Potential Effects of Nutraceuticals in Retinopathy of Prematurity
Abstract
:1. Introduction
2. Pathogenesis of ROP
2.1. Risk Factors
2.2. Pathological Vascular Changes in ROP and Its Metabolism
2.3. Animal Models for ROP
3. Nutritional Supplements
3.1. Oil
3.1.1. Polyunsaturated Fatty Acids (PUFAs) and Long-Chain Polyunsaturated Fatty Acid (LCPUFAs)
3.2. Flavonoids
3.2.1. Green Tea
3.2.2. Bilberries
3.2.3. Quercetin
3.2.4. Baicalin
3.2.5. Luteolin
3.2.6. Deguelin
3.3. Non-Flavonoids
3.3.1. Caffeic Acid
3.3.2. Curcumin
3.3.3. Resveratrol
3.3.4. Honokiol
3.4. Alkaloids: Caffeine
3.5. Alkylpyrazines: Tetramethylpyrazine (TMP)
3.6. Carotenoids: Lutein and Zeaxanthin
3.7. Nonsteroidal Compounds: Decursin
3.8. Organic Compounds: Oleanolic Acid
3.9. Photodynamic Compounds: Hypericin
3.10. Plant Phenolic Compounds: Apocynin
3.11. Chinese Herbal Formulas and Other Plant Extracts
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ALA | α-linoleic acid |
| ATP | Adenosine triphosphate |
| AV | Avascular |
| Bcl-2 | B-cell lymphoma 2 |
| BRB | Blood–retinal barrier |
| CNV | Choroidal neovascularization |
| COX-2 | Cyclooxygenease-2 |
| DHA | Docosahexaenoic acid |
| ERK | Extracellular signal-regulated kinase |
| eNOS | Endothelial nitric oxide synthase |
| EPA | Eicosapentaenoic acid |
| Epo | Erythropoietin |
| FO | Fish oil |
| GFAP | Glial fibrillary acidic protein |
| GLUTs | Sodium-independent glucose transporters |
| HAR | Hyperglycemia-associated retinopathy |
| HIF-1 | Hypoxia-inducible factor-1 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IGF-1 | Insulin-like growth factors-1 |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| JNK1 | c-Jun N-terminal protein kinase 1 |
| LA | Linoleic acid |
| MMP-2 | Matrix metalloproteinase-2 |
| MUFAs | Monounsaturated fatty acids |
| n-3 | Omega 3 |
| n-6 | Omega 6 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor-κB |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NV | Neovascular |
| ODC | Ornithine decarboxylase |
| OIR | Oxygen-induced retinopathy |
| OO | Olive oil |
| PGE2 | Prostaglandin E2 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PUFAs | Polyunsaturated fatty acids |
| ROP | Retinopathy of prematurity |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| SO | Soybean oil |
| TNF-α | Tumor necrosis factor α |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
| VEGF | Vascular endothelial growth factor |
References
- Kong, L.; Fry, M.; Al-Samarraie, M.; Gilbert, C.; Steinkuller, P.G. An Update on Progress and the Changing Epidemiology of Causes of Childhood Blindness Worldwide. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2012, 16, 501–507. [Google Scholar]
- Natarajan, G.; Blair, M.P.; Shapiro, M.J.; Berrocal, A.M.; Murray, T.G.; Martinez-Castellanos, M.A.; Hubbard, G.B. Neurodevelopmental Outcomes of Preterm Infants with Retinopathy of Prematurity by Treatment. Pediatrics 2020, 145, 4. [Google Scholar]
- Baker, J.P. The Incubator and the Medical Discovery of the Premature Infant. J. Perinatol. 2000, 20, 321–328. [Google Scholar]
- Hartnett, M.E. Advances in Understanding and Management of Retinopathy of Prematurity. Surv. Ophthalmol. 2017, 62, 257–276. [Google Scholar]
- Terry, T.L. Extreme Prematurity and Fibroblastic Overgrowth of Persistent Vascular Sheath Behind Each Crystalline Lens*: I. Preliminary Report. Am. J. Ophthalmol. 1942, 25, 203–204. [Google Scholar]
- Campbell, K. Intensive Oxygen Therapy as a Possible Cause of Retrolental Fibroplasia; a Clinical Approach. Med. J. Aust. 1951, 2, 48. [Google Scholar]
- Patz, A.; Leroy, E.H.; Edgar, D.L.C. Studies on the Effect of High Oxygen Administration in Retrolental Fibroplasia*: I. Nursery Observations. Am. J. Ophthalmol. 1952, 35, 1248–1253. [Google Scholar]
- Crosse, V.M.; Evans, P.J. Prevention of Retrolental Fibroplasia. Ama Arch. Ophthalmol. 1952, 48, 83–87. [Google Scholar]
- Raghuveer, T.S.; Zackula, R. Strategies to Prevent Severe Retinopathy of Prematurity: A 2020 Update and Meta-Analysis. NeoReviews 2020, 21, 4249. [Google Scholar]
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-Associated Visual Impairment and Estimates of Retinopathy of Prematurity at Regional and Global Levels for 2010. Pediatric Res. 2013, 74, 35–49. [Google Scholar]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.-Y. Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar]
- Liu, J.; Connie, H.Y.L.; Amy, C.Y.L. Therapeutic Strategies for Retinopathy of Prematurity. Hong Kong J. Ophthalmol. 2015, 19, 8–15. [Google Scholar]
- Palmer, E.A.; Robert, J.H.; Velma, D.; Dale, L.P.; Graham, E.Q.; Carole, G.S.; Carol, P.K.; Betty, T. 15-Year Outcomes Following Threshold Retinopathy of Prematurity: Final Results from the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity. Arch. Ophthalmol. (Chicago, Ill.: 1960) 2005, 123, 311–318. [Google Scholar]
- Clark, D.; Mandal, K. Treatment of Retinopathy of Prematurity. Early Hum. Dev. 2008, 84, 95–99. [Google Scholar]
- Lenis, T.L.; Gunzenhauser, R.C.; Fung, S.S.; Dhindsa, Y.K.; Sarraf, D.; Pineles, S.L.; Tsui, I. Myopia and Anterior Segment Optical Coherence Tomography Findings in Laser-Treated Retinopathy of Prematurity Eyes. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2020, 24, 86.e1–86.e7. [Google Scholar]
- Cavallaro, G.; Filippi, L.; Bagnoli, P.; La Marca, G.; Cristofori, G.; Raffaeli, G.; Padrini, L.; Araimo, G.; Fumagalli, M.; Groppo, M.; et al. The Pathophysiology of Retinopathy of Prematurity: An Update of Previous and Recent Knowledge. Acta Ophthalmol. 2014, 92, 2–20. [Google Scholar]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z. Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar]
- Wu, W.-C.; Yeh, P.-T.; Chen, S.-N.; Yang, C.-M.; Lai, C.-C.; Kuo, H.-K. Effects and Complications of Bevacizumab Use in Patients with Retinopathy of Prematurity: A Multicenter Study in Taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar]
- Castellanos, M.A.M.; Schwartz, S.; García-Aguirre, G.; Quiroz-Mercado, H. Short-Term Outcome after Intravitreal Ranibizumab Injections for the Treatment of Retinopathy of Prematurity. Br. J. Ophthalmol. 2013, 97, 816–819. [Google Scholar]
- Martínez-Castellanos, M.A.; Schwartz, S.; Hernández-Rojas, M.L.; Kon-Jara, V.A.; García-Aguirre, G.; Guerrero-Naranjo, J.L.; Chan, R.V.P.; Quiroz–Mercado, H. Long-Term Effect of Antiangiogenic Therapy for Retinopathy of Prematurity up to 5 Years of Follow-Up. Retina 2013, 33, 329–338. [Google Scholar]
- Sato, T.; Wada, K.; Aarhori, H.; Kuno, N.; Imoto, K.; Iwahashi-Shima, C.; Kusaka, S. Serum Concentrations of Bevacizumab (Avastin) and Vascular Endothelial Growth Factor in Infants with Retinopathy of Prematurity. Am. J. Ophthalmol. 2012, 153, 327–333.e1. [Google Scholar]
- Hoerster, R.; Muether, P.; Dahlke, C.; Mehler, K.; Oberthür, A.; Kirchhof, B.; Fauser, S. Serum Concentrations of Vascular Endothelial Growth Factor in an Infant Treated with Ranibizumab for Retinopathy of Prematurity. Acta Ophthalmol. 2013, 91, e74–e75. [Google Scholar]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.N.; Shah, V.; Shah, P.S.; Kelly, E.N.; Canadian Neonatal Network. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar]
- Shahidi, F. Nutraceuticals, Functional Foods and Dietary Supplements in Health and Disease. J. Food Drug Anal. 2012, 20, 226–230. [Google Scholar]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487. [Google Scholar]
- Kalra, E.K. Nutraceutical-Definition and Introduction. Aaps Pharmsci. 2003, 5, 27–28. [Google Scholar]
- Ansari, S.H.; Chauhan, B.; Kalam, N.; Kumar, G. Current Concepts and Prospects of Herbal Nutraceutical: A Review. J. Adv. Pharm. Technol. Res. 2013, 4, 4. [Google Scholar]
- Ballard, O.; Ardythe, L.M. Human Milk Composition: Nutrients and Bioactive Factors. Pediatric Clin. 2013, 60, 49–74. [Google Scholar]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.P.; Chiang, M.F. Retinopathy of Prematurity: A Review of Risk Factors and Their Clinical Significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar]
- A, P.E.; Flynn, J.T.; Hardy, R.J.; Phelps, D.L.; Phillips, C.L.; Schaffer, D.B.; Tung, B.; Elsas, F.J.; Botsford, J.M.; Braune, K.W.; et al. Incidence and Early Course of Retlnonathy of Prematurity. Ophthalmology 1991, 98, 1628–1640. [Google Scholar]
- Ashton, N.; Ward, B.; Serpell, G. Effect of Oxygen on Developing Retinal Vessels with Particular Reference to the Problem of Retrolental Fibroplasia. Br. J. Ophthalmol. 1954, 38, 397. [Google Scholar]
- Network, SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research. Target Ranges of Oxygen Saturation in Extremely Preterm Infants. N. Engl. J. Med. 2010, 362, 1959–1969.
