A Potential Serum Biomarker for Screening Lung Cancer Risk in High Level Environmental Radon Areas: A Pilot Study
Abstract
:1. Introduction
2. Materials and Method
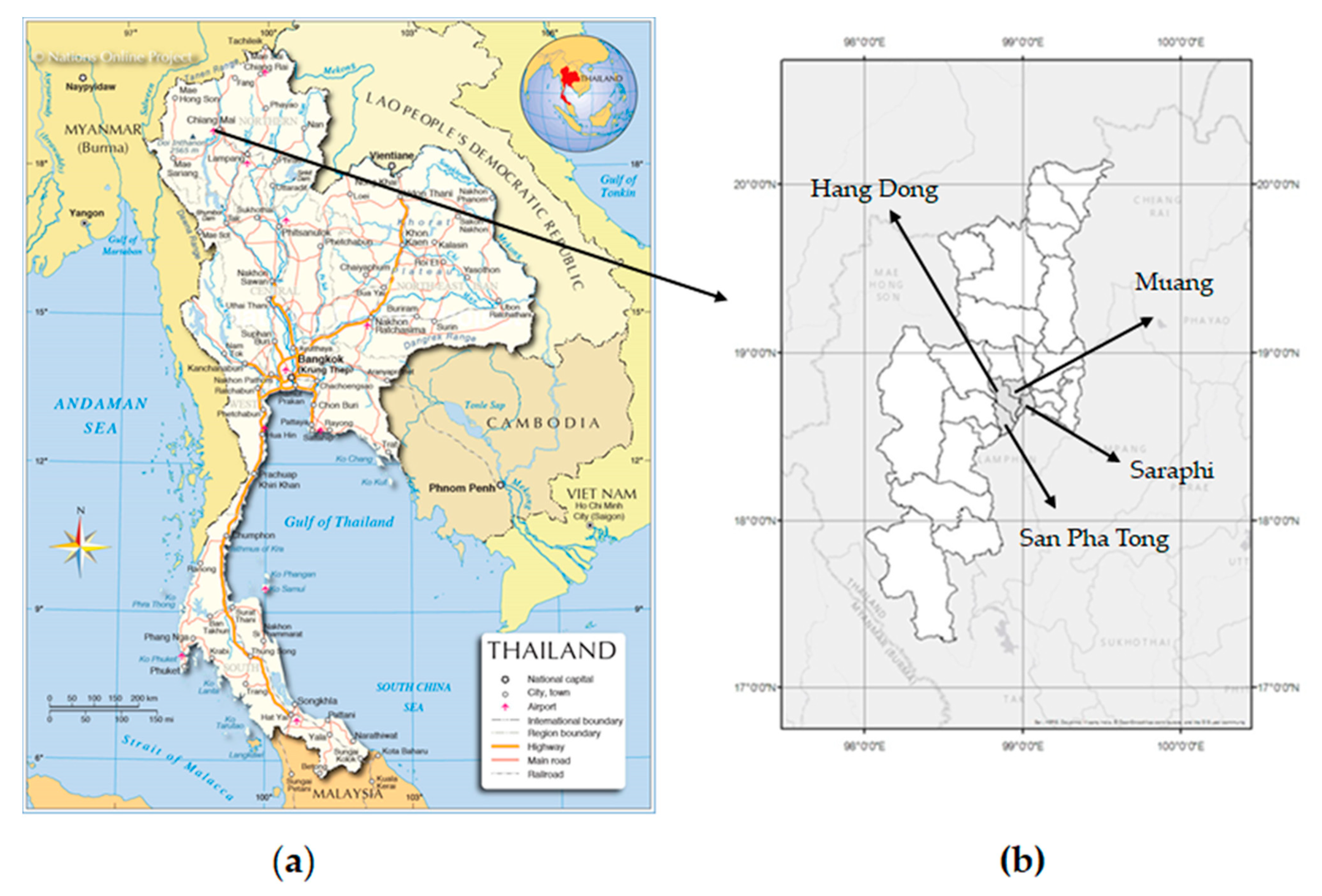
2.1. Study Area
2.2. Study Design
2.3. Sample Collection
2.4. Biochemical Analyses
2.5. Statistical Analysis
