O2 and Other High-Energy Molecules in Photosynthesis: Why Plants Need Two Photosystems
Abstract
:1. Introduction
ΔrGo’ = +2870 kJ/mol, ΔrGo = +2875 kJ/mol = ΔrGo”.
ΔrGo’ = +2640 kJ/mol, ΔrGo” = +2600 kJ/mol,
ΔrGo’ = −204 kJ/mol, ΔrGo” = −252 kJ/mol,
2. Results
2.1. The Laws of Thermodynamics and Photosynthesis
2.2. Misconceptions from Redox Potentials
- There is no voltage or electric potential difference [36] of [−0.144 V − (−0.505 V)] = 0.36 V between the locations of Pheo and QA in PSII, nor an associated electric field acting on electrons. The redox potential of Eo’Pheo, Pheo-· = −0.505 V depends on the free energies of both Pheo and Pheo−˙, and Eo’QA, QA-· = −0.144 V on those of both QA and QA−˙ (see Equations (S63) and (S57)). Since QA and QA−˙ are not simultaneously present in a given PSII, there is no physical basis for predicting a static voltage of 0.36 V. When Pheo−˙ sits at a distance d from QA, the electric potential difference is roughly −e/d, which is unrelated to the redox-potential difference of 0.36 V. As in simple batteries, where positively charged ions move to the positive electrode [32], the movement of charged species in redox processes is not determined by electric fields or voltages but by free-energy (e.g., bond-energy) differences. An example of a redox reaction occurring without a voltage [37] is shown in Figure S5a and Equation (S95). The predicted voltage exists only if conductive electrodes, see Figures S5b–d and S6, are immersed into (or connected via salt bridges to) half cells each containing the reduced and conjugate oxidized species simultaneously in similar amounts.