- Stenson, B.J.; O Tarnow-Mordi, W.; A Darlow, B.; Simes, J.; Juszczak, E.; Askie, L.M.; Battin, M.R.; Bowler, U.; Broadbent, R.S.; Cairns, P.; et al. Oxygen Saturation and Outcomes in Preterm Infants. N. Engl. J. Med. 2013, 368, 2094–2104. [Google Scholar]
- Fu, Z.; Chatarina, A.L.; Raffael, L.Z.; Wang, Y.; Sun, Y.; Gong, C.-H.; Liu, S.S.M.; Samuel, B.B.I. ArellaPhotoreceptor Glucose Metabolism Determines Normal Retinal Vascular Growth. Embo Mol. Med. 2018, 10, 76–90. [Google Scholar]
- Vannadil, H.; Moulick, P.; Khan, M.; Shankar, S.; Kaushik, J.; Sati, A. Hyperglycaemia as a Risk Factor for the Development of Retinopathy of Prematurity: A Cohort Study. Med. J. Armed Forces India 2020, 76, 95–102. [Google Scholar]
- Fu, Z.; Sun, Y.; Cakir, B.; Tomita, Y.; Huang, S.; Wang, Z.; Liu, C.-H.; Cho, S.S.; Britton, W.; Kern, T.S.; et al. Targeting Neurovascular Interaction in Retinal Disorders. Int. J. Mol. Sci. 2020, 21, 1503. [Google Scholar]
- Country, M.W. Retinal Metabolism: A Comparative Look at Energetics in the Retina. Brain Res. 2017, 1672, 50–57. [Google Scholar]
- Wong-Riley, M. Energy Metabolism of the Visual System. Eye Brain 2010, 2, 99. [Google Scholar]
- Burmester, T.; Hankeln, T. What Is the Function of Neuroglobin? J. Exp. Biol. 2009, 212, 1423–1428. [Google Scholar]
- Narayan, D.S.; Chidlow, G.; Wood, J.P.; Casson, R.J. Glucose Metabolism in Mammalian Photoreceptor Inner and Outer Segments. Clin. Exp. Ophthalmol. 2017, 45, 730–741. [Google Scholar]
- Fu, Z.; Chen, C.T.; Cagnone, G.; Heckel, E.; Sun, Y.; Cakir, B.; Tomita, Y.; Huang, S.; Li, Q.; Britton, W.; et al. Dyslipidemia in Retinal Metabolic Disorders. Embo Mol. Med. 2019, 11, e10473. [Google Scholar]
- Joyal, J.-S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.-S.J.J.S.; Patel, J.-S.J.G.; et al. Retinal Lipid and Glucose Metabolism Dictates Angiogenesis through the Lipid Sensor Ffar1. Nat. Med. 2016, 22, 439–445. [Google Scholar]
- Smith, L. Pathogenesis of Retinopathy of Prematurity. Growth Horm. Igf Res. 2004, 14, 140–144. [Google Scholar]
- Hartnett, M.E. Pathophysiology and Mechanisms of Severe Retinopathy of Prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of Polyol Pathway to Diabetes-Induced Oxidative Stress. J. Am. Soc. Nephrol. 2003, 14 (Suppl. 3), S233–S236. [Google Scholar]
- Fu, Z.; Li, S.-Y.; Kociok, N.; Wong, D.; Chung, S.K.; Lo, A.C.-Y. Aldose Reductase Deficiency Reduced Vascular Changes in Neonatal Mouse Retina in Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5698–5712. [Google Scholar]
- Fu, Z.; Nian, S.; Li, S.-Y.; Wong, D.; Chung, S.K.; Lo, A.C.-Y. Deficiency of Aldose Reductase Attenuates Inner Retinal Neuronal Changes in a Mouse Model of Retinopathy of Prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1503–1513. [Google Scholar]
- Fu, Z.; Yan, G.; Chatarina, L.; Ann, H.; Lois, E.H.S. Adiponectin in Retinopathy. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2016, 1862, 1392–1400. [Google Scholar]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty Acid Carbon Is Essential for Dntp Synthesis in Endothelial Cells. Nature 2015, 520, 192–197. [Google Scholar]
- E Smith, L.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; A D’Amore, P. Oxygen-Induced Retinopathy in the Mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Grossniklaus, H.E.; Kang, S.J.; Berglin, L. Animal Models of Choroidal and Retinal Neovascularization. Prog. Retin. Eye Res. 2010, 29, 500–519. [Google Scholar]
- Penn, J.S.; Tolman, B.L.; A Lowery, L. Variable Oxygen Exposure Causes Preretinal Neovascularization in the Newborn Rat. Investig. Ophthalmol. Vis. Sci. 1993, 34, 576–585. [Google Scholar]
- Hartnett, M.E. The Prematurity of Recommending Particular Polyunsaturated Fatty Acid Supplements for Retinopathy of Prematurity. JAMA Ophthalmol. 2018, 136, 277–278. [Google Scholar]
- Shimazawa, M.; Nakajima, Y.; Mashima, Y.; Hara, H. Docosahexaenoic Acid (Dha) Has Neuroprotective Effects against Oxidative Stress in Retinal Ganglion Cells. Brain Res. 2009, 1251, 269–275. [Google Scholar]
- Kim, J.H.; Lee, B.J.; Kim, J.H.; Yu, Y.S.; Kim, K.-W. Anti-Angiogenic Effect of Caffeic Acid on Retinal Neovascularization. Vasc. Pharmacol. 2009, 51, 262–267. [Google Scholar]
- Vavilala, D.T.; O’Bryhim, B.E.; Ponnaluri, V.K.; White, R.S.; Radel, J.; Symons, R.C.; Mukherji, M. Honokiol Inhibits Pathological Retinal Neovascularization in Oxygen-Induced Retinopathy Mouse Model. Biochem. Biophys. Res. Commun. 2013, 438, 697–702. [Google Scholar]
- Kim, W.T.; Suh, E.S. Retinal Protective Effects of Resveratrol Via Modulation of Nitric Oxide Synthase on Oxygen-Induced Retinopathy. Korean J. Ophthalmol. 2010, 24, 108–118. [Google Scholar]
- Kim, J.H.; Kim, J.H.; Lee, Y.M.; Ahn, E.M.; Kim, K.W.; Yu, Y.S. Decursin Inhibits Retinal Neovascularization Via Suppression of Vegfr-2 Activation. Mol. Vis. 2009, 15, 1868–1875. [Google Scholar]
- Lee, D.-H.; Lee, J.; Jeon, J.; Kim, K.-J.; Yun, J.-H.; Jeong, H.-S.; Lee, E.H.; Koh, Y.J.; Cho, C.-H. Oleanolic Acids Inhibit Vascular Endothelial Growth Factor Receptor 2 Signaling in Endothelial Cells: Implication for Anti-Angiogenic Therapy. Mol. Cells 2018, 41, 771. [Google Scholar]
- Fu, Z.; A Lofqvist, C.; Shao, Z.; Sun, Y.; Joyal, J.-S.; Hurst, C.G.; Cui, R.Z.; Evans, L.P.; Tian, K.; SanGiovanni, J.P.; et al. Dietary Ω-3 Polyunsaturated Fatty Acids Decrease Retinal Neovascularization by Adipose–Endoplasmic Reticulum Stress Reduction to Increase Adiponectin. Am. J. Clin. Nutr. 2015, 101, 879–888. [Google Scholar]
- Jo, H.; Jung, S.H.; Bin Yim, H.; Lee, S.J.; Kang, K.D. The Effect of Baicalin in a Mouse Model of Retinopathy of Prematurity. BMB Rep. 2015, 48, 271. [Google Scholar]
- Matsunaga, N.; Yuichi, C.; Masamitsu, S.; Shigeru, Y.; Hideaki, H. Vaccinium Myrtillus (Bilberry) Extracts Reduce Angiogenesis in Vitro and in Vivo. Evid. Based Complementary Altern. Med. 2010, 7, 47–56. [Google Scholar]
- Kim, J.H.; Yu, Y.S.; Shin, J.Y.; Lee, H.-Y.; Kim, K.-W. Deguelin Inhibits Retinal Neovascularization by Down-Regulation of Hif-1α in Oxygen-Induced Retinopathy. J. Cell. Mol. Med. 2008, 12, 2407–2415. [Google Scholar]
- Minami, M.; Hasebe, Y.; Nakanishi-Ueda, T.; Iwai, S.; Ueda, T.; Dorey, C.K.; Oguchi, K.; Yasuhara, H.; Koide, R. Inhibition of Oxygen-Induced Retinal Neovascularization in the Neonatal Rat by Green Tea Extract. J. Clin. Biochem. Nutr. 2003, 33, 23–31. [Google Scholar]
- Saito, Y.; Hasebe-Takenaka, Y.; Ueda, T.; Nakanishi-Ueda, T.; Kosuge, S.; Aburada, M.; Shimada, T.; Ikeya, Y.; Onda, H.; Ogura, H.; et al. Effects of Green Tea Fractions on Oxygen-Induced Retinal Neovascularization in the Neonatal Rat. J. Clin. Biochem. Nutr. 2007, 41, 43–49. [Google Scholar]
- Park, S.W.; Cho, C.S.; Jun, H.O.; Ryu, N.H.; Kim, J.S.; Yu, Y.S.; Kim, J.H. Anti-Angiogenic Effect of Luteolin on Retinal Neovascularization Via Blockade of Reactive Oxygen Species Production. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7718–7726. [Google Scholar]
- Chen, Y.; Li, F.; Meng, X.; Li, X. Suppression of Retinal Angiogenesis by Quercetin in a Rodent Model of Retinopathy of Prematurity. Zhonghua Yi Xue Za Zhi 2015, 95, 1113–1115. [Google Scholar]
- Kim, S.J.; Gu, Y.R.; Kim, Y.J.; Yu, H.G. Effect of Curcumin in a Mouse Model of Oxygen-Induced Retinopathy. J. Korean Ophthalmol. Soc. 2013, 54, 1588–1593. [Google Scholar]
- Li, W.; Jiang, D. Effect of Resveratrol on Bcl-2 and Vegf Expression in Oxygen-Induced Retinopathy of Prematurity. J. Pediatr. Ophthalmol. Strabismus 2012, 49, 230–235. [Google Scholar]
- Zhang, S.; Zhou, R.; Li, B.; Li, H.; Wang, Y.; Gu, X.; Tang, L.; Wang, C.; Zhong, D.; Ge, Y.; et al. Caffeine Preferentially Protects against Oxygen-Induced Retinopathy. Faseb J. 2017, 31, 3334–3348. [Google Scholar]
- Liang, X.-L.; Zhou, H.; Ding, Y.; Li, J.; Yang, C.; Luo, Y.; Li, S.; Sun, G.; Liao, X.; Min, W. Tmp Prevents Retinal Neovascularization and Imparts Neuroprotection in an Oxygen-Induced Retinopathy Model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2157–2169. [Google Scholar]
- Fu, Z.; Meng, S.S.; Burnim, S.B.; Smith, L.E.; Lo, A.C.-Y. Lutein Facilitates Physiological Revascularization in a Mouse Model of Retinopathy of Prematurity. Clin. Exp. Ophthalmol. 2017, 45, 529–538. [Google Scholar]
- Saito, Y.; Uppal, A.; Byfield, G.; Budd, S.; Hartnett, M.E. Activated Nad (P) H Oxidase from Supplemental Oxygen Induces Neovascularization Independent of Vegf in Retinopathy of Prematurity Model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1591–1598. [Google Scholar]
