Composition of Stallion Seminal Plasma and Its Impact on Oxidative Stress Markers and Spermatozoa Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Semen Collection and Processing
2.2. Motility Analysis
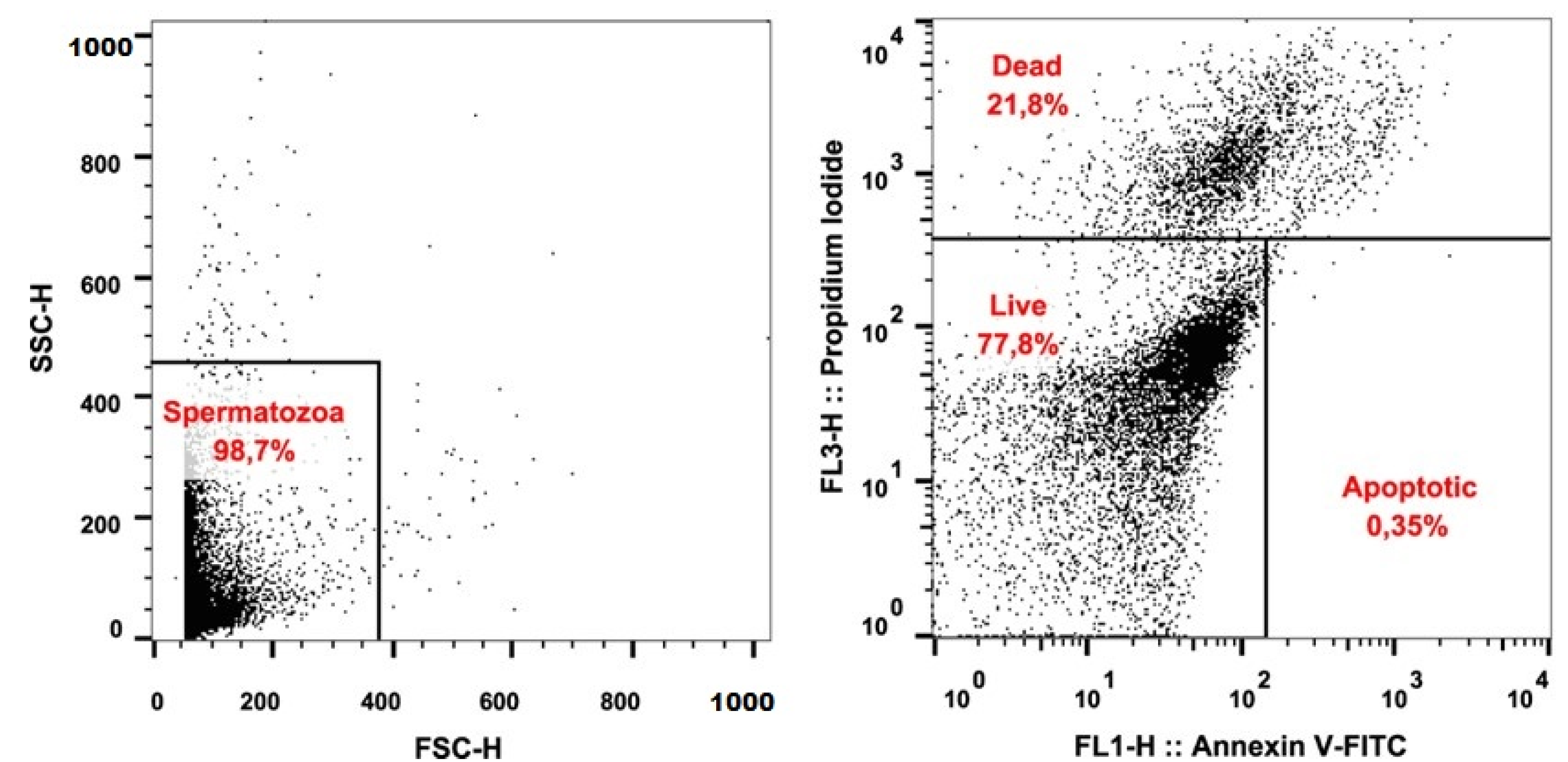
2.3. Detection of Apoptosis
2.4. Assessment of Sperm DNA Integrity
2.5. Mitochondrial Activity
2.6. Detection of Micro and Macro-Elements in Seminal Plasma
2.7. Ferric Reducing Ability of Plasma (FRAP)
2.8. Superoxide Dismutase (SOD)
2.9. Glutathione Peroxidase (GPx)
2.10. Total Oxidant Status (TOS)
2.11. Lipid Peroxidation (LPO)
2.12. Biochemical Analysis of Seminal Plasma
2.13. Statistical Analysis
3. Results
3.1. Sperm Quality
3.2. Markers of Oxidative Stress in Seminal Plasma
3.3. Biochemical Composition of Stallion Seminal Plasma
3.4. Chemical Composition of Stallion Seminal Plasma
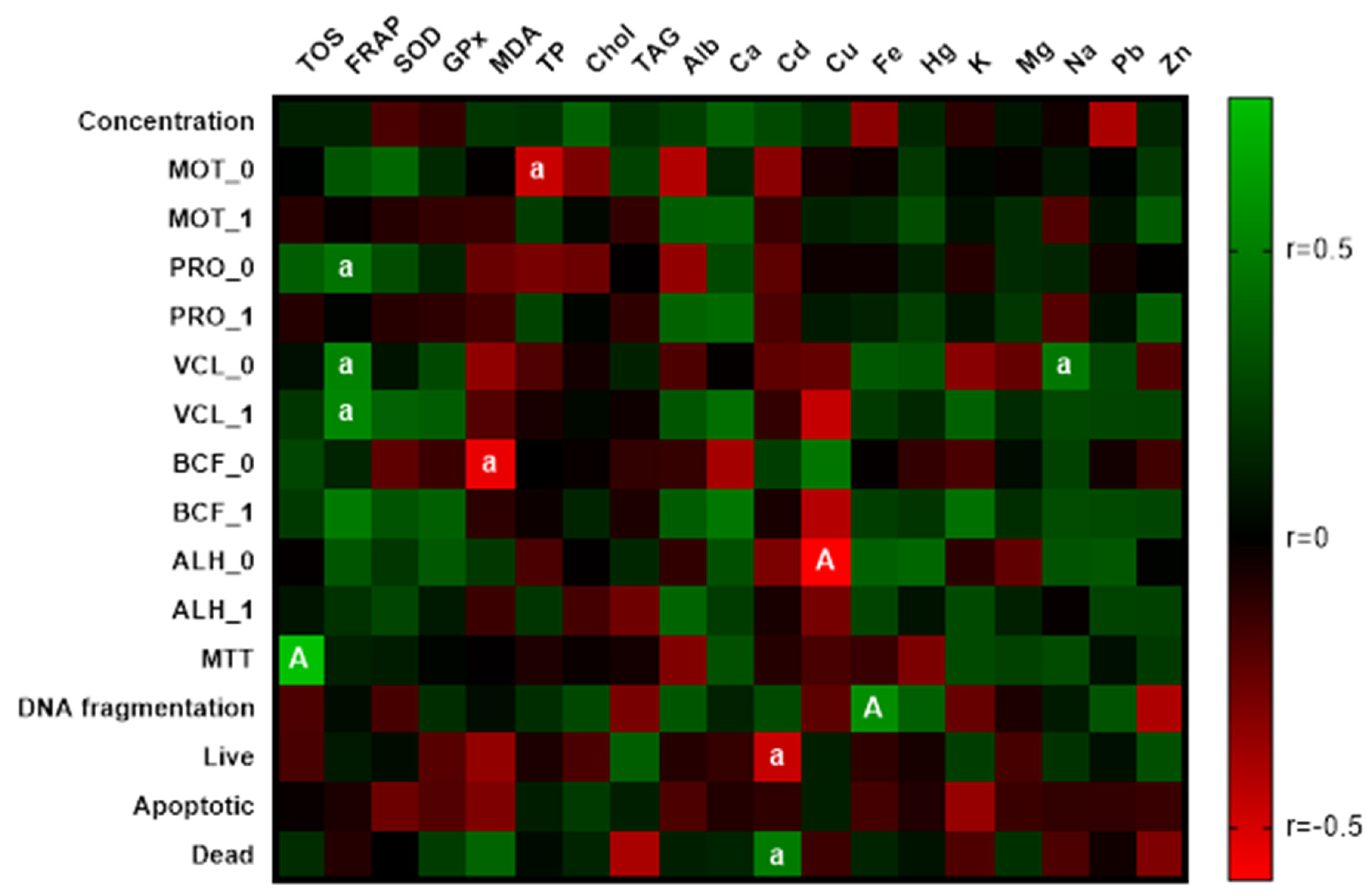
3.5. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magistrini, M.; Lindeberg, H.; Koskinen, E.; Beau, P.; Seguin, F. Biophysical and 1H Magnetic Resonance Spectroscopy Characteristics of Fractionated Stallion Ejaculates. J. Reprod. Fertil. Suppl. 2000, 56, 101–110. [Google Scholar]
- Pojprasath, T.; Lohachit, C.; Techakumphu, M.; Stout, T.; Tharasanit, T. Improved Cryopreservability of Stallion Sperm Using a Sorbitol-Based Freezing Extender. Theriogenology 2011, 75, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Papas, M.; Catalán, J.; Fernandez-Fuertes, B.; Arroyo, L.; Bassols, A.; Miró, J.; Yeste, M. Specific Activity of Superoxide Dismutase in Stallion Seminal Plasma Is Related to Sperm Cryotolerance. Antioxidants 2019, 8, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kareskoski, M.; Katila, T. Components of Stallion Seminal Plasma and the Effects of Seminal Plasma on Sperm Longevity. Anim. Reprod. Sci. 2008, 107, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Halo, M.; Tirpák, F.; Kováčik, A.; Lípová, P.; Greń, A.; Massányi, P. Biochemical Parameters of Seminal Plasma Affect Motility Traits of Stallion Spermatozoa. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 472–474. [Google Scholar] [CrossRef]
- Halo, M.J.; Tirpak, F.; Tvrda, E.; Blaszczyk, M.; Lipova, P.; Binkowski, Ł.; Massanyi, P. Microelements and macroelements in seminal plasma affect oxidative balance of stallion semen. In Proceedings of the MendelNet 2017—International PhD Students Conference, Brno, Czech Republic, 8–9 November 2017; Mendel University in Brno: Brno, Czech Republic, 2017; pp. 685–690. [Google Scholar]
