effect of sodium tanshinone IIA sulfate and simvastatin on elevated serum levels of inflammatory markers in patients with coronary heart disease: A study protocol for a randomized controlled trial. Evid. Based. Complement. Alternat. Med. 2013, 2013, 756519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Peng, W.; Liu, W.; Cai, W.; Xia, Z.; Zhang, H.; Xing, A.Z. Cardioprotective roles of the Chinese medicinal formula Bao-Xin-TANG on acute myocardial infarction in rats. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.T.; Ng, T.B.; Yeung, H.W.; Xu, G.J. Immunomodulatory effect of a polysaccharide-enriched preparation of Codonopsis pilosula roots. Gen. Pharmacol. 1996, 27, 1347–1350. [Google Scholar] [CrossRef]
- Chang, K.-S.; Lee, N.-H.; Kuo, W.-W.; Hu, W.-S.; Chang, M.-H.; Tsai, F.-J.; Tsai, K.-H.; Yang, Y.-S.; Chen, T.-S.; Huang, C.-Y. Dung-Shen downregulates the synergistic apoptotic effects of angiotensin II plus Leu 27-IGF II on cardiomyoblasts. Acta Cardiol. Sin. 2014, 30, 56–66. [Google Scholar]
- Luo, J.; Xu, H.; Chen, K. Systematic review of compound danshen dropping pill: A Chinese patent medicine for acute myocardial infarction. Evid. Based. Complement. Alternat. Med. 2013, 2013, 808076. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-H.; Lee, N.-H.; Chen, G.-Y.; Hu, W.-S.; Tsai, C.-Y.; Chang, M.-H.; Jong, G.-P.; Kuo, C.-H.; Tzang, B.-S.; Tsai, F.-J.; et al. Dung-shen (Codonopsis pilosula) attenuated the cardiac-impaired insulin-like growth factor II receptor pathway on myocardial cells. Food Chem. 2013, 138, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Gulick, J.; Subramaniam, A.; Neumann, J.; Robbins, J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J. Biol. Chem. 1991, 266, 9180–9185. [Google Scholar] [CrossRef]
- Hescheler, J.; Fleischmann, B.; Lentini, S.; Maltsev, V.; Rohwedel, J.; Wobus, A.; Addicks, K. Embryonic stem cells: A model to study structural and functional properties in cardiomyogenesis. Cardiovasc. Res. 1997, 36, 149–162. [Google Scholar] [CrossRef]
- Pucéat, M. Protocols for cardiac differentiation of embryonic stem cells. Methods 2008, 45, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.D.; Lee, H.L.; Yang, Y.J. Cell transplantation for myocardial injury: A preliminary comparative study. Cytotherapy 2010, 12, 692–700. [Google Scholar] [CrossRef]
- Morkin, E. Control of cardiac myosin heavy chain gene expression. Microsc. Res. Tech. 2000, 50, 522–531. [Google Scholar] [CrossRef]
- Katano, W.; Moriyama, Y.; Takeuchi, J.K.; Koshiba-Takeuchi, K. Cardiac septation in heart development and evolution. Dev. Growth Differ. 2019, 61, 114–123. [Google Scholar] [CrossRef]
- Sylva, M.; van den Hoff, M.J.; Moorman, A.F. Development of the human heart. Am. J. Med. Genet. A 2014, 164A, 1347–1371. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takahashi, T.; Esaki, M.; Ushikoshi, H.; Nagano, S.; Fujiwara, H.; Kosai, K.-I. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ. J. 2004, 68, 691–702. [Google Scholar] [CrossRef][Green Version]
- Schneider, M.D.; Gaussin, V.; Lyons, K.M. Tempting fate: BMP signals for cardiac morphogenesis. Cytokine Growth Factor Rev. 2003, 14, 1–4. [Google Scholar] [CrossRef]