3. Results
3.1. Characteristics of LC and HC Groups
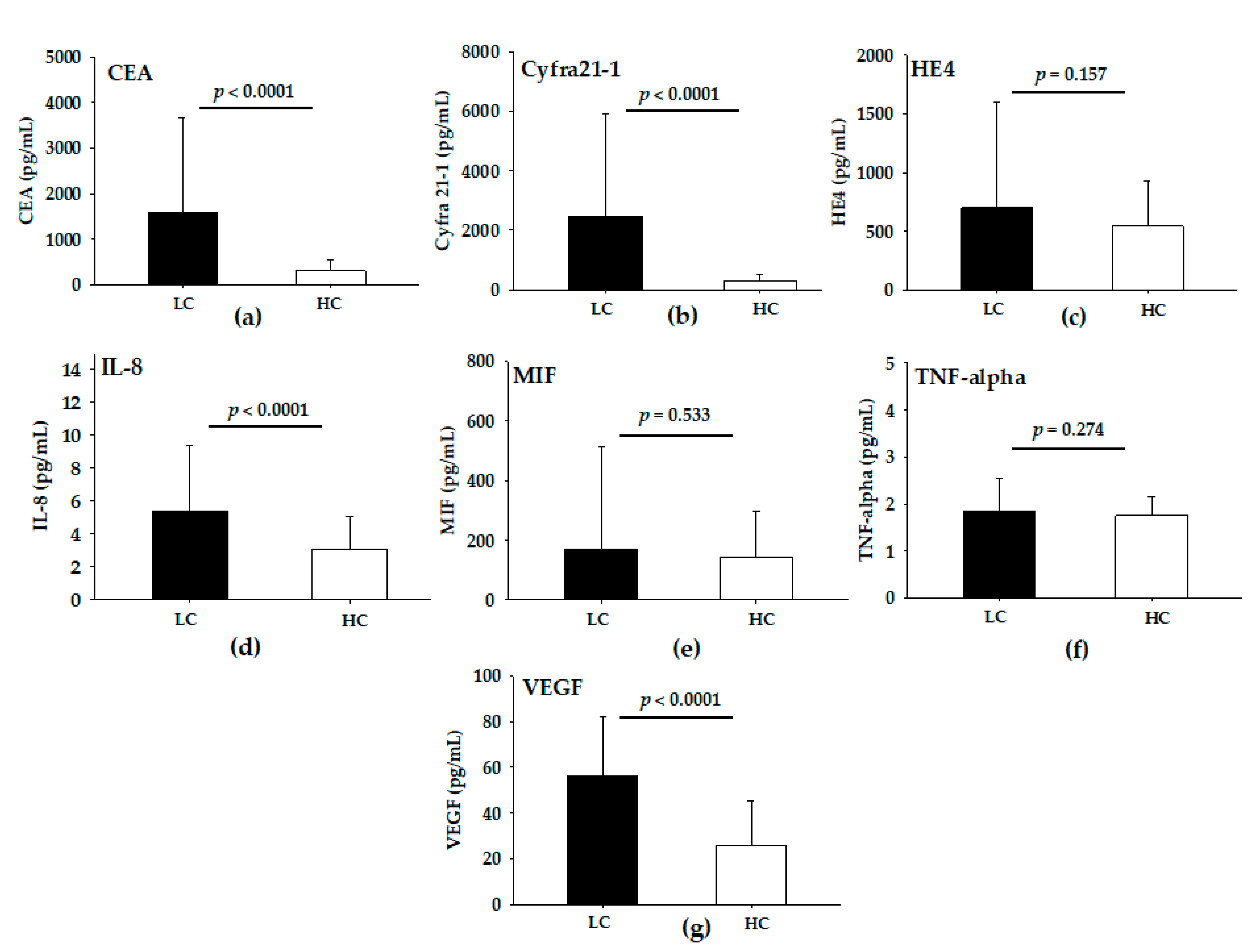
3.2. Levels of Serum Analytes in LC and HC Groups
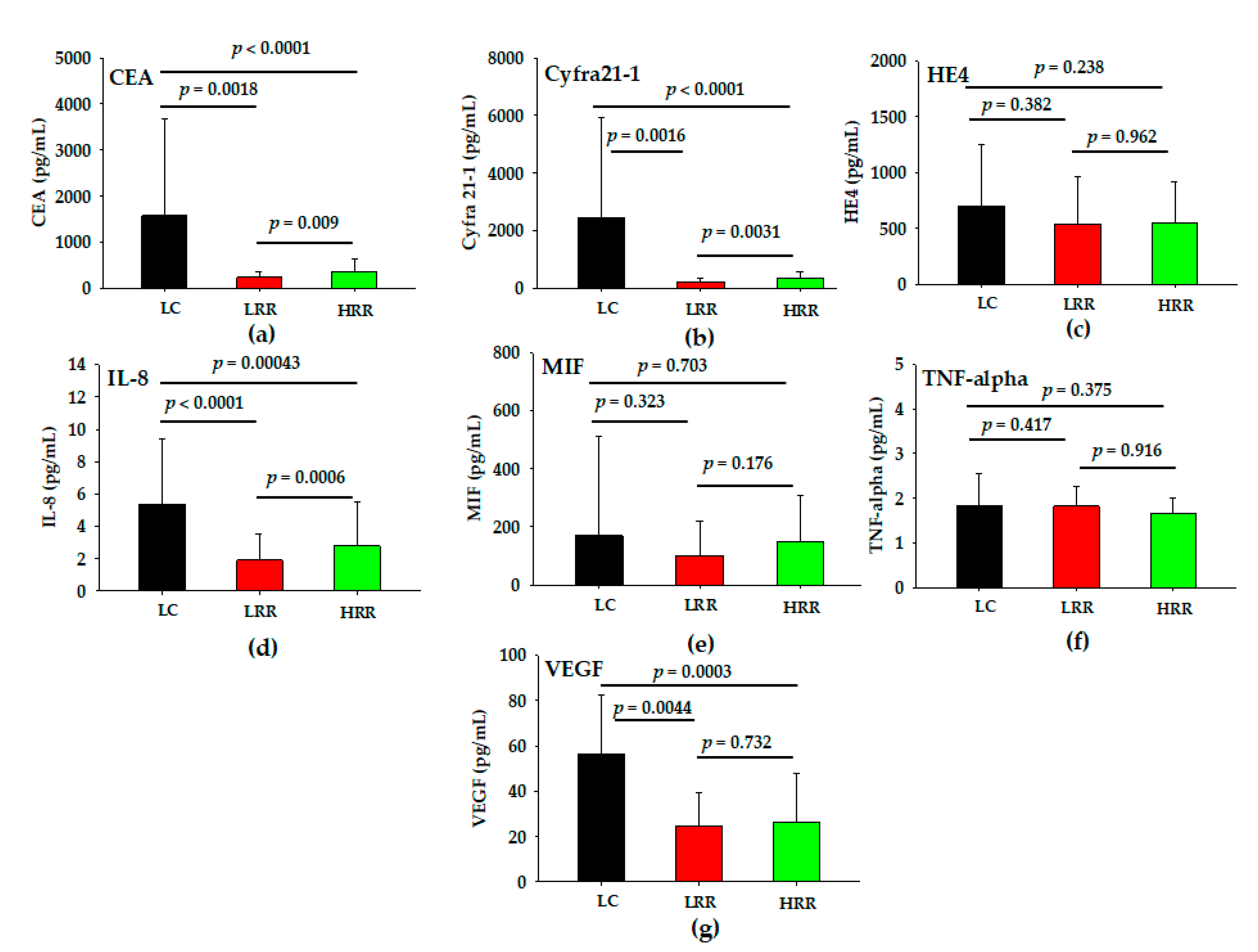
3.3. Levels of Serum Analytes in LC, LRR and HRR Groups
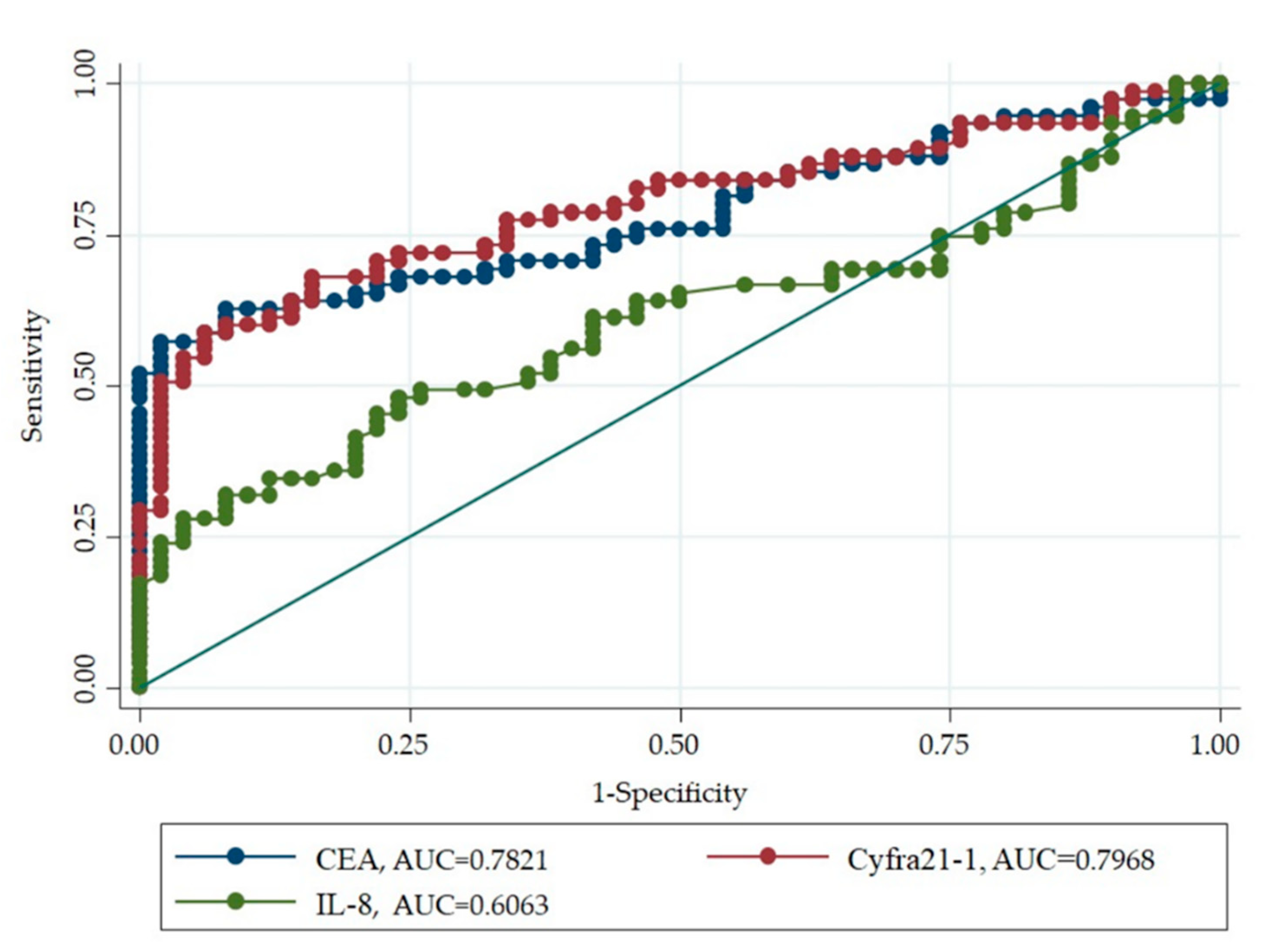
3.4. Diagnostic Ability of Serum Biomarker for LC Risk in High Level Environmental Radon Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistic 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, X.; Liu, J.; Chen, Z.; Wang, G. Serum Cyfra21-1 as a biomarker in patients with nonsmall cell lung cancer. J. Can. Res. Ther. 2014, 10, 215–217. [Google Scholar]
- Mao, Y.; Yang, D.; He, J.; Krasna, M.J. Epidemiology of lung cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 439–445. [Google Scholar] [CrossRef]
- Thailand-Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/populations/764-thailand-fact-sheets.pdf (accessed on 20 September 2021).