- Talluri, T.R.; Mal, G.; Ravi, S.K. Biochemical Components of Seminal Plasma and Their Correlation to the Fresh Seminal Characteristics in Marwari Stallions and Poitou Jacks. Vet. World 2017, 10, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzec-Wróblewska, U.; Kamiński, P.; Lakota, P. Influence of Chemical Elements on Mammalian Spermatozoa. Folia Biol. 2012, 58, 7–15. [Google Scholar]
- Aloosh, M.; Hassani, M.; Nikoobakht, M. Seminal Plasma Magnesium and Premature Ejaculation: A Case-Control Study. BJU Int. 2006, 98, 402–404. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional Ecology of Heavy Metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm Cohort-Specific Zinc Signature Acquisition and Capacitation-Induced Zinc Flux Regulate Sperm-Oviduct and Sperm-Zona Pellucida Interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef] [Green Version]
- Azadmanesh, J.; Borgstahl, G.E.O. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintus, E.; Ros-Santaella, J.L. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef]
- Pellavio, G.; Laforenza, U. Human Sperm Functioning Is Related to the Aquaporin-Mediated Water and Hydrogen Peroxide Transport Regulation. Biochimie 2021, 188, 45–51. [Google Scholar] [CrossRef]
- Greifová, H.; Jambor, T.; Tokárová, K.; Speváková, I.; Knížatová, N.; Lukáč, N. Resveratrol Attenuates Hydrogen Peroxide-Induced Oxidative Stress in TM3 Leydig Cells in Vitro. J. Environ. Sci. Health Part A 2020, 55, 585–595. [Google Scholar] [CrossRef]
- Gosalvez, J.; Tvrda, E.; Agarwal, A. Free Radical and Superoxide Reactivity Detection in Semen Quality Assessment: Past, Present, and Future. J. Assist. Reprod. Genet. 2017, 34, 697–707. [Google Scholar] [CrossRef]
- Hafez, E.S.E.; Hafez, B. Reproduction in Farm Animals; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal Plasma Proteins: What Role Do They Play? Am. J. Reprod. Immunol. 2011, 66 (Suppl. 1), 11–22. [Google Scholar] [CrossRef] [Green Version]
- Akcay, E.; Reilas, T.; Andersson, M.; Katila, T. Effect of Seminal Plasma Fractions on Stallion Sperm Survival after Cooled Storage. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2006, 53, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.S.; Foster, D.N.; Troedsson, M.H.T. Equine Seminal Plasma Reduces Sperm Binding to Polymorphonuclear Neutrophils (PMNs) and Improves the Fertility of Fresh Semen Inseminated into Inflamed Uteri. Reproduction 2004, 127, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Brinsko, S.P.; Crockett, E.C.; Squires, E.L. Effect of Centrifugation and Partial Removal of Seminal Plasma on Equine Spermatozoal Motility after Cooling and Storage. Theriogenology 2000, 54, 129–136. [Google Scholar] [CrossRef]