- The energy release in a redox reaction such as Pheo−˙ + QA → … cannot be attributed to electron transfer from a high-energy donor to a lower-energy acceptor [3]. It is shown in the SI (see Equation (S52)) that the acceptor accepting the electron must be of high enough energy for the reaction to be spontaneous (ΔrGo’ < 0), since that acceptor (QA in our example) is a reactant.
- A redox potential depends on the bond and hydration energies of at least two chemical species [32], the oxidized and reduced molecules or ions in the half reaction, see Figure S3b, (as well as on the ionization energy for cations and the electron affinity of anions).
- Redox-potential energies are not energy levels of electrons. For instance, the electron energy in the electrodes of two separate electrochemical half cells connected only by a wire of negligible resistance is equal even when the half-cell redox potentials differ [3]. The energy of an electron in a half reaction depends on its environment and presents a difficult problem even in simple metal electrodes (keywords: work function, Fermi level, inner/outer/surface potentials) [38]; it would be even more challenging to analyze for electrons in molecules. ‘Free’ electrons are only intermediates and do not show up in the overall redox reaction such as Pheo−˙ + QA → Pheo + QA−˙. Therefore, the energetics of batteries [32] and biochemical reactions [3] can be analyzed without requiring quantification of the unknown free energy of ‘free’ electrons.
2.3. Why Electrons Move without Redox Voltages
2.4. Misconceptions about Electron Donors
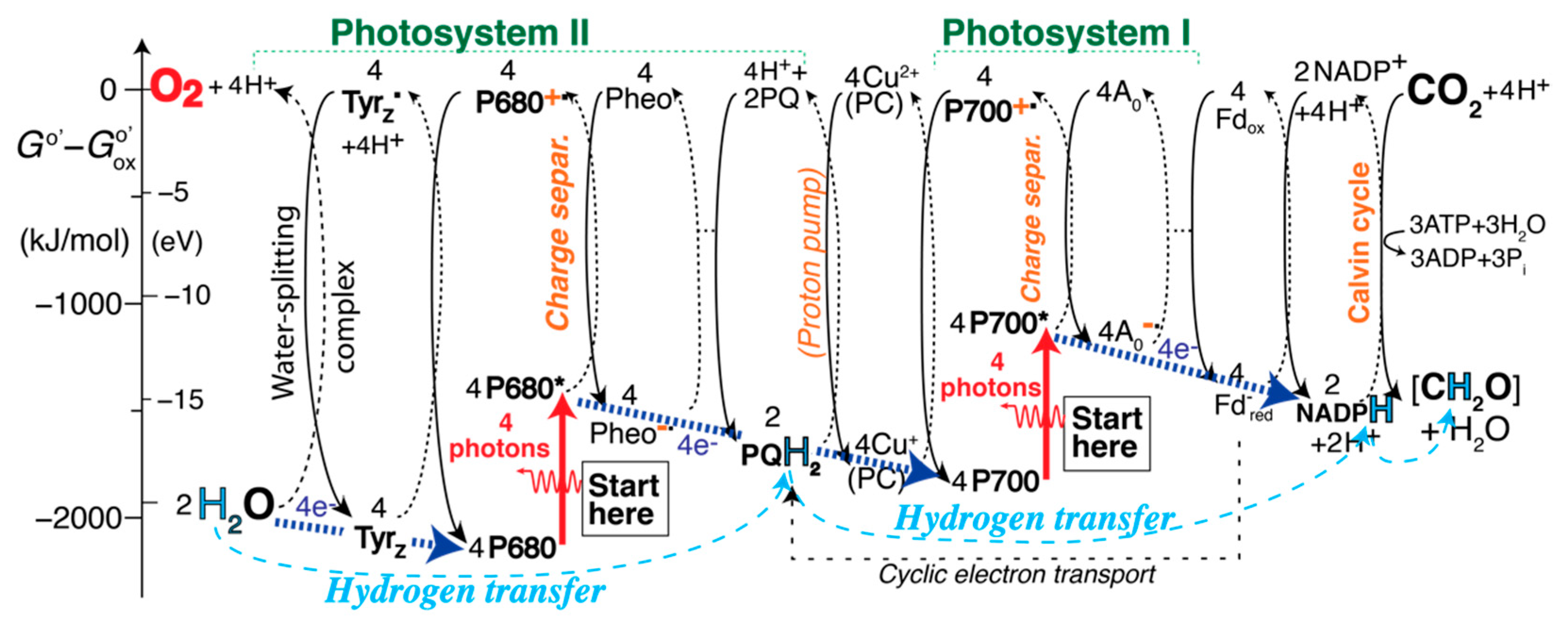
2.5. The Self-Explanatory Expanded Z (EZ)-Scheme: Processes in Photosystem II (PSII)