- Higuchi, A.; Yamada, H.; Yamada, E.; Jo, N.; Matsumura, M. Hypericin Inhibits Pathological Retinal Neovascularization in a Mouse Model of Oxygen-Induced Retinopathy. Mol. Vis. 2008, 14, 249. [Google Scholar]
- Kim, J.; Lee, Y.M.; Jung, W.; Park, S.-B.; Kim, C.-S.; Kim, J.S. Aster Koraiensis Extract and Chlorogenic Acid Inhibit Retinal Angiogenesis in a Mouse Model of Oxygen-Induced Retinopathy. Evid. Based Complementary Altern. Med. 2018, 2018, 6402650. [Google Scholar]
- Liu, X.; Wang, B.; Sun, Y.; Jia, Y.; Xu, Z. Astragalus Root Extract Inhibits Retinal Cell Apoptosis and Repairs Damaged Retinal Neovascularization in Retinopathy of Prematurity. Cell Cycle 2019, 18, 3147–3159. [Google Scholar]
- Lee, Y.M.; Lee, Y.-R.; Kim, C.-S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Cnidium Officinale Extract and Butylidenephthalide Inhibits Retinal Neovascularization in Vitro and in Vivo. BMC Complementary Altern. Med. 2016, 16, 231. [Google Scholar]
- Lee, Y.M.; Lee, Y.-R.; Kim, C.-S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy. Int. J. Mol. Sci. 2015, 16, 29900–29910. [Google Scholar]
- Lee, Y.M.; Kim, C.-S.; Jo, K.; Sohn, E.J.; Kim, J.S.; Kim, J. Inhibitory Effect of Samul-Tang on Retinal Neovascularization in Oxygen-Induced Retinopathy. BMC Complementary Altern. Med. 2015, 15, 1–7. [Google Scholar]
- Lee, Y.M.; Kim, C.-S.; Sohn, E.; Jo, K.; Lim, H.R.; Kim, S.K.; Kim, J.S.; Kim, J. Sipjeondaebo-Tang, a Traditional Herbal Formula, Inhibits Retinal Neovascularization in a Mouse Model of Oxygen-Induced Retinopathy. Tohoku J. Exp. Med. 2014, 234, 229–236. [Google Scholar]
- Pawlik, D.; Lauterbach, R.; Turyk, E. Fish-Oil Fat Emulsion Supplementation May Reduce the Risk of Severe Retinopathy in Vlbw Infants. Pediatrics 2011, 127, 223–228. [Google Scholar]
- Beken, S.; Dilli, D.; Fettah, N.D.; Kabataş, E.U.; Zenciroğlu, A.; Okumuş, N. The Influence of Fish-Oil Lipid Emulsions on Retinopathy of Prematurity in Very Low Birth Weight Infants: A Randomized Controlled Trial. Early Hum. Dev. 2014, 90, 27–31. [Google Scholar]
- Tu, C.-F.; Lee, C.-H.; Chen, H.-N.; Tsao, L.-Y.; Chen, J.-Y.; Hsiao, C.-C. Effects of Fish Oil-Containing Lipid Emulsions on Retinopathy of Prematurity in Very Low Birth Weight Infants. Pediatrics Neonatol. 2020, 224–230. [Google Scholar]
- Gawecka, A.; Michalkiewicz, J.; Kornacka, M.K.; Luckiewicz, B.; Kubiszewska, I. Immunologic Properties Differ in Preterm Infants Fed Olive Oil vs Soy-Based Lipid Emulsions During Parenteral Nutrition. J. Parenter. Enter. Nutr. 2008, 32, 448–453. [Google Scholar]
- Ferreira, M.W.; Da Costa, D.V.; Leal, C.A.G.; Figueiredo, H.C.P.; Rosa, P.V. Dietary Oil Sources on the Innate Immunity and Resistance of Nile Tilapia, Oreochromis Niloticus, to Streptococcus Agalactiae Challenge. J. World Aquac. Soc. 2015, 46, 252–262. [Google Scholar]
- Waitzberg, D.L.; Lotierzo, P.H.; Logullo, A.F.; Torrinhas, R.S.M.; Pereira, C.C.A.; Meier, R. Parenteral Lipid Emulsions and Phagocytic Systems. Br. J. Nutr. 2002, 87, S49–S57. [Google Scholar]
- Yaqoob, P. Lipids and the Immune Response: From Molecular Mechanisms to Clinical Applications. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 133–150. [Google Scholar]
- Goodwin, J.S.; Ceuppens, J.L. Regulation of the Immune Response by Prostaglandins. J. Clin. Immunol. 1983, 3, 295–315. [Google Scholar]
- Yaqoob, P.; Calder, P.C. Effects of Dietary Lipid Manipulation Upon Inflammatory Mediator Production by Murine Macrophages. Cell. Immunol. 1995, 163, 120–128. [Google Scholar]
- De Caterina, R. N–3 Fatty Acids in Cardiovascular Disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar]
- Najm, S.; Chatarina, L.; Gunnel, H.; Eva, E.; Pia, L.; Anna-Lena, H.; Alexandre, L.; Karin, S.; Anders, K.N.; Mats, X.A. Effects of a Lipid Emulsion Containing Fish Oil on Polyunsaturated Fatty Acid Profiles, Growth and Morbidities in Extremely Premature Infants: A Randomized Controlled Trial. Clin. Nutr. Espen 2017, 20, 17–23. [Google Scholar]
- Göbel, Y.; Koletzko, B.; Böhles, H.-J.; Engelsberger, I.; Forget, D.; Le Brun, A.; Peters, J.; Zimmermann, A. Parenteral Fat Emulsions Based on Olive and Soybean Oils: A Randomized Clinical Trial in Preterm Infants. J. Pediatric Gastroenterol. Nutr. 2003, 37, 161–167. [Google Scholar]
- Goulet, O.; Sophie d, P.; Helena, A.; Fathi, D.; Virginie, C.; Gilbert, B.; Louis-Gérald, A.; Odile, C.; Alexia, L.B.; Guy, D. Long-Term Efficacy and Safety of a New Olive Oil–Based Intravenous Fat Emulsion in Pediatric Patients: A Double-Blind Randomized Study. Am. J. Clin. Nutr. 1999, 70, 338–345. [Google Scholar]
- Gibson, R.A.; Maria, M. The Role of Long Chain Polyunsaturated Fatty Acids (Lcpufa) in Neonatal Nutrition. Acta Paediatr. 1998, 87, 1017–1022. [Google Scholar]
- Koletzko, B.; Carlo, A.; Susan, E.C.T.C.; Gerard, H.; Martha, N.; Ricardo, U.; Yuichiro, Y.; Peter, W. Long Chain Polyunsaturated Fatty Acids (Lc-Pufa) and Perinatal Development. Acta Paediatr. 2001, 90, 460–464. [Google Scholar]
- SanGiovanni, J.P.; Socorro, P.-C.; Graham, A.C.; Catherine, S.B.; Johanna, T.D. Meta-Analysis of Dietary Essential Fatty Acids and Long-Chain Polyunsaturated Fatty Acids as They Relate to Visual Resolution Acuity in Healthy Preterm Infants. Pediatrics 2000, 105, 1292–1298. [Google Scholar]
- Birch, E.E.; Sharon, G.; Dennis, R.H.; Ricardo, U.; David, G.B. A Randomized Controlled Trial of Early Dietary Supply of Long-Chain Polyunsaturated Fatty Acids and Mental Development in Term Infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N. Effects of Long-Chain Polyunsaturated Fatty Acid Supplementation on Neurodevelopment in Childhood: A Review of Human Studies. Prostaglandins Leukot. Essent. Fat. Acids (Plefa) 2010, 82, 305–314. [Google Scholar]
- Lapillonne, A.; Moltu, S.J. Long-Chain Polyunsaturated Fatty Acids and Clinical Outcomes of Preterm Infants. Ann. Nutr. Metab. 2016, 69, 35–44. [Google Scholar]
- Uauy, R.; Patricia, M. Long-Chain Polyunsaturated Fatty Acids Supplementation in Preterm Infants. Curr. Opin. Pediatrics 2015, 27, 165–171. [Google Scholar]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; A Pravda, E.; Majchrzak, S.; Carper, D.; et al. Increased Dietary Intake of Ω-3-Polyunsaturated Fatty Acids Reduces Pathological Retinal Angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar]
- SanGiovanni, J.P.; Chew, E.Y. The Role of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Health and Disease of the Retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar]
- Stahl, A.; Przemyslaw, S.; Kip, M.C.; John, P.S.; Jing, C.; Christopher, M.A.; Keirnan, L.W.; Nathan, M.K.; Roberta, J.D.; Molly, R.S. Pparγ Mediates a Direct Antiangiogenic Effect of Ω3-Pufas in Proliferative Retinopathy. Circ. Res. 2010, 107, 495–500. [Google Scholar]
- Sapieha, P.; Andreas, S.; Jing, C.; Molly, R.S.; Keirnan, L.W.; Nathan, M.K.; Roberta, J.D.; Kip, M.C.; Christopher, M.A.; Elvira, L. 5-Lipoxygenase Metabolite 4-Hdha Is a Mediator of the Antiangiogenic Effect of Ω-3 Polyunsaturated Fatty Acids. Sci. Transl. Med. 2011, 3, ra12–ra69. [Google Scholar]
- Khalesi, N.; Bordbar, A.; Khosravi, N.; Kabirian, M.; Karimi, A. The Efficacy of Omega-3 Supplement on Prevention of Retinopathy of Prematurity in Premature Infants: A Randomized Double-Blinded Controlled Trial. Curr. Pharm. Des. 2018, 24, 1845–1848. [Google Scholar]
- Löfqvist, C.A.; Svetlana, N.; Gunnel, H.; Eva, E.; Karin, S.; Anders, K.N.; Mats, X.A.; Anna-Lena, H.; Lois, E.S.; Ann, H. Association of Retinopathy of Prematurity with Low Levels of Arachidonic Acid: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 271–277. [Google Scholar]
- Baack, M.L.; Puumala, S.E.; Messier, S.E.; Pritchett, D.K.; Harris, W.S. What Is the Relationship between Gestational Age and Docosahexaenoic Acid (Dha) and Arachidonic Acid (Ara) Levels? Prostaglandins Leukot. Essent. Fat. Acids 2015, 100, 5–11. [Google Scholar]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased Postnatal Docosahexaenoic and Arachidonic Acid Blood Levels in Premature Infants Are Associated with Neonatal Morbidities. J. Pediatr. 2011, 159, 743–749 e2. [Google Scholar]
- Crespy, V.; Williamson, G. A Review of the Health Effects of Green Tea Catechins in in Vivo Animal Models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar]
- Cooper, R.; Morré, D.M.; Morré, D.M. Medicinal Benefits of Green Tea: Part II. Review of Anticancer Properties. J. Altern. Complementary Med. 2005, 11, 639–652. [Google Scholar]