- Dimofski, P.; Meyre, D.; Dreumont, N.; Leininger-Muller, B. Consequences of Paternal Nutrition on Offspring Health and Disease. Nutrients 2021, 13, 2818. [Google Scholar] [CrossRef]
- Jambor, T.; Greifova, H.; Kovacik, A.; Kovacikova, E.; Massanyi, P.; Forgacs, Z.; Lukac, N. Identification of in Vitro Effect of 4-Octylphenol on the Basal and Human Chorionic Gonadotropin (HCG) Stimulated Secretion of Androgens and Superoxide Radicals in Mouse Leydig Cells. J. Environ. Sci. Health Part A 2019, 54, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Binkowski, L.J.; Sloboda, M.; Dudzik, P.; Klak, M.; Stawarz, R. Pollution of Artesian Wells in the Urban Areas of Krakow, Europe. Fresenius Environ. Bull. 2017, 26, 846–853. [Google Scholar]
- Botsou, F.; Sungur, A.; Kelepertzis, E.; Soylak, M. Insights into the Chemical Partitioning of Trace Metals in Roadside and Off-Road Agricultural Soils along Two Major Highways in Attica’s Region, Greece. Ecotoxicol. Environ. Saf. 2016, 132, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Kilic, S.; Soylak, M. Determination of Trace Element Contaminants in Herbal Teas Using ICP-MS by Different Sample Preparation Method. J. Food Sci. Technol. 2020, 57, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.H.; Kharkan, J.; Binkowski, L.J.; Moshgani, M.; Błaszczyk, M.; Mansouri, B. Cadmium and Chromium Levels in Water and Edible Herbs in a Risk Assessment Study of Rural Residents Living in Eastern Iran. Environ. Sci. Pollut. Res. 2020, 27, 9901–9909. [Google Scholar] [CrossRef]
- Perillo, L.; Arfuso, F.; Piccione, G.; Dara, S.; Tropia, E.; Cascone, G.; Licitra, F.; Monteverde, V. Quantification of Some Heavy Metals in Hair of Dairy Cows Housed in Different Areas from Sicily as a Bioindicator of Environmental Exposure—A Preliminary Study. Animals 2021, 11, 2268. [Google Scholar] [CrossRef]
- Kovacik, A.; Arvay, J.; Tusimova, E.; Harangozo, L.; Tvrda, E.; Zbynovska, K.; Cupka, P.; Andrascikova, S.; Tomas, J.; Massanyi, P. Seasonal Variations in the Blood Concentration of Selected Heavy Metals in Sheep and Their Effects on the Biochemical and Hematological Parameters. Chemosphere 2017, 168, 365–371. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Binkowski, Ł.J.; Rogoziński, P.; Błaszczyk, M.; Semla, M.; Melia, P.M.; Stawarz, R. Relationship between Air Pollution and Metal Levels in Cancerous and Non-Cancerous Lung Tissues. J. Environ. Sci. Health Part A 2016, 51, 1303–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janicka, M.; Binkowski, Ł.J.; Błaszczyk, M.; Paluch, J.; Wojtaś, W.; Massanyi, P.; Stawarz, R. Cadmium, Lead and Mercury Concentrations and Their Influence on Morphological Parameters in Blood Donors from Different Age Groups from Southern Poland. J. Trace Elem. Med. Biol. 2015, 29, 342–346. [Google Scholar] [CrossRef]
- Kovacik, A.; Tirpak, F.; Tomka, M.; Miskeje, M.; Tvrda, E.; Arvay, J.; Andreji, J.; Slanina, T.; Gabor, M.; Hleba, L. Trace Elements Content in Semen and Their Interactions with Sperm Quality and RedOx Status in Freshwater Fish Cyprinus Carpio: A Correlation Study. J. Trace Elem. Med. Biol. 2018, 50, 399–407. [Google Scholar] [CrossRef]
- Halo Jr, M.; Massányi, M.; Tokárová, K.; Tirpák, F.; Greifová, H.; Solár, D.; Halo, M.; Massányi, P. High Taurine Concentrations Negatively Effect Stallion Spermatozoa Parameters in Vitro. Acta Fytotech. Zootech. 2021, 24, 15–19. [Google Scholar] [CrossRef]
- Vizzari, F.; Massányi, M.; Knížatová, N.; Corino, C.; Rossi, R.; Ondruška, Ľ.; Tirpák, F.; Halo, M.; Massányi, P. Effects of Dietary Plant Polyphenols and Seaweed Extract Mixture on Male-Rabbit Semen: Quality Traits and Antioxidant Markers. Saudi J. Biol. Sci. 2021, 28, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Slanina, T.; Miškeje, M.; Tirpák, F.; Baszczyk, M.; Stawarz, R.; Massányi, P. Effect of Taurine on Turkey (Meleagris Gallopavo) Spermatozoa Viability and Motility. Czech J. Anim. Sci. 2018, 63, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Tirpak, F.; Slanina, T.; Tomka, M.; Zidek, R.; Halo, M.; Ivanic, P.; Gren, A.; Formicki, G.; Stachanczyk, K.; Lukac, N.; et al. Exposure to Non-Ionizing Electromagnetic Radiation of Public Risk Prevention Instruments Threatens the Quality of Spermatozoids. Reprod. Domest. Anim. 2019, 54, 150–159. [Google Scholar] [CrossRef]
- Kuželová, L.; Vašíček, J.; Rafay, J.; Chrenek, P. Detection of Macrophages in Rabbit Semen and Their Relationship with Semen Quality. Theriogenology 2017, 97, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Johannisson, A.; Wallgren, M.; Rodríguez-Martínez, H. Assessment of Fresh and Frozen–Thawed Boar Semen Using an Annexin-V Assay: A New Method of Evaluating Sperm Membrane Integrity. Theriogenology 2003, 60, 677–689. [Google Scholar] [CrossRef]
- Tvrdá, E.; Arroyo, F.; Ďuračka, M.; López-Fernández, C.; Gosálvez, J. Dynamic Assessment of Human Sperm DNA Damage II: The Effect of Sperm Concentration Adjustment during Processing. J. Assist. Reprod. Genet. 2019, 36, 799–807. [Google Scholar] [CrossRef]