- Wiwatanadate, P. Lung cancer related to environmental and occupational hazards and epidemiology in Chiang Mai, Thailand. Gene Environ. 2011, 33, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Rankantha, A.; Chitapanarux, I.; Pongnikorn, D.; Prasitwattanaseree, S.; Bunyatisai, W.; Sripan, P.; Traisathit, P. Risk patterns of lung cancer mortality in northern Thailand. BMC Public Health. 2018, 18, 1138. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Oranratnachai, S.; Puataweepong, P.; Tangsujarivijit, V.; Cherntanomwong, P. Lung cancer in Thailand. J. Thorac. Oncol. 2020, 15, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Handbook on Indoor Radon; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- United Nations. Scientific Committee on the Effect of Atomic Radiation. In Source and Effects of Ionizing Radiation: United Nations Scientific Committee on the Effect of Atomic Radiation: UNSCEAR 2008 Report to the Genera; Assembly with Scientific Annexes; United Nations: New York, NY, USA, 2010. [Google Scholar]
- Sabbarese, C.; Ambrosino, F.; D’Onofrio, A. Development of radon transport model in different types of dwellings to assess indoor activity concentration. J. Environ. Radioact. 2021, 227, 106501. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoughool, M.; Krewski, D. Health effects of radon: A review of the literature. Int. J. Radiat. Biol. 2009, 85, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Autsavapromporn, N.; Klunklin, P.; Threeatana, C.; Tuntiwechapikul, W.; Hosada, M.; Tokonami, S. Short telomere length as a biomarker risk of lung cancer development induced by high radon levels: A pilot study. Int. J. Environ. Res. Public Health 2018, 15, 2152. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zheng, X.; Yang, Z.; Sun, M.; Zhang, G.; An, X.; Pan, L.; Zhang, S. Serum carcinoembryonic antigen, neuron-specific enolase as biomarkers for diagnosis of nonsmall cell lung cancer. J. Can. Res. Ther. 2016, 12, C34–C36. [Google Scholar]
- Zeng, Q.; Liu, M.; Zhou, N.; Liu, L.; Song, X. Serum human epididymis protein4 (HE4) may be a better tumor marker in early lung cancer. Clin. Chim. Acta 2016, 455, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Tas, F.; Duranyildiz, D.; Oguz, H.; Camlica, H.; Yasasever, V.; Topuz, E. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels is small cell lung cancer. Cancer Investig. 2006, 24, 492–496. [Google Scholar] [CrossRef]
- Balla, M.M.; Desai, S.; Purwar, P.; Kumar, A.; Bhandarkar, P.; Shejul, Y.K.; Pramesh, C.S.; Laskar, S.; Pandey, B.N. Differential diagnosis of lung cancer, its metastasis and chronic obstructive pulmonary disease based on serum VEGF, Il-8 and MMP-9. Sci. Rep. 2016, 6, 36065. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Pozo, A.; Sánchez-Navarro, I.; Calvo, E.; Teresa Agulló-Ortuño, M.T.; López-Vacas, R.; Díaz, E.; Camafeita, E.; Nistal, M.; Madero, R.; Espinosa, E.; et al. PTRF/Cavin-1 and MIF proteins are identified as non-small cell lung cancer biomarkers by label-free proteomics. PLoS ONE 2012, 7, e33752. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xu, Z.; Sun, S.; Yu, Y.; Lv, D.; Cao, C.; Deng, Z. TGF-β1, IL-6 and TNF-α in bronchoalveolar lavage fluid: Useful markers for lung cancer? Sci. Rep. 2014, 4, 5595. [Google Scholar] [CrossRef] [Green Version]
- National Statistical Office Thailand. Population and Housing Census 2019. Available online: http://web.nso.go.th/index.htm (accessed on 20 September 2021).
- Department of Mineral Resources. Geology of Thailand. Available online: http://www.dmr.go.th/main.php?filename=Mineral_re2015_EN (accessed on 15 September 2021).