- Cooper, R.; Morré, D.M.; Morré, D.M. Medicinal Benefits of Green Tea: Part I. Review of Noncancer Health Benefits. J. Altern. Complementary Med. 2005, 11, 521–528. [Google Scholar]
- Zhao, B. The Health Effects of Tea Polyphenols and Their Antioxidant Mechanism. J. Clin. Biochem. Nutr. 2006, 38, 59–68. [Google Scholar]
- Cao, Y.; Cao, R. Angiogenesis Inhibited by Drinking Tea. Nature 1999, 398, 381. [Google Scholar]
- Tsuda, T. Dietary Anthocyanin-Rich Plants: Biochemical Basis and Recent Progress in Health Benefits Studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins Via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar]
- Kim, J.; Kim, C.-S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium Myrtillus Extract Prevents or Delays the Onset of Diabetes--Induced Blood–Retinal Barrier Breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of Interleukin-1β Mediated Inflammatory Response in Human Astrocytes by Flavonoids: Implications in Neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.-M. Ros and Brain Diseases: The Good, the Bad, and the Ugly. Oxidative Med. Cell. Longev. 2013, 2013, 963520. [Google Scholar]
- Chen, Y.; Li, X.-X.; Xing, N.-Z.; Cao, X.-G. Quercetin Inhibits Choroidal and Retinal Angiogenesis in Vitro. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 373–378. [Google Scholar]
- Li, F.; Bai, Y.; Zhao, M.; Huang, L.; Li, S.; Li, X.; Chen, Y. Quercetin Inhibits Vascular Endothelial Growth Factor-Induced Choroidal and Retinal Angiogenesis in Vitro. Ophthalmic Res. 2015, 53, 109–116. [Google Scholar]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar]
- Lin, C.-C.; Shieh, D.-E. The Anti-Inflammatory Activity of Scutellaria Rivularis Extracts and Its Active Components, Baicalin, Baicalein and Wogonin. Am. J. Chin. Med. 1996, 24, 31–36. [Google Scholar]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free Radical Scavenging and Antioxidant Activities of Flavonoids Extracted from the Radix of Scutellaria Baicalensis Georgi. Biochim. Et Biophys. Acta (Bba)-Gen. Subj. 1999, 1472, 643–650. [Google Scholar]
- Nagashima, S.; Hirotani, M.; Yoshikawa, T. Purification and Characterization of Udp-Glucuronate: Baicalein 7-O-Glucuronosyltransferase from Scutellaria Baicalensis Georgi. Cell Suspension Cultures. Phytochemistry 2000, 53, 533–538. [Google Scholar]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The Correlation between Active Oxygens Scavenging and Antioxidative Effects of Flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar]
- Xiong, P.; Chen, X.; Guo, C.; Zhang, N.; Ma, B. Baicalin and Deferoxamine Alleviate Iron Accumulation in Different Brain Regions of Parkinson’s Disease Rats. Neural Regen. Res. 2012, 7, 2092. [Google Scholar]
- Huang, H.-L.; Ya-Jing, W.; Qing-Yu, Z.; Bin, L.; Fang-Yuan, W.; Jing-Jing, L.; Run-Zhi, Z. Hepatoprotective Effects of Baicalein against Ccl4-Induced Acute Liver Injury in Mice. World J. Gastroenterol. Wjg 2012, 18, 6605. [Google Scholar]
- Ueda, S.; Nakamura, H.; Masutani, H.; Sasada, T.; Takabayashi, A.; Yamaoka, Y.; Yodoi, J. Baicalin Induces Apoptosis Via Mitochondrial Pathway as Prooxidant. Mol. Immunol. 2002, 38, 781–791. [Google Scholar]
- Chan, F.L.; Choi, H.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of Apoptosis in Prostate Cancer Cell Lines by a Flavonoid, Baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar]
- Zhou, Q.-M.; Wang, S.; Zhang, H.; Lu, Y.-Y.; Wang, X.-F.; Motoo, Y.; Su, S.-B. The Combination of Baicalin and Baicalein Enhances Apoptosis Via the Erk/P38 Mapk Pathway in Human Breast Cancer Cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar]
- Zhu, J.; Wang, J.-F.; Sheng, Y.; Zou, Y.; Bo, L.; Wang, F.; Lou, J.; Fan, X.; Bao, R.; Wu, Y.; et al. Baicalin Improves Survival in a Murine Model of Polymicrobial Sepsis Via Suppressing Inflammatory Response and Lymphocyte Apoptosis. PLoS ONE 2012, 7, e35523. [Google Scholar]
- Lin, M.; Li, L.; Li, L.; Pokhrel, G.; Qi, G.; Rong, R.; Zhu, T. The Protective Effect of Baicalin against Renal Ischemia-Reperfusion Injury through Inhibition of Inflammation and Apoptosis. BMC Complementary Altern. Med. 2014, 14, 19. [Google Scholar]
- Tu, X.-K.; Yang, W.-Z.; Shi, S.-S.; Wang, C.-H.; Chen, C.-M. Neuroprotective Effect of Baicalin in a Rat Model of Permanent Focal Cerebral Ischemia. Neurochem. Res. 2009, 34, 1626–1634. [Google Scholar]
- Paris, R. Presence of a Luteolin Glycoside in Weld (Reseda Luteola). Ann. Pharm. Françaises 1955, 13, 485–487. [Google Scholar]
- Seelinger, G.; Irmgard, M.; Ute, W.; Christoph, M.S. Anti-Carcinogenic Effects of the Flavonoid Luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-Inflammatory Activity of Structurally Related Flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar]
- Choi, J.S.; Islam, N.; Ali, Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The Effects of C-Glycosylation of Luteolin on Its Antioxidant, Anti-Alzheimer’s Disease, Anti-Diabetic, and Anti-Inflammatory Activities. Arch. Pharmacal. Res. 2014, 37, 1354–1363. [Google Scholar]
- Ziyan, L.; Yongmei, Z.; Nan, Z.; Ning, T.; Baolin, L. Evaluation of the Anti-Inflammatory Activity of Luteolin in Experimental Animal Models. Planta Med. 2007, 73, 221–226. [Google Scholar]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an Anti-Inflammatory and Neuroprotective Agent: A Brief Review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar]
- Udeani, G.; Gerhauser, C.; Thomas, C.F.; Moon, R.C.; Kosmeder, J.W.; Kinghorn, A.D.; Moriarty, R.M.; Pezzuto, J.M. Cancer Chemopreventive Activity Mediated by Deguelin, a Naturally Occurring Rotenoid. Cancer Res. 1997, 57, 3424–3428. [Google Scholar]
- Lee, H.-Y.; Oh, S.-H.; Woo, J.K.; Kim, W.-Y.; Van Pelt, C.S.; Price, R.E.; Cody, D.; Tran, H.; Pezzuto, J.M.; Moriarty, R.M.; et al. Chemopreventive Effects of Deguelin, a Novel Akt Inhibitor, on Tobacco-Induced Lung Tumorigenesis. J. Natl. Cancer Inst. 2005, 97, 1695–1699. [Google Scholar]
- Murillo, G.; Kosmeder, J.W.; Pezzuto, J.M.; Mehta, R.G. Deguelin Suppresses the Formation of Carcinogen-Induced Aberrant Crypt Foci in the Colon of Cf-1 Mice. Int. J. Cancer 2003, 104, 7–11. [Google Scholar]
- Gerhauser, C.; Lee, S.K.; Kosmeder, J.W.; Moriarty, R.M.; Hamel, E.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Regulation of Ornithine Decarboxylase Induction by Deguelin, a Natural Product Cancer Chemopreventive Agent. Cancer Res. 1997, 57, 3429–3435. [Google Scholar]
- Chun, K.-H.; Kosmeder, J.W.; Sun, S.; Pezzuto, J.M.; Lotan, R.; Hong, W.K.; Lee, H.-Y. Effects of Deguelin on the Phosphatidylinositol 3-Kinase/Akt Pathway and Apoptosis in Premalignant Human Bronchial Epithelial Cells. J. Natl. Cancer Inst. 2003, 95, 291–302. [Google Scholar]
- Lee, H.-Y.; Suh, Y.-A.; Kosmeder, J.W.; Pezzuto, J.M.; Hong, W.K.; Kurie, J.M. Deguelin-Induced Inhibition of Cyclooxygenase-2 Expression in Human Bronchial Epithelial Cells. Clin. Cancer Res. 2004, 10, 1074–1079. [Google Scholar]
- Murillo, G.; Salti, G.; Ii, J.K.; Pezzuto, J.; Mehta, R. Deguelin Inhibits the Growth of Colon Cancer Cells through the Induction of Apoptosis and Cell Cycle Arrest. Eur. J. Cancer 2002, 38, 2446–2454. [Google Scholar]
- Dell’Eva, R.; Claudia, A.; Simona, M.; Douglas, M.N.; Adriana, A.; Nicoletta, F. The Akt Inhibitor Deguelin, Is an Angiopreventive Agent Also Acting on the Nf-Κb Pathway. Carcinogenesis 2007, 28, 404–413. [Google Scholar]
- Li, M.; Yu, X.; Li, W.; Liu, T.; Deng, G.; Liu, W.; Liu, H.; Gao, F. Deguelin Suppresses Angiogenesis in Human Hepatocellular Carcinoma by Targeting Hgf-C-Met Pathway. Oncotarget 2018, 9, 152. [Google Scholar]
- Oh, S.-H.; Woo, J.-K.; Jin, Q.; Kang, H.-J.; Jeong, J.-W.; Kim, K.-W.; Hong, W.K.; Lee, H.-Y. Identification of Novel Antiangiogenic Anticancer Activities of Deguelin Targeting Hypoxia-Inducible Factor-1 Alpha. Int. J. Cancer 2008, 122, 5–14. [Google Scholar]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Park, K.H.; Kang, H.J.; Lee, H.-Y.; Kim, K.-W. Antiangiogenic Effect of Deguelin on Choroidal Neovascularization. J. Pharmacol. Exp. Ther. 2008, 324, 643–647. [Google Scholar]
- Toyoda, T.; Tsukamoto, T.; Takasu, S.; Shi, L.; Hirano, N.; Ban, H.; Kumagai, T.; Tatematsu, M. Anti-Inflammatory Effects of Caffeic Acid Phenethyl Ester (Cape), a Nuclear Factor-Κb Inhibitor, on Helicobacter Pylori-Induced Gastritis in Mongolian Gerbils. Int. J. Cancer 2009, 125, 1786–1795. [Google Scholar]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar]