- Halo, M.; Tirpák, F.; Dano, A.; Zbynovská, K.; Kovácik, A.; Ondruška, L.; Gren, A.; Lukác, N.; Massányi, P. Zinc Affects Rabbit Spermatozoa in Vitro: Effects on Motility and Viability. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 901–904. [Google Scholar]
- Tirpak, F.; Slanina, T.; Kovacik, A.; Ondruska, L.; Massanyi, P.; Halo, M.; Massanyi, P. Low Taurine Concentrations Possitively Affect Rabbit Spermatozoa Properties in Later Time Intervals. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 128–131. [Google Scholar] [CrossRef] [Green Version]
- Binkowski, Ł.J.; Błaszczyk, M.; Przystupińska, A.; Ożgo, M.; Massanyi, P. Metal Concentrations in Archaeological and Contemporary Mussel Shells (Unionidae): Reconstruction of Past Environmental Conditions and the Present State. Chemosphere 2019, 228, 756–761. [Google Scholar] [CrossRef]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Greifová, H.; Abdramanov, A.; Lukáč, N. Curcumin Has Protective and Antioxidant Properties on Bull Spermatozoa Subjected to Induced Oxidative Stress. Anim. Reprod. Sci. 2016, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Tokarova, K.; Vasicek, J.; Jurcik, R.; Balazi, A.; Kovacikova, E.; Kovacik, A.; Chrenek, P.; Capcarova, M. Low Dose Exposure of Patulin and Protective Effect of Epicatechin on Blood Cells in Vitro. J. Environ. Sci. Health Part B 2019, 54, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Kňažická, Z.; Lukáčová, J.; Schneidgenová, M.; Goc, Z.; Greń, A.; Szabó, C.; Massányi, P.; Lukáč, N. The Impact of Lead and Cadmium on Selected Motility, Prooxidant and Antioxidant Parameters of Bovine Seminal Plasma and Spermatozoa. J. Environ. Sci. Health Part A 2013, 48, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.C.G.D. Equine Reproductive Physiology, Breeding and Stud Management, 5th ed.; CABI: Wallingford, UK, 2020. [Google Scholar]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- El Sisy, G.A.; Abo El-Maaty, A.M.; Rawash, Z.M. Comparative Blood and Seminal Plasma Oxidant/Antioxidant Status of Arab Stallions with Different Ages and Their Relation to Semen Quality. Asian Pac. J. Reprod. 2016, 5, 428–433. [Google Scholar] [CrossRef]
- Massányi, P.; Trandzik, J.; Nad, P.; Lukac, N.; Skalicka, M.; Korenekova, B.; Cigankova, V.; Toman, R.; Halo, M.; Strapak, P. Semen Concentration of Trace Elements in Stallions and Relation to the Spermatozoa Quality. Trace Elem. Electrolytes 2004, 21, 229–231. [Google Scholar] [CrossRef]