- Oyanagi, J.; Koh, Y.; Sato, K.; Mori, K.; Teraoka, S.; Akamatsu, H.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer 2019, 132, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Autsavapromporn, N.; Dukaew, N.; Wongnoppavicvich, A.; Chewaskulyong, B.; Roytrakul, S.; Klunklin, P.; Phantawong, K.; Chitapanarux, I.; Sripan, P.; Kritsananuwat, R.; et al. Identification of novel biomarkers for lung cancer risk in high levels of radon by proteomics: A pilot study. Radiat. Prot. Dosimetry 2019, 184, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Hanagiri, T.; Sugaya, M.; Takenaka, M.; Oka, S.; Baba, T.; Shigematsu, Y.; Nagata, Y.; Shimokawa, H.; Uramoto, H.; Takenoyama, M.; et al. Preoperative Cyfra21-1 and CEA as prognostic factors in patients with srage I non-small cell lung cancer. Lung Cancer 2011, 74, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Dalaveris, E.; Kerenidi, T.; Katsabeki-katsafli, A.; Kiropoulos, T.; Tanou, K.; Gourgoulianis, K.I.; Kostikas, K. VEGF, TNF-α and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer 2009, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Xu, Y.; Ding, R.; Qiu, K.; Zhang, R.; Wang, H.; Huang, L.; Xie, X.; Yan, H.; Deng, Y.; et al. Extensive serum biomarker analysis in patients with non-small-cell lung carcinoma. Lung Cancer 2020, 126, 154868. [Google Scholar] [CrossRef]
- Balla, M.M.S.; Patwardhan, S.; Melwani, P.K.; Purwar, P.; Kumar, A.; Pramesh, C.S.; Laskar, S.; Pandey, B.N. Prognosis of metastasis based on age and serum analytes after follow-up of non-metastatic lung cancer patients. Transl. Oncol. 2021, 14, 100933. [Google Scholar] [CrossRef] [PubMed]
| Characteristics. | LC (n = 75) | HC | ||
|---|---|---|---|---|
| LRR (n = 25) | HRR (n = 50) | Total (n = 75) | ||
| Age in years, mean (SD) | 60.3 (10.8) | 61.2 (7.1) | 58.8 (8.8) | 59.6.(8.3) |
| Gender | ||||
| Male | 38 | 15 | 23 | 38 |
| Female | 37 | 10 | 27 | 37 |
| Biomarker | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|
| CEA | 57.3 | 98 | 0.7821 |
| Cyfra21-1 | 58.6 | 94 | 0.7968 |
| IL-8 | 48 | 76 | 0.6063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Autsavapromporn, N.; Klunklin, P.; Chitapanarux, I.; Jaikang, C.; Chewaskulyong, B.; Sripan, P.; Hosoda, M.; Tokonami, S. A Potential Serum Biomarker for Screening Lung Cancer Risk in High Level Environmental Radon Areas: A Pilot Study. Life 2021, 11, 1273. https://doi.org/10.3390/life11111273
Autsavapromporn N, Klunklin P, Chitapanarux I, Jaikang C, Chewaskulyong B, Sripan P, Hosoda M, Tokonami S. A Potential Serum Biomarker for Screening Lung Cancer Risk in High Level Environmental Radon Areas: A Pilot Study. Life. 2021; 11(11):1273. https://doi.org/10.3390/life11111273
Chicago/Turabian StyleAutsavapromporn, Narongchai, Pitchayaponne Klunklin, Imjai Chitapanarux, Churdsak Jaikang, Busyamas Chewaskulyong, Patumrat Sripan, Masahiro Hosoda, and Shinji Tokonami. 2021. "A Potential Serum Biomarker for Screening Lung Cancer Risk in High Level Environmental Radon Areas: A Pilot Study" Life 11, no. 11: 1273. https://doi.org/10.3390/life11111273
APA StyleAutsavapromporn, N., Klunklin, P., Chitapanarux, I., Jaikang, C., Chewaskulyong, B., Sripan, P., Hosoda, M., & Tokonami, S. (2021). A Potential Serum Biomarker for Screening Lung Cancer Risk in High Level Environmental Radon Areas: A Pilot Study. Life, 11(11), 1273. https://doi.org/10.3390/life11111273