- Chao, P.-C.; Hsu, C.-C.; Yin, M.-C. Anti-Inflammatory and Anti-Coagulatory Activities of Caffeic Acid and Ellagic Acid in Cardiac Tissue of Diabetic Mice. Nutr. Metab. 2009, 6, 33. [Google Scholar]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-Apoptotic Activity of Caffeic Acid, Ellagic Acid and Ferulic Acid in Normal Human Peripheral Blood Mononuclear Cells: A Bcl-2 Independent Mechanism. Biochim. Et Biophys. Acta (Bba)-Gen. Subj. 2006, 1760, 283–289. [Google Scholar]
- Singh, S.; Bharat, B.A. Activation of Transcription Factor Nf-Κb Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar]
- Shishodia, S.; Hesham, M.A.; Raymond, L.; Bharat, B.A. Curcumin (Diferuloylmethane) Inhibits Constitutive Nf-Κb Activation, Induces G1/S Arrest, Suppresses Proliferation, and Induces Apoptosis in Mantle Cell Lymphoma. Biochem. Pharmacol. 2005, 70, 700–713. [Google Scholar]
- Rafiee, P.; Victoria, M.N.; Sharon, M.; Michael, W.; Martin, F.; David, G.B.; Reza, S. Effect of Curcumin on Acidic Ph-Induced Expression of Il-6 and Il-8 in Human Esophageal Epithelial Cells (Het-1a): Role of Pkc, Mapks, and Nf-Κb. Am. J. Physiol. -Gastrointest. Liver Physiol. 2009, 296, G388–G398. [Google Scholar]
- Cohen, A.N.; Mysore, S.V.; Eri, S.S.; Marilene, B.W. Suppression of Interleukin 6 and 8 Production in Head and Neck Cancer Cells with Curcumin Via Inhibition of Iκβ Kinase. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 190–197. [Google Scholar]
- Kim, J.M.; Araki, S.; Kim, D.J.; Park, C.B.; Takasuka, N.; Baba-Toriyama, H.; Ota, T.; Nir, Z.; Khachik, F.; Shimidzu, N.; et al. Chemopreventive Effects of Carotenoids and Curcumins on Mouse Colon Carcinogenesis after 1, 2-Dimethylhydrazine Initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar]
- LoTempio, M.M.; Veena, M.S.; Steele, H.L.; Ramamurthy, B.; Ramalingam, T.S.; Cohen, A.N.; Chakrabarti, R.; Srivatsan, E.S.; Wang, M.B. Curcumin Suppresses Growth of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2005, 11, 6994–7002. [Google Scholar]
- Rao, C.V.; Simi, B.; Reddy, B.S. Inhibition by Dietary Curcumin of Azoxymethane-Induced Ornithine Decarboxylase, Tyrosine Protein Kinase, Arachidonic Acid Metabolism and Aberrant Crypt Foci Formation in the Rat Colon. Carcinogenesis 1993, 14, 2219–2225. [Google Scholar]
- Billerey-Larmonier, C.; Uno, J.K.; Larmonier, N.; Midura, A.J.; Timmermann, B.; Ghishan, F.K.; Kiela, P.R. Protective Effects of Dietary Curcumin in Mouse Model of Chemically Induced Colitis Are Strain Dependent. Inflamm. Bowel Dis. 2008, 14, 780–793. [Google Scholar]
- Nones, K.; Knoch, B.; Dommels, Y.; Paturi, G.; A Butts, C.; McNabb, W.C.; Roy, N.C. Multidrug Resistance Gene Deficient (Mdr1a–/–) Mice Have an Altered Caecal Microbiota That Precedes the Onset of Intestinal Inflammation. J. Appl. Microbiol. 2009, 107, 557–566. [Google Scholar]
- Hasan, S.; Zingg, J.-M.; Kwan, P.; Noble, T.; Smith, D.; Meydani, M. Curcumin Modulation of High Fat Diet-Induced Atherosclerosis and Steatohepatosis in Ldl Receptor Deficient Mice. Atherosclerosis 2014, 232, 40–51. [Google Scholar]
- Baum, L.; Cheung, S.K.; Mok, V.C.; Lam, L.C.; Leung, V.P.Y.; Hui, E.; Cheng, W.K.F.; Chow, M.; Ho, P.C.; Lam, S. Curcumin Effects on Blood Lipid Profile in a 6-Month Human Study. Pharmacol. Res. 2007, 56, 509–514. [Google Scholar]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M. Curcumin Potentiates Cholesterol-Lowering Effects of Phytosterols in Hypercholesterolaemic Individuals. A Randomised Controlled Trial. Metabolism 2018, 82, 22–35. [Google Scholar]
- Kim, S.G.; Veena, M.S.; Basak, S.K.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin Treatment Suppresses Ikkβ Kinase Activity of Salivary Cells of Patients with Head and Neck Cancer: A Pilot Study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar]
- Sahebkar, A. Dual Effect of Curcumin in Preventing Atherosclerosis: The Potential Role of Pro-Oxidant–Antioxidant Mechanisms. Nat. Prod. Res. 2015, 29, 491–492. [Google Scholar]
- Alwi, I.; Santoso, T.; Suyono, S.; Sutrisna, B.; Suyatna, F.D.; Kresno, S.B.; Ernie, S. The Effect of Curcumin on Lipid Level in Patients with Acute Coronary Syndrome. Acta Med. Indones 2008, 40, 201–210. [Google Scholar]
- Ringman, J.M.; A Frautschy, S.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-Week Randomized, Double Blind, Placebo-Controlled Study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase Ii Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase Iia Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of Atherogenic Risk in Patients with Type 2 Diabetes by Curcuminoid Extract: A Randomized Controlled Trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar]
- Lal, B.; Kapoor, A.K.; Asthana, O.P.; Agrawal, P.K.; Prasad, R.; Kumar, P.; Srimal, R.C. Efficacy of Curcumin in the Management of Chronic Anterior Uveitis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 318–322. [Google Scholar]
- Pia, A.; Allegri, P.; Mastromarino, A.; Neri, P. Management of Chronic Anterior Uveitis Relapses: Efficacy of Oral Phospholipidic Curcumin Treatment. Long-Term Follow-Up. Clin. Ophthalmol. (Auckl. Nz) 2010, 4, 1201. [Google Scholar]
- Biswas, N.R.; Gupta, S.K.; Das, G.K.; Kumar, N.; Mongre, P.K.; Haldar, D.; Beri, S. Evaluation of Ophthacare® Eye Drops—a Herbal Formulation in the Management of Various Ophthalmic Disorders. Phytother. Res. 2001, 15, 618–620. [Google Scholar]
- Woo, J.M.; Shin, D.-Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin Protects Retinal Pigment Epithelial Cells against Oxidative Stress Via Induction of Heme Oxygenase-1 Expression and Reduction of Reactive Oxygen. Mol. Vis. 2012, 18, 901. [Google Scholar]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal Protection and Distribution of Curcumin in Vitro and in Vivo. Front. Pharmacol. 2018, 9, 670. [Google Scholar]
- Zini, R.; Morin, C.; A Bertelli, A.; Tillement, J.-P. Effects of Resveratrol on the Rat Brain Respiratory Chain. Drugs Exp. Clin. Res. 1999, 25, 87–97. [Google Scholar]
- Sinha, K.; Chaudhary, G.; Gupta, Y.K. Protective Effect of Resveratrol against Oxidative Stress in Middle Cerebral Artery Occlusion Model of Stroke in Rats. Life Sci. 2002, 71, 655–665. [Google Scholar]
- Alarcón-De-La-Lastra, C.; Villegas, I. Resveratrol as an Antioxidant and Pro-Oxidant Agent: Mechanisms and Clinical Implications; Portland Press Ltd.: London, UK, 2007. [Google Scholar]
- Ateş, Ö.; Çayli, S.; Altinoz, E.; Gurses, I.; Yucel, N.; Sener, M.; Kocak, A.; Yologlu, S. Neuroprotection by Resveratrol against Traumatic Brain Injury in Rats. Mol. Cell. Biochem. 2007, 294, 137–144. [Google Scholar]
- Bastianetto, S.; Caroline, M.; Rémi, Q. Neuroprotective Action of Resveratrol. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Hoda, N.; Khuwaja, G.; Raza, S.S.; Khan, A.; Javed, H.; Vaibhav, K.; Islam, F.; et al. Resveratrol Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Dopamine Depletion in Rat Model of Parkinson’s Disease. Brain Res. 2010, 1328, 139–151. [Google Scholar]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. Resveratrol Neuroprotection in Stroke and Traumatic Cns Injury. Neurochem. Int. 2015, 89, 75–82. [Google Scholar]
- Li, F.; Gong, Q.; Dong, H.; Shi, J. Resveratrol, a Neuroprotective Supplement for Alzheimer’s Disease. Curr. Pharm. Des. 2012, 18, 27–33. [Google Scholar]
- Kubota, S.; Kurihara, T.; Ebinuma, M.; Kubota, M.; Yuki, K.; Sasaki, M.; Noda, K.; Ozawa, Y.; Oike, Y.; Ishida, S.; et al. Resveratrol Prevents Light-Induced Retinal Degeneration Via Suppressing Activator Protein-1 Activation. Am. J. Pathol. 2010, 177, 1725–1731. [Google Scholar]
- Ahn, K.S.; Sethi, G.; Shishodia, S.; Sung, B.; Arbiser, J.L.; Aggarwal, B.B. Honokiol Potentiates Apoptosis, Suppresses Osteoclastogenesis, and Inhibits Invasion through Modulation of Nuclear Factor-Kappab Activation Pathway. Mol. Cancer Res. 2006, 4, 621–633. [Google Scholar]
- Fried, L.E.; Arbiser, J.L. Honokiol, a Multifunctional Antiangiogenic and Antitumor Agent. Antioxid. Redox Signal 2009, 11, 1139–1148. [Google Scholar]
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a Small Molecular Weight Natural Product, Inhibits Angiogenesis in Vitro and Tumor Growth in Vivo. J. Biol. Chem. 2003, 278, 35501–35507. [Google Scholar]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In Vitro Antibacterial and Anti-Inflammatory Effects of Honokiol and Magnolol against Propionibacterium Sp. Eur. J. Pharm. 2004, 496, 189–195. [Google Scholar]
- Lin, Y.-R.; Chen, H.-H.; Ko, C.-H.; Chan, M.-H. Neuroprotective Activity of Honokiol and Magnolol in Cerebellar Granule Cell Damage. Eur. J. Pharmacol. 2006, 537, 64–69. [Google Scholar]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A Novel Natural Agent for Cancer Prevention and Therapy. Curr. Mol. Med. 2012, 12, 1244–1252. [Google Scholar]