- Usuga, A.; Rojano, B.; Restrepo, G. Effect of Seminal Plasma Components on the Quality of Fresh and Cryopreserved Stallion Semen. J. Equine Vet. Sci. 2017, 58, 103–111. [Google Scholar] [CrossRef]
- Ball, B.A. Oxidative Stress, Osmotic Stress and Apoptosis: Impacts on Sperm Function and Preservation in the Horse. Anim. Reprod. Sci. 2008, 107, 257–267. [Google Scholar] [CrossRef]
- Tirpák, F.; Greifová, H.; Lukáč, N.; Stawarz, R.; Massányi, P. Exogenous Factors Affecting the Functional Integrity of Male Reproduction. Life 2021, 11, 213. [Google Scholar] [CrossRef]
- Wnuk, M.; Lewinska, A.; Oklejewicz, B.; Bartosz, G.; Tischner, M.; Bugno-Poniewierska, M. Redox Status of Equine Seminal Plasma Reflects the Pattern and Magnitude of DNA Damage in Sperm Cells. Theriogenology 2010, 74, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Pesch, S.; Bergmann, M.; Bostedt, H. Determination of Some Enzymes and Macro- and Microelements in Stallion Seminal Plasma and Their Correlations to Semen Quality. Theriogenology 2006, 66, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signalling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [PubMed] [Green Version]
- Singh, A.P.; Rajender, S. CatSper channel, sperm function and male fertility. Reprod. Biomed. Online 2015, 30, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Baumber, J.; Sabeur, K.; Vo, A.; Ball, B.A. Reactive Oxygen Species Promote Tyrosine Phosphorylation and Capacitation in Equine Spermatozoa. Theriogenology 2003, 60, 1239–1247. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The Role of Antioxidants in Sperm Freezing: A Review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Töpfer-Petersen, E.; Ekhlasi-Hundrieser, M.; Kirchhoff, C.; Leeb, T.; Sieme, H. The Role of Stallion Seminal Proteins in Fertilisation. Anim. Reprod. Sci. 2005, 89, 159–170. [Google Scholar] [CrossRef] [PubMed]

| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Concentration (109/mL) | 0.24 | 0.08 | 0.11 | 0.37 |
| MOT_0 (%) | 58.61 | 12.41 | 28.45 | 77.50 |
| MOT_1 (%) | 47.22 | 15.32 | 29.56 | 78.43 |
| PRO_0 (%) | 22.20 | 8.73 | 10.36 | 43.55 |
| PRO_1 (%) | 32.57 | 18.14 | 10.89 | 68.42 |
| VCL_0 (µm/s) | 186.24 | 25.89 | 140.90 | 218.84 |
| VCL_1 (µm/s) | 121.63 | 36.88 | 78.82 | 179.07 |
| BCF_0 (Hz) | 32.37 | 2.11 | 27.42 | 35.23 |
| BCF_1 (Hz) | 3.37 | 0.85 | 2.39 | 4.72 |
| ALH_0 (µm) | 5.19 | 0.59 | 4.18 | 6.02 |
| ALH_1 (µm) | 27.41 | 2.67 | 23.50 | 31.96 |
| MTT (Abs) | 0.28 | 0.11 | 0.14 | 0.63 |
| DNA fragmentation (%) | 10.99 | 4.14 | 3.62 | 20.00 |