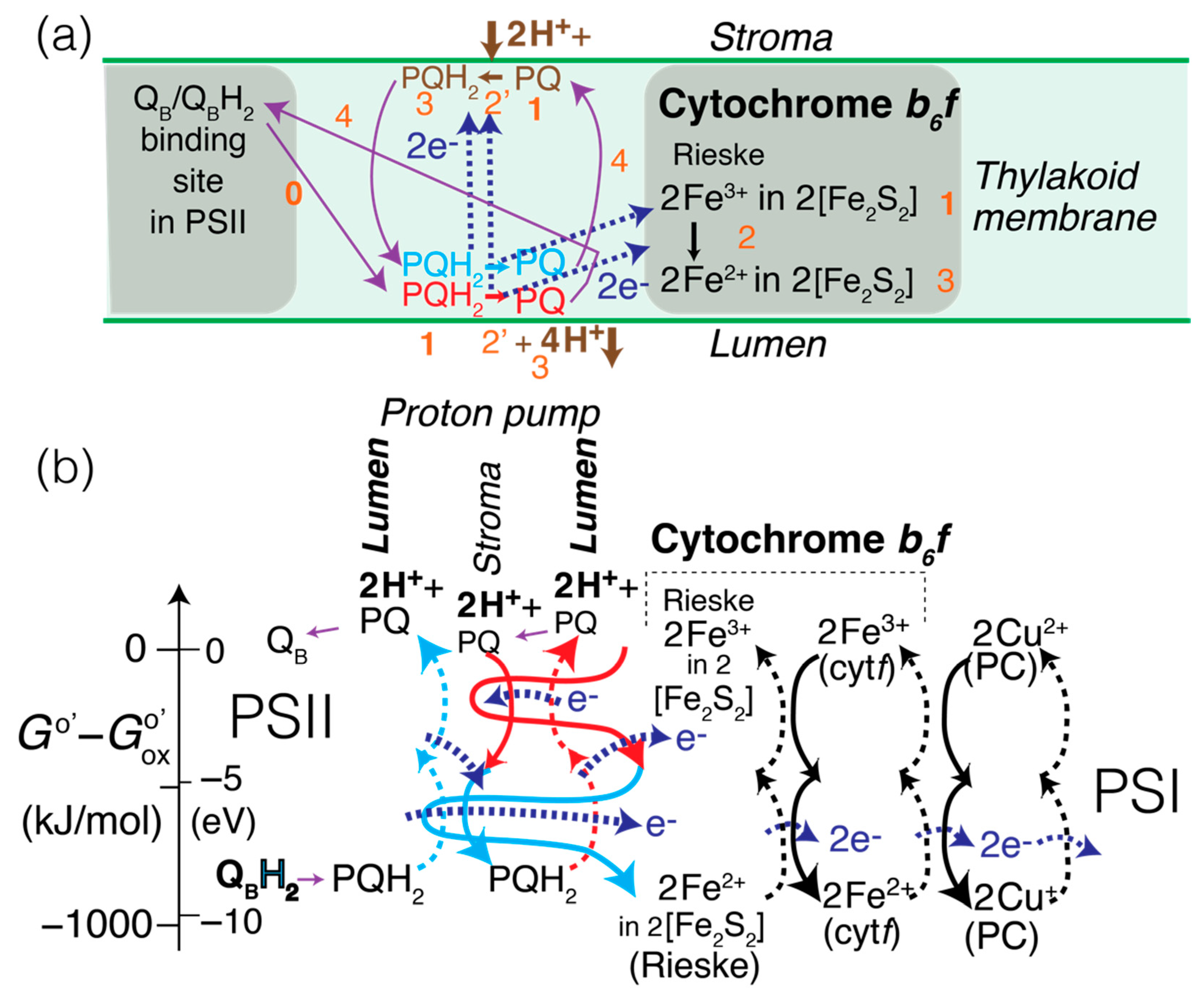
2.6. Processes Involving Cytochrome b6f, in the EZ-Scheme
→ PQlumen side + 2 H+lumen + (PQH2)stroma side
→ PQlumen side + 2 H+lumen + 2 [Fe2+Fe3+S2] (Rieske).
2.7. Processes Involving Photosystem I (PSI) in the EZ-Scheme
2.8. “Hydrogenation” (Reduction) of CO2 to Carbohydrates
2.9. Cyclic Electron Transport
2.10. The Complete EZ-Scheme of Oxygenic Photosynthesis
2.11. Vertical Shifts in the EZ-Scheme
2.12. An Alternative EZ-Scheme: Energy Flow
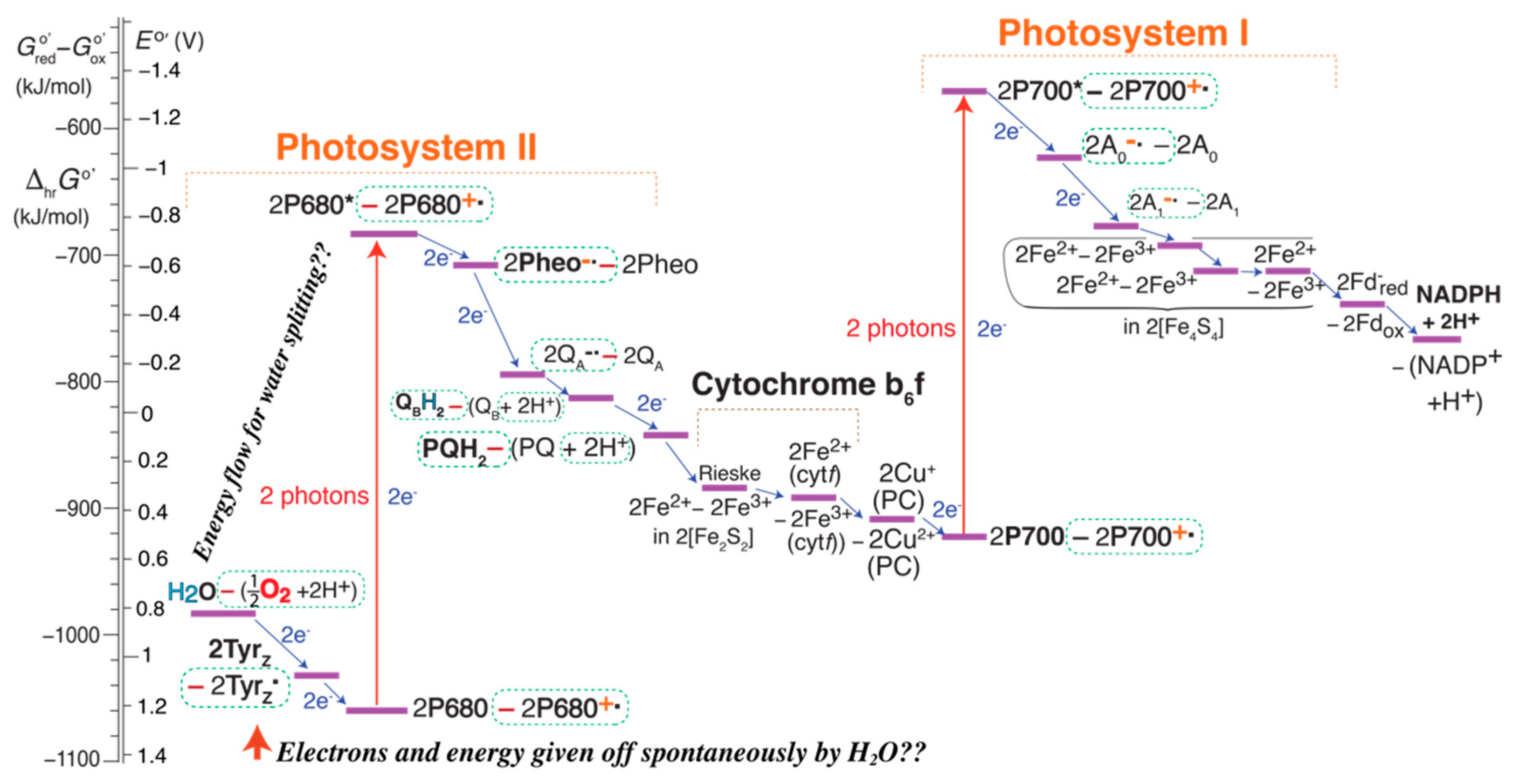
2.13. The Corrected Z-Scheme
3. Discussion
3.1. The Superiority of the EZ-Scheme
3.2. PSI Has Too Little Energy for Water Splitting, Due to Low Ionization Energy
3.3. Little of the Energy of the Photons Absorbed by PSII Directly Benefits the Plant
ΔrGo = +31 kJ/mol, ΔrGo” = +27 kJ/mol
ΔrGo’ < −125 kJ/mol, ΔrGo” < −90 kJ/mol.
ΔrGo” = +922 kJ/mol
ΔrGo” = +695 kJ/mol
ΔrGo” = +635 kJ/mol
ΔrGo” = +200 kJ/mol
3.4. Two Photosystems Because of the High Energy of O2
3.5. Photosynthesis with Only One Photosystem
ΔrGo = +426 kJ/mol.
3.6. Photon Energy Stored in O2 vs. Glucose
2 electron-pair bonds: −971 −804 −806 −498 kJ/mol
3.7. Energetics of H2O Relative to O2 and QH2
3.8. Photosynthetic Efficiency: Is It Meaningful?
3.9. Challenging the Paradigm of Electron Transport as Energy Transport
3.9.1. No “High-Energy Electrons” in Photosynthesis
3.9.2. Energy Flow Runs Counter to Electron Flow
3.9.3. Hydrogen Transfer, Not Electron Transport, as the Main Function of Photosynthesis
3.9.4. The Bicycle Chain: An Analogy for Electron Transport
3.10. Synopsis: A Valid Description of Energy and Hydrogen Flow in Photosynthesis
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A0 | An acceptor near P700 in PSI |