- Santiago, A.R.; Filipa, I.B.; Paulo, F.S.; Gonçalo, C.; António, F.A.; Rodrigo, A.C.; Catarina, A.G. Role of Microglia Adenosine A2a Receptors in Retinal and Brain Neurodegenerative Diseases. Mediat. Inflamm. 2014, 2014, 465694. [Google Scholar]
- Saura, J.; Angulo-Pueyo, E.; Ejarque, A.; Casadó, V.; Tusell, J.M.; Moratalla, R.; Chen, J.-F.; Schwarzschild, M.A.; Lluís, C.; Franco, R.; et al. Adenosine A2a Receptor Stimulation Potentiates Nitric Oxide Release by Activated Microglia. J. Neurochem. 2005, 95, 919–929. [Google Scholar]
- Chen, J.-F.; Kui, X.; Jacobus, P.P.; Roland, S.; Yue-Hang, X.; Mark, B.; Patricia, K.S.; Kay, C.; Neal, C.; Michael, A.S. Neuroprotection by Caffeine and A2a Adenosine Receptor Inactivation in a Model of Parkinson’s Disease. J. Neurosci. 2001, 21, RC143. [Google Scholar]
- Dall’Lgna, O.P.; O Porciúncula, L.; O Souza, D.; A Cunha, R.; Lara, D.R. Neuroprotection by Caffeine and Adenosine A2a Receptor Blockade of Β-Amyloid Neurotoxicity. Br. J. Pharmacol. 2003, 138, 1207–1209. [Google Scholar]
- Herlenius, E.; Lagercrantz, H.; Yamamoto, Y. Adenosine Modulates Inspiratory Neurons and the Respiratory Pattern in the Brainstem of Neonatal Rats. Pediatric Res. 1997, 42, 46–53. [Google Scholar]
- Natarajan, G.; Lulic-Botica, M.; Aranda, J. Pharmacology Review: Clinical Pharmacology of Caffeine in the Newborn. NeoReviews 2007, 8, e214–e221. [Google Scholar]
- Lodha, A.K.; Seshia, M.; McMillan, D.D.; Barrington, K.J.; Yang, J.; Lee, S.K.; Shah, P.S. Association of Early Caffeine Administration and Neonatal Outcomes in Very Preterm Neonates. JAMA Pediatr. 2015, 169, 33–38. [Google Scholar]
- Doyle, L.W.; Cheong, J.; Hunt, R.W.; Lee, K.J.; Thompson, D.K.; Davis, P.G.; Rees, S.; Anderson, P.J.; Inder, T.E. Caffeine and Brain Development in Very Preterm Infants. Ann. Neurol. 2010, 68, 734–742. [Google Scholar]
- Hoecker, C.; Nelle, M.; Poeschl, J.; Beedgen, B.; Linderkamp, O. Caffeine Impairs Cerebral and Intestinal Blood Flow Velocity in Preterm Infants. Pediatrics 2002, 109, 784–787. [Google Scholar]
- Chavez-Valdez, R.; Wills-Karp, M.; Ahlawat, R.; A Cristofalo, E.; Nathan, A.; Gauda, E.B. Caffeine Modulates Tnf-A Production by Cord Blood Monocytes: The Role of Adenosine Receptors. Pediatr. Res. 2009, 65, 203. [Google Scholar]
- Patel, R.M.; Leong, T.; Carlton, D.P.; Vyas-Read, S. Early Caffeine Therapy and Clinical Outcomes in Extremely Preterm Infants. J. Perinatol. 2013, 33, 134–140. [Google Scholar]
- Gillot, I.; Gouyon, J.; Guignard, J. Renal Effects of Caffeine in Preterm Infants. Neonatology 1990, 58, 133–136. [Google Scholar]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Long-Term Effects of Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar]
- Schmidt, B.; Peter, J.A.; Lex, W.D.; Deborah, D.; Ruth, E.G.; Elizabeth, V.A.; Peter, G.D.; Win, T.; Diane, M. Alfonso SolimaSurvival without Disability to Age 5 Years after Neonatal Caffeine Therapy for Apnea of Prematurity. JAMA 2012, 307, 275–282. [Google Scholar]
- Doyle, L.W.; Barbara, S.; Peter, J.A.; Peter, G.D.; Diane, M.; Ruth, E.G.; Karel, O.; Koravangattu, S.; Eric, H.; Robin, R. Reduction in Developmental Coordination Disorder with Neonatal Caffeine Therapy. J. Pediatr. 2014, 165, 356–359 e2. [Google Scholar]
- Dobson, N.R.; Patel, R.M.; Smith, P.B.; Kuehn, D.R.; Clark, J.; Vyas-Read, S.; Herring, A.; Laughon, M.M.; Carlton, D.; Hunt, C.E. Trends in Caffeine Use and Association between Clinical Outcomes and Timing of Therapy in Very Low Birth Weight Infants. J. Pediatr. 2014, 164, 992–998 e3. [Google Scholar]
- Zhao, Y.; Liu, Y.; Chen, K. Mechanisms and Clinical Application of Tetramethylpyrazine (an Interesting Natural Compound Isolated from Ligusticum Wallichii): Current Status and Perspective. Oxidative Med. Cell. Longev. 2016, 2016, 2124638. [Google Scholar]
- Guo, M.; Liu, Y.; Shi, D. Cardiovascular Actions and Therapeutic Potential of Tetramethylpyrazine (Active Component Isolated from Rhizoma Chuanxiong): Roles and Mechanisms. Biomed. Res. Int. 2016, 2016, 894283. [Google Scholar]
- Qian, W.; Xiong, X.; Fang, Z.; Lu, H.; Wang, Z. Protective Effect of Tetramethylpyrazine on Myocardial Ischemia-Reperfusion Injury. Evid. Based Complementary Altern. Med. 2014, 2014, 107501. [Google Scholar]
- Mu, Q.-C.; Huang, H.-Y.; Gao, H.-J.; Liu, P.-F.; Li, P.-W.; Huang, Z.-Y.; Yu, F.-B.; Lei, T.; Chen, Y.; Cheng, Y. Ligustrazine Monomer against Cerebral Ischemia/Reperfusion Injury. Neural. Regen. Res. 2015, 10, 832. [Google Scholar]
- Kao, T.-K.; Ou, Y.-C.; Kuo, J.-S.; Chen, W.-Y.; Liao, S.-L.; Wu, C.-W.; Chen, C.-J.; Ling, N.-N.; Zhang, Y.-H.; Peng, W.-H. Neuroprotection by Tetramethylpyrazine against Ischemic Brain Injury in Rats. Neurochem. Int. 2006, 48, 166–176. [Google Scholar]
- Cheng, X.; Zhang, L.; Hu, J.; Sun, L.; Du, G. Neuroprotective Effects of Tetramethylpyrazine on Hydrogen Peroxide-Induced Apoptosis in Pc12 Cells. Cell Biol. Int. 2007, 31, 438–443. [Google Scholar]
- Juan, S.-H.; Chen, C.-H.; Hsu, Y.-H.; Hou, C.-C.; Chen, T.-H.; Lin, H.; Chu, Y.-L.; Sue, Y.-M. Tetramethylpyrazine Protects Rat Renal Tubular Cell Apoptosis Induced by Gentamicin. Nephrol. Dial. Transplant. 2007, 22, 732–739. [Google Scholar]
- Liao, S.-L.; Kao, T.-K.; Chen, W.-Y.; Lin, Y.-S.; Chen, S.-Y.; Raung, S.-L.; Wu, C.-W.; Lu, H.-C.; Chen, C.-J. Tetramethylpyrazine Reduces Ischemic Brain Injury in Rats. Neurosci. Lett. 2004, 372, 40–45. [Google Scholar]
- Zhang, Z.; Wei, T.; Hou, J.; Li, G.; Yu, S.; Xin, W. Tetramethylpyrazine Scavenges Superoxide Anion and Decreases Nitric Oxide Production in Human Polymorphonuclear Leukocytes. Life Sci. 2003, 72, 2465–2472. [Google Scholar]
- Li, S.-Y.; Fung, F.K.C.; Fu, Z.J.; Wong, D.; Chan, H.H.L.; Lo, A.C.Y. Anti-Inflammatory Effects of Lutein in Retinal Ischemic/Hypoxic Injury: In Vivo and in Vitro Studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5976–5984. [Google Scholar]
- Li, S.-Y.; Lo, A.C.-Y. Lutein Protects Rgc-5 Cells against Hypoxia and Oxidative Stress. Int. J. Mol. Sci. 2010, 11, 2109–2117. [Google Scholar]
- Zhang, C.; Wang, Z.; Zhao, J.; Li, Q.; Huang, C.; Zhu, L.; Lu, D. Neuroprotective Effect of Lutein on Nmda-Induced Retinal Ganglion Cell Injury in Rat Retina. Cell. Mol. Neurobiol. 2016, 36, 531–540. [Google Scholar]
- Woo, T.T.Y.; Li, S.-Y.; Lai, W.W.K.; Wong, D.; Lo, A.C.-Y. Neuroprotective Effects of Lutein in a Rat Model of Retinal Detachment. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 41–51. [Google Scholar]
- Li, S.-Y.; Yang, D.; Fu, Z.J.; Woo, T.; Wong, D.; Lo, A.C.Y. Lutein Enhances Survival and Reduces Neuronal Damage in a Mouse Model of Ischemic Stroke. Neurobiol. Dis. 2012, 45, 624–632. [Google Scholar]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and Zeaxanthin and Their Potential Roles in Disease Prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar]
- Alves-Rodrigues, A.; Shao, A. The Science Behind Lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar]
- Dani, C.; Ilaria, L.; Federica, F.; Saverio, F.; Hubert, M.; Petra, W.; Sergio, D.M.; Chiara, O.; Antonio, B. Ciantelli MassimiliaLutein and Zeaxanthin Supplementation in Preterm Infants to Prevent Retinopathy of Prematurity: A Randomized Controlled Study. J. Matern. Fetal Neonatal Med. 2012, 25, 523–527. [Google Scholar]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; Mackey, A.D.; A Dimmit, R.; E Hartmann, E.; et al. Effect of Carotenoid Supplementation on Plasma Carotenoids, Inflammation and Visual Development in Preterm Infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar]
- Manzoni, P.; Guardione, R.; Bonetti, P.; Priolo, C.; Maestri, A.; Mansoldo, C.; Mostert, M.; Anselmetti, G.; Sardei, D.; Bellettato, M.; et al. Lutein and Zeaxanthin Supplementation in Preterm Very Low-Birth-Weight Neonates in Neonatal Intensive Care Units: A Multicenter Randomized Controlled Trial. Am. J. Perinatol 2013, 30, 25–32. [Google Scholar]
- Nidhi, B.; Ramaprasad, T.R.; Baskaran, V. Dietary Fatty Acid Determines the Intestinal Absorption of Lutein in Lutein Deficient Mice. Food Res. Int. 2014, 64, 256–263. [Google Scholar]
- Nidhi, B.; Mamatha, B.S.; Baskaran, V. Olive Oil Improves the Intestinal Absorption and Bioavailability of Lutein in Lutein-Deficient Mice. Eur. J. Nutr. 2014, 53, 117–126. [Google Scholar]
- Xu, H.; Yong, K.K.; Seung, Y.S.; MRU; Sook, Y.L.; Su, Y.L. Decursin Production from Hairy Root Culture of Angelica Gigas. J. Korea Soc. Appl. Biol. Chem. 2008, 51, 349–351. [Google Scholar]