| Live (%) | 77.68 | 7.64 | 63.08 | 90.85 |
| Apoptotic (%) | 2.77 | 1.35 | 0.69 | 5.85 |
| Dead (%) | 19.55 | 7.56 | 8.46 | 34.76 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| TOS (μmol H2O2/g TP) | 0.04 | 0.02 | 0.01 | 0.12 |
| FRAP (μmol Fe2+/g TP) | 25.19 | 11.88 | 0.75 | 42.93 |
| GPx (U/g TP) | 22.53 | 6.34 | 15.82 | 42.79 |
| SOD (U/mg TP) | 1.28 | 0.62 | 0.51 | 3.38 |
| MDA (µmol/g TP) | 1.89 | 0.92 | 1.02 | 4.47 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| TP (g/L) | 18.59 | 5.34 | 8.06 | 30.85 |
| Alb (g/L) | 2.25 | 0.57 | 1.41 | 3.18 |
| Chol (mmol/L) | 0.59 | 0.05 | 0.53 | 0.71 |
| TAG (mmol/L) | 0.82 | 0.60 | 0.24 | 2.24 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Ca (mg/kg) | 169.90 | 62.59 | 72.00 | 288.00 |
| Cd (μg/kg) | 15.79 | 25.23 | 10.00 | 120.00 |
| Cu (mg/kg) | 0.97 | 0.34 | 0.26 | 1.72 |
| Fe (mg/kg) | 2.60 | 2.46 | 1.32 | 11.42 |
| Hg (µg/kg) | 18.87 | 2.81 | 14.29 | 25.52 |
| K (g/kg) | 0.91 | 0.12 | 0.62 | 1.12 |
| Mg (mg/kg) | 43.85 | 13.89 | 24.07 | 69.04 |
| Na (g/kg) | 3.47 | 0.34 | 2.84 | 4.08 |
| Pb (mg/kg) | 0.12 | 0.04 | 0.107 | 0.280 |
| Zn (mg/kg) | 64.51 | 7.84 | 48.40 | 77.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirpák, F.; Halo, M., Jr.; Tokárová, K.; Binkowski, L.J.; Vašíček, J.; Svoradová, A.; Błaszczyk-Altman, M.; Kováčik, A.; Tvrdá, E.; Chrenek, P.; et al. Composition of Stallion Seminal Plasma and Its Impact on Oxidative Stress Markers and Spermatozoa Quality. Life 2021, 11, 1238. https://doi.org/10.3390/life11111238
Tirpák F, Halo M Jr., Tokárová K, Binkowski LJ, Vašíček J, Svoradová A, Błaszczyk-Altman M, Kováčik A, Tvrdá E, Chrenek P, et al. Composition of Stallion Seminal Plasma and Its Impact on Oxidative Stress Markers and Spermatozoa Quality. Life. 2021; 11(11):1238. https://doi.org/10.3390/life11111238
Chicago/Turabian StyleTirpák, Filip, Marko Halo, Jr., Katarína Tokárová, Lukasz J. Binkowski, Jaromír Vašíček, Andrea Svoradová, Martyna Błaszczyk-Altman, Anton Kováčik, Eva Tvrdá, Peter Chrenek, and et al. 2021. "Composition of Stallion Seminal Plasma and Its Impact on Oxidative Stress Markers and Spermatozoa Quality" Life 11, no. 11: 1238. https://doi.org/10.3390/life11111238
APA StyleTirpák, F., Halo, M., Jr., Tokárová, K., Binkowski, L. J., Vašíček, J., Svoradová, A., Błaszczyk-Altman, M., Kováčik, A., Tvrdá, E., Chrenek, P., Lukáč, N., & Massányi, P. (2021). Composition of Stallion Seminal Plasma and Its Impact on Oxidative Stress Markers and Spermatozoa Quality. Life, 11(11), 1238. https://doi.org/10.3390/life11111238