- Armiñán, A.; Gandía, C.; Bartual, M.; García-Verdugo, J.M.; Lledó, E.; Mirabet, V.; Llop, M.; Barea, J.; Montero, J.A.; Sepúlveda, P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009, 18, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, C.A.; Naya, F.J. The function of the MEF2 family of transcription factors in cardiac development, cardiogenomics, and direct reprogramming. J. Cardiovasc. Dev. Dis. 2016, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, K.; Lee, J.; Kim, H.S.; Ihm, C.H.; Iio, A.; Ogawa, M.; Nishikawa, S.; Kodama, I.; Morisaki, T. Chamber-specific differentiation of Nkx2.5-positive cardiac precursor cells from murine embryonic stem cells. FASEB J. 2003, 17, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Haendeler, J.; Badorff, C.; Brandes, R.P.; Hoffmann, J.; Pandur, P.; Zeiher, A.M.; Kühl, M.; Dimmeler, S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J. Biol. Chem. 2005, 280, 16838–16842. [Google Scholar] [CrossRef]
- Pandur, P.; Läsche, M.; Eisenberg, L.M.; Kühl, M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 2002, 418, 636–641. [Google Scholar] [CrossRef]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal medicine for cardiovascular diseases: Efficacy, mechanism, and safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Kin, J.H. Cardiovascular diseases and panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng Res. 2012, 35, 16–26. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, L. Search for a pharmacological role of Dangshen in alimentary system. J. Beijing Tradit. Chin. Med. 1991, 14, 47–48. [Google Scholar]
- Tingyu, F.; Bo, J.; Qing, W. New Chinese-English Dictionary of Traditional Chinese Medicine, 2nd ed.; Chinese Medical Science and Technology Press: Beijing, China, 2013; ISBN 978-7506760553. [Google Scholar]
- Liu, J.H.; Bao, Y.M.; Song, J.J.; An, L.J. Codonopsis pilosula (Franch) Nannf total alkaloids potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Acta. Pharmacol. Sin. 2003, 24, 913–917. [Google Scholar]



| Control (n = 6) | 417W (n = 6) | |||||
|---|---|---|---|---|---|---|
| 1 week | 3 weeks | 6 weeks | 1 week | 3 weeks | 6 weeks | |
| FS (%) | 26.9 ± 6.0 | 21.3 ± 7.1 | 24.3 ± 6.4 | 25.8 ± 7.1 | 30.0 ± 5.2 | 29.8 ± 6.9 |
| FAC (%) | 46.9 ± 8.7 | 35.7 ± 8.5 | 37.4 ± 11.3 | 45.2 ± 9.3 | 56.8 ± 9.0 | 50.6 ± 8.0 |
| EF (%) | 59.4 ± 19.9 | 52.2 ± 12.4 | 52.1 ± 13.6 | 56.8 ± 9.8 | 73.2 ± 9.2 | 67.5 ± 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-N.; Kan, C.-D.; Lee, L.-T.; Huang, L.L.H.; Hsiao, Y.-L.; Chang, A.H.; Liu, W.; Lin, C.; Lin, C.-W. Herbal Extract from Codonopsis pilosula (Franch.) Nannf. Enhances Cardiogenic Differentiation and Improves the Function of Infarcted Rat Hearts. Life 2021, 11, 422. https://doi.org/10.3390/life11050422
Wang J-N, Kan C-D, Lee L-T, Huang LLH, Hsiao Y-L, Chang AH, Liu W, Lin C, Lin C-W. Herbal Extract from Codonopsis pilosula (Franch.) Nannf. Enhances Cardiogenic Differentiation and Improves the Function of Infarcted Rat Hearts. Life. 2021; 11(5):422. https://doi.org/10.3390/life11050422
Chicago/Turabian StyleWang, Jieh-Neng, Chung-Dann Kan, Lain-Tze Lee, Lynn L. H. Huang, Ya-Li Hsiao, Allen H. Chang, Wanchun Liu, Cheng Lin, and Chou-Wen Lin. 2021. "Herbal Extract from Codonopsis pilosula (Franch.) Nannf. Enhances Cardiogenic Differentiation and Improves the Function of Infarcted Rat Hearts" Life 11, no. 5: 422. https://doi.org/10.3390/life11050422
APA StyleWang, J.-N., Kan, C.-D., Lee, L.-T., Huang, L. L. H., Hsiao, Y.-L., Chang, A. H., Liu, W., Lin, C., & Lin, C.-W. (2021). Herbal Extract from Codonopsis pilosula (Franch.) Nannf. Enhances Cardiogenic Differentiation and Improves the Function of Infarcted Rat Hearts. Life, 11(5), 422. https://doi.org/10.3390/life11050422