- Lee, S.; Yeon, S.L.; Sang, H.J.; Kuk, H.S.; Bak-Kwang, K.; Sam, S.K. Anti-Tumor Activities of Decursinol Angelate and Decursin Fromangelica Gigas. Arch. Pharmacal. Res. 2003, 26, 727–730. [Google Scholar]
- Jiang, C.; Guo, J.; Wang, Z.; Xiao, B.; Lee, H.-J.; Lee, E.-O.; Kim, S.; Lü, J. Decursin and Decursinol Angelate Inhibit Estrogen-Stimulated and Estrogen-Independent Growth and Survival of Breast Cancer Cells. Breast Cancer Res. 2007, 9, R77. [Google Scholar]
- Jung, M.H.; Lee, S.H.; Ahn, E.-M.; Lee, Y.M. Decursin and Decursinol Angelate Inhibit Vegf-Induced Angiogenesis Via Suppression of the Vegfr-2-Signaling Pathway. Carcinogenesis 2009, 30, 655–661. [Google Scholar]
- Choi, S.-R.; Lee, J.-H.; Kim, J.-Y.; Park, K.-W.; Jeong, I.Y.; Shim, K.-H.; Lee, M.-K.; Seo, K.-I. Decursin from Angelica Gigas Nakai Induces Apoptosis in Rc-58t/H/Sa# 4 Primary Human Prostate Cancer Cells Via a Mitochondria-Related Caspase Pathway. Food Chem. Toxicol. 2011, 49, 2517–2523. [Google Scholar]
- Moon, S.-K.; Kim, W.-J.; Lee, S.-J.; Choi, Y.D. Decursin Inhibits Growth of Human Bladder and Colon Cancer Cells Via Apoptosis, G1-Phase Cell Cycle Arrest and Extracellular Signal-Regulated Kinase Activation. Int. J. Mol. Med. 2010, 25, 635–641. [Google Scholar]
- Ahn, K.-S.; Sim, W.-S.; Kim, I.-H. Decursin: A Cytotoxic Agent and Protein Kinase C Activator from the Root of Angelica Gigas. Planta Med. 1996, 62, 7–9. [Google Scholar]
- Lee, H.J.; Lee, E.O.; Lee, J.H.; Lee, K.S.; Kim, K.H.; Kim, S.-H.; Lü, J. In Vivo Anti-Cancer Activity of Korean Angelica Gigas and Its Major Pyranocoumarin Decursin. Am. J. Chin. Med. 2009, 37, 127–142. [Google Scholar]
- Yim, D.; Singh, R.P.; Agarwal, C.; Lee, S.; Chi, H.; Agarwal, R. A Novel Anticancer Agent, Decursin, Induces G1 Arrest and Apoptosis in Human Prostate Carcinoma Cells. Cancer Res. 2005, 65, 1035–1044. [Google Scholar]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and Decursinol Angelate: Molecular Mechanism and Therapeutic Potential in Inflammatory Diseases. Inflamm. Res. 2018, 67, 209–218. [Google Scholar]
- Kim, J.-H.; Ji-Hye, J.; Sung-Tak, J.; Ho, K.; Jiyeon, O.; Kyoungho, S.; Sang-In, K.; Kyung-Sik, S.; Won-ha, L. Decursin Inhibits Induction of Inflammatory Mediators by Blocking Nuclear Factor-Κb Activation in Macrophages. Mol. Pharmacol. 2006, 69, 1783–1790. [Google Scholar]
- Kim, W.-J.; Lee, M.-Y.; Kim, J.-H.; Suk, K.; Lee, W.-H. Decursinol Angelate Blocks Transmigration and Inflammatory Activation of Cancer Cells through Inhibition of Pi3k, Erk and Nf-Κb Activation. Cancer Lett. 2010, 296, 35–42. [Google Scholar]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Polymorphisms in the Toll-Like Receptor and the Il-23/Il-17 Pathways Were Associated with Susceptibility to Inflammatory Bowel Disease in a Danish Cohort. PLoS ONE 2015, 10, 12. [Google Scholar]
- Ziberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.M.; Bishayee, A.; Farooqi, A.A.; Sureda, A. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar]
- Pollier, J.; Goossens, A. Oleanolic Acid. Phytochemistry 2012, 77, 10–15. [Google Scholar]
- Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Oleanolic Acid Activates Nrf2 and Protects from Acetaminophen Hepatotoxicity Via Nrf2-Dependent and Nrf2-Independent Processes. Biochem. Pharm. 2009, 77, 1273–1282. [Google Scholar]
- Liu, J.; Wu, Q.; Lu, Y.-F.; Pi, J. New Insights into Generalized Hepatoprotective Effects of Oleanolic Acid: Key Roles of Metallothionein and Nrf2 Induction. Biochem. Pharm. 2008, 76, 922–928. [Google Scholar]
- Klaassen, C.D.; Reisman, S.A. Nrf2 the Rescue: Effects of the Antioxidative/Electrophilic Response on the Liver. Toxicol. Appl. Pharm. 2010, 244, 57–65. [Google Scholar]
- Liu, J. Pharmacology of Oleanolic Acid and Ursolic Acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar]
- Feng, J.; Zhang, P.; Chen, X.; He, G. Pi3k and Erk/Nrf2 Pathways Are Involved in Oleanolic Acid-Induced Heme Oxygenase-1 Expression in Rat Vascular Smooth Muscle Cells. J. Cell Biochem. 2011, 112, 1524–1531. [Google Scholar]
- Wang, X.; Ye, X.-L.; Liu, R.; Chen, H.-L.; Bai, H.; Liang, X.; Zhang, X.-D.; Wang, Z.; Li, W.-L.; Hai, C. Antioxidant Activities of Oleanolic Acid in Vitro: Possible Role of Nrf2 and Map Kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar]
- Ovesná, Z.; Kozics, K.; Slameňová, D. Protective Effects of Ursolic Acid and Oleanolic Acid in Leukemic Cells. Mutat. Res. 2006, 600, 131–137. [Google Scholar]
- Sultana, N.; Ata, A. Oleanolic Acid and Related Derivatives as Medicinally Important Compounds. J. Enzym. Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar]
- Valtchanova-Matchouganska, A.; Nadar, A.; Rammanan, P.; Shode, F. Cardiovascular, Antihyperlipidemic and Antioxidant Effects of Oleanolic and Ursolic Acids in Experimental Hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar]
- Valtchanova-Matchouganska, A.; Shode, F.; Ramnanan, P.; Nadar, A. Antihypertensive, Antiatherosclerotic and Antioxidant Activity of Triterpenoids Isolated from Olea Europaea, Subspecies Africana Leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar]
- Li, H.-F.; Wang, X.-A.; Xiang, S.-S.; Hu, Y.-P.; Jiang, L.; Shu, Y.-J.; Li, M.-L.; Wu, X.-S.; Zhang, F.; Ye, Y.-Y.; et al. Oleanolic Acid Induces Mitochondrial-Dependent Apoptosis and G0/G1 Phase Arrest in Gallbladder Cancer Cells. Drug Des. Dev. Ther. 2015, 9, 3017. [Google Scholar]
- Li, X.; Song, Y.; Zhang, P.; Zhu, H.; Chen, L.; Xiao, Y.; Xing, Y. Oleanolic Acid Inhibits Cell Survival and Proliferation of Prostate Cancer Cells in Vitro and in Vivo through the Pi3k/Akt Pathway. Tumor Biol. 2016, 37, 7599–7613. [Google Scholar]
- Furtado, R.A.; Rodrigues, É.P.; Araújo, F.R.R.; Oliveira, W.L.; Furtado, M.A.; Castro, M.B.; Cunha, W.R.; Tavares, D.C. Ursolic Acid and Oleanolic Acid Suppress Preneoplastic Lesions Induced by 1, 2-Dimethylhydrazine in Rat Colon. Toxicol. Pathol. 2008, 36, 576–580. [Google Scholar]
- Karioti, A.; Bilia, A.R. Hypericins as Potential Leads for New Therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar]
- Vandenbogaerde, A.L.; Kamuhabwa, A.; Delaey, E.; Himpens, B.E.; Merlevede, W.J.; De Witte, P.A. Photocytotoxic Effect of Pseudohypericin Versus Hypericin. J. Photochem. Photobiol. B Biol. 1998, 45, 87–94. [Google Scholar]
- Thomas, C.; MacGill, R.S.; Miller, G.C.; Pardini, R.S. Photoactivation of Hypericin Generates Singlet Oxygen in Mitochondria and Inhibits Succinoxidase. Photochem. Photobiol. 1992, 55, 47–53. [Google Scholar]
- Thomas, C.; Pardini, R.S. Oxygen Dependence of Hypericin-Induced Phototoxicity to Emt6 Mouse Mammary Carcinoma Cells. Photochem. Photobiol. 1992, 55, 831–837. [Google Scholar]
- Davids, L.M.; Kleemann, B.; Kacerovská, D.; Pizinger, K.; Kidson, S.H. Hypericin Phototoxicity Induces Different Modes of Cell Death in Melanoma and Human Skin Cells. J. Photochem. Photobiol. B Biol. 2008, 91, 67–76. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One—Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar]
- Pu, X.-y.; Jian-ping, L.; Tao, X.; Ruo-feng, S.; Xue-hong, W.; Lan-ying, H.; Yu, L.; Yan-mei, X. Study on Activity in Vitro of Hypericin against Highly Pathogenic Prrsv. Chin. Vet. Sci. 2008, 9, 21. [Google Scholar]
- Wang, S.-y.; Ji-hong, C. Studies on the Inhibitory Effects of Hypericin on the Adsorption Ability of Foot-and-Mouth Virus to Host Cells in Vitro. J. Tradit. Chin. Vet. Med. 2009, 1, 5–8. [Google Scholar]
- Kubin, A.; Wierrani, F.; Burner, U.; Alth, G.; Grunberger, W. Hypericin-the Facts About a Controversial Agent. Curr. Pharm. Des. 2005, 11, 233–253. [Google Scholar]
- Hart, B.A.T.; Copray, S.; Philippens, I. Apocynin, a Low Molecular Oral Treatment for Neurodegenerative Disease. Biomed. Res. Int. 2014, 2014, 298020. [Google Scholar]
- Wagner, M.C.; Yeligar, S.M.; Brown, L.A.; Hart, C.M. Pparγ Ligands Regulate Nadph Oxidase, Enos, and Barrier Function in the Lung Following Chronic Alcohol Ingestion. Alcohol. Clin. Exp. Res. 2012, 36, 197–206. [Google Scholar]
- Hwang, J.; Kleinhenz, D.J.; Lassègue, B.; Griendling, K.K.; Dikalov, S.; Hart, C.M. Peroxisome Proliferator-Activated Receptor-Γ Ligands Regulate Endothelial Membrane Superoxide Production. Am. J. Physiol. Cell Physiol. 2005, 288, C899–C905. [Google Scholar]
- Hougee, S.; Hartog, A.; Sanders, A.; Graus, Y.M.; Hoijer, M.A.; Garssen, J.; Berg, W.B.V.D.; Van Beuningen, H.M.; Smit, H.F. Oral Administration of the Nadph-Oxidase Inhibitor Apocynin Partially Restores Diminished Cartilage Proteoglycan Synthesis and Reduces Inflammation in Mice. Eur. J. Pharmacol. 2006, 531, 264–269. [Google Scholar]
- Wilkinson, B.L.; Cramer, P.E.; Varvel, N.H.; Reed-Geaghan, E.; Jiang, Q.; Szabo, A.; Herrup, K.; Lamb, B.T.; Landreth, G.E. Ibuprofen Attenuates Oxidative Damage through Nox2 Inhibition in Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 197-e21. [Google Scholar]
- Schilling, T.; Eder, C. Amyloid-Β-Induced Reactive Oxygen Species Production and Priming Are Differentially Regulated by Ion Channels in Microglia. J. Cell. Physiol. 2011, 226, 3295–3302. [Google Scholar]
- Shimohamaa, S.; Taninob, H.; Kawakamib, N.; Okamurac, N.; Kodamac, H.; Yamaguchid, T.; Hayakawad, T.; Nunomurae, A.; Chibae, S.; Perryf, G.; et al. Activation of Nadph Oxidase in Alzheimer’s Disease Brains. Biochem. Biophys. Res. Commun. 2000, 273, 5–9. [Google Scholar]
- Barger, S.W.; Goodwin, M.E.; Porter, M.M.; Beggs, M.L. Glutamate Release from Activated Microglia Requires the Oxidative Burst and Lipid Peroxidation. J. Neurochem. 2007, 101, 1205–1213. [Google Scholar]
- Choi, S.-H.; Aid, S.; Kim, H.-W.; Jackson, S.H.; Bosetti, F. Inhibition of Nadph Oxidase Promotes Alternative and Anti-Inflammatory Microglial Activation During Neuroinflammation. J. Neurochem. 2012, 120, 292–301. [Google Scholar]
- Lull, M.E.; Levesque, S.; Surace, M.J.; Block, M.L. Chronic Apocynin Treatment Attenuates Beta Amyloid Plaque Size and Microglial Number in Happ (751) Sl Mice. PLoS ONE 2011, 6, e20153. [Google Scholar]

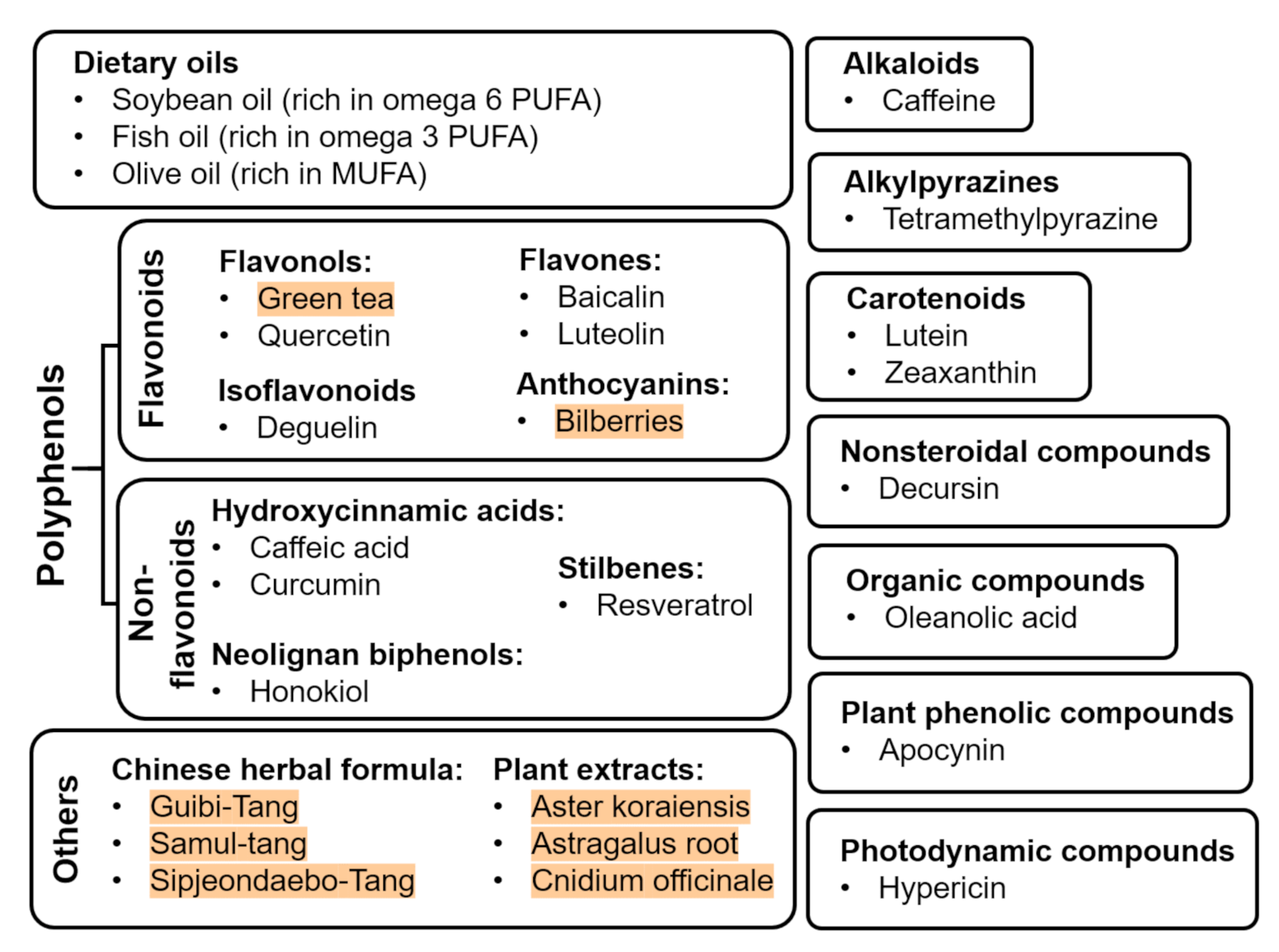

| Family of Compound | Dietary Source/Compound | Dosage | Supplementation Period | Cell Culture/Animal Model | Effects | Reference(s) | |
|---|---|---|---|---|---|---|---|
| In vitro | Dietary oil | Docosahexaenoic acid (DHA) | 0.5 to 10 μM | / | Retinal ganglion cell (RGC-5) (H2O2 induced hypoxia) |
| [55] |
| Non- flavonoids | Caffeic acid | 10 to 200 μM | / | Human retina microvascular endothelial cells (VEGF-induced proliferation) |
| [56] | |
| 100 μM | / | Human retina microvascular endothelial cells (H2O2-induced hypoxia) |
| ||||
| Honokiol | 20 μM | / | Human retinal pigment epithelial cell lines (Hypoxic chamber) |
| [57] | ||
| Resveratrol | 5 to 100 μg/ml | / | Primary culture of dissociated-dispersed retinal cells (Hyperoxia induction by 100% O2 incubation for 6 h) |
| [58] | ||
| In vitro | Nonsteroidal compounds | Decursin | 1 to 50 μM | / | Human retinal pigment epithelial cell lines (VEGF-induced proliferation) |
| [59] |
| Organic compounds | Oleanolic acid | 0.1 to 50 μM | / | Human umbilical vein endothelial cells (VEGF induced proliferation) |
| [60] | |
| In vivo | Dietary oils | 2% ω-3 LCPUFAs (1% DHA and 1% EPA) or 2% ω-6 LCPUFAs (AA) | 10% (w/w) safflower oil containing either 2% ω-3 LCPUFAs (1% DHA and 1% EPA) or 2% ω-6 LCPUFAs (AA) (Oral) | P1 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [61] |
| Flavonoids | Baicalin | 1 or 10 mg/kg/day (Intraperitoneal) | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [62] | |
| Bilberry extract | 300 ng per eye (Intravitreal) | P12 | Mouse OIR model (75% O2, P7 to P12) |
| [63] | ||
| In vivo | Flavonoids | Deguelin | 0.1 μM (Intravitreal) | P14 | Mouse OIR model (75% O2, P7 to P12) |
| [64] |
| Green tea extract | 12.5% or 25% GTE (Oral) | P6 to P17 | Rat OIR model (P0 to P12) |
| [65] | ||
| Green tea fraction (With less content of catechins and caffeine) | 0.01 or 0.05 g/mL/day GTF (Oral) | P6 to P17 | Rat OIR model (P0 to P12) |
| [66] | ||
| Luteolin | 0.1 or 10 μM (Intravitreal) | P14 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [67] | ||
| Quercetin | 20 mg/kg (Intraperitoneal) | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [68] | ||
| Non-flavonoids | Caffeic acid | 100 μM (Intravitreal) | P14 | Mouse OIR model (75% O2, P7 to P12) |
| [56] | |
| In vivo | Non- flavonoids | Curcumin | 50 or 100 mg/kg/day (Intraperitoneal) | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [69] |
| 0.1 or 1 μg (Intravitreal) | P13 | ||||||
| Honokiol | 10–20 mg/kg/day (Intraperitoneal) | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [57] | ||
| Resveratrol | 30 mg/kg/day (Intravitreal) | P14 to P21 | Rat OIR model (P1 to P14) |
| [58] | ||
| 10, 30, or 60 mg/kg/day (intragastrical) | P12 to P17 | Rat OIR model (P7 to P12) |
| [70] | |||
| Alkaloids | Caffeine | 0.1, 0.3, and 1 g/L in drinking water for lactating mothers (uptake via maternal breast milk) | P0 to P17,P7 to P12, orP12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [71] | |
| In vivo | Alkylpyrazines | Tetramethylpyrazine | 200 mg/kg/day | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [72] |
| Carotenoids | Lutein and Zeaxanthin | 0.2 mg/kg/day (Intraperitoneal) | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [73] | |
| Nonsteroidal compounds | Decursin | 5 µM (Intravitreal) | P14 | Mouse OIR model (75% O2, P7 to P12) |
| [59] | |
| In vivo | Organic compounds | Oleanolic acid | 62.5 or 125 mg/kg (Intraperitoneal) | P11 and P15 | Mouse OIR model (85% O2, P8 to P11) |
| [60] |
| Plant phenolic compounds | Apocynin | 10 mg/kg/day (Intraperitoneal) | P12 to P17 | Rat OIR model (P0 to P14) |
| [74] | |
| Photodynamic compounds | Hypericin | 15 mg/kg/day of St. John Wort (Oral) | P12 to P17 | Mouse OIR model (75% O2, P7 to P12) |
| [75] | |
| 15, 45, and 135 µg/kg/day of hypericin (Oral) | |||||||
| Plant extracts | Aster koraiensis Extract | 25 or 50 mg/kg/day (Intraperitoneal) | P12 to P16 | Mouse OIR model (75% O2, P7 to P12) |
| [76] | |
| Astragalus root extract | 10, 20 or 40 mg/kg/day (Intragastrical) | 4 weeks | Mouse OIR model (75%O2) |
| [77] | ||
| In vivo | Plant extracts | Cnidium officinale extract | 100 mg/kg/day (Intraperitoneal) | P12 to P16 | Mouse OIR model (75% O2, P7 to P12) |
| [78] |
| Chinese herbal formula | Guibi-tang | 50 or 100 mg/kg/day (Intraperitoneal) | P12 to P16 | Mouse OIR model (75% O2, P7 to P12) |
| [79] | |
| Samul-tang | 10 or 50 mg/kg/day (Intraperitoneal) | P12 to P16 | Mouse OIR model (75% O2, P7 to P12) |
| [80] | ||
| Sipjeondaebo-tang | 50 or 100 mg/kg/day (Intraperitoneal) | P12 to P16 | Mouse OIR model (75% O2, P7 to P12) |
| [81] |
| Family of compound | Dietary Source/Compound | Dosage | Supplementation Period | Candidates for Clinical Study | Effects | Reference(s) |
|---|---|---|---|---|---|---|
| Dietary oils | 20% Clinoleic: 50% of soybean and olive oil emulsion 10% Omegaven: 50% of fish-oil emulsion | (Intravenous, Daily) Birth weight of <1000 g: 0.15 g of Omegaven and 0.35 g of Clinoleic Birth weight of >1000 g: 0.35 g of Omegaven and 0.65 g of Clinoleic (Maximum: 1.0–1.2 g of Omegaven and 2.0–2.3 g of Clinoleic) | From the first day of life | Clinical, preterm infants <32 weeks’ gestational age, weighed <1250 g |
| [82] |
| 20% Intralipid: soybean oil 200 g/dL20% SMOFlipid: soybean oil 60 g/dL, MCT 60 g/dL, olive oil 50 g/dL, fish oil 30 g/dL | (Intravenous, Daily) Initial dose: 0.5 g of lipids per kg of body weight (birth weight of <1000 g) or 1.0 g of lipids per kg of body weight (birth weight of >1000 g) (Maximum of 3.0 g of lipids per kg of body weight/day) | From the first day of life | Clinical, preterm infants <32 weeks’ gestational age, weighed <1250 g |
| [83] | |
| 20% Lipovenoes medium-chain triglycerides: 50% soybean oil SMOFlipid: 30% soybean oil, 25% olive oil, and 15% fish oil | 3 g/kg body weight/day (Intravenous) | Within 24 h of birth for a duration of at least 7 consecutive days | Clinical, preterm infants weighed <1500 g |
| [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsang, J.K.W.; Wolf, S.A.; Pompoes, I.M.; Joussen, A.M.; Lam, W.C.; Yang, D.; Lo, A.C.Y. Potential Effects of Nutraceuticals in Retinopathy of Prematurity. Life 2021, 11, 79. https://doi.org/10.3390/life11020079
Tsang JKW, Wolf SA, Pompoes IM, Joussen AM, Lam WC, Yang D, Lo ACY. Potential Effects of Nutraceuticals in Retinopathy of Prematurity. Life. 2021; 11(2):79. https://doi.org/10.3390/life11020079
Chicago/Turabian StyleTsang, Jessica K. W., Susanne A. Wolf, Inga M. Pompoes, Antonia M. Joussen, Wai Ching Lam, Di Yang, and Amy C. Y. Lo. 2021. "Potential Effects of Nutraceuticals in Retinopathy of Prematurity" Life 11, no. 2: 79. https://doi.org/10.3390/life11020079
APA StyleTsang, J. K. W., Wolf, S. A., Pompoes, I. M., Joussen, A. M., Lam, W. C., Yang, D., & Lo, A. C. Y. (2021). Potential Effects of Nutraceuticals in Retinopathy of Prematurity. Life, 11(2), 79. https://doi.org/10.3390/life11020079