| A0−˙ | Acceptor A0 after it has taken up an electron; a radical anion |
| A1 | Phylloquinone, also known as phytomenadione, a fat-soluble naphthoquinone derivative in PSI |
| [CH2O] | Generic carbohydrate |
| Cytf | Cytochrome b6f, a dimeric enzyme in the thylakoid membrane in a chloroplast |
| Fd | Ferredoxin, a soluble iron–sulfur protein on the stroma side of the thylakoid membrane, containing a [Fe2S2] cluster |
| Fe2+ in [Fe2S2] (Rieske) | Reduced form [Fe2+Fe3+S2] of the [Fe2S2] or 2Fe-2S cluster in a Rieske iron–sulfur protein |
| [Fe4S4] | Iron–sulfur clusters, also denoted as 4Fe-4S, in PSI |
| FNR | Ferredoxin–NADP+ reductase (or ferredoxin:NADP+ oxidoreductase), an enzyme catalyzing the reduction (“hydrogenation”) of NADP+ coupled with the oxidation of reduced ferredoxin |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| NADPH | The reduced form of NADP+; NADPH + H+ is a slightly lower-energy biochemical analogue of H2 [3] |
| P680 | PSII primary electron donor, a pigment (special chlorophyll dimer) with an absorption maximum near a wavelength of 680 nm |
| P680* | The electronically excited state of P680 after photon absorption |
| P680+˙ | P680* after loss of an electron; a radical cation; the oxidized counterpart of both P680 and P680* |
| P700 | PSI primary electron donor, the reaction-center chlorophyll-a dimer, with an absorption maximum near 700 nm |
| P700* | The electronically excited state of P700 after photon absorption |
| P700+˙ | P700* after loss of an electron; a radical cation; the oxidized counterpart of both P700 and P700* |
| PC | Plastocyanin, a soluble protein with a redox-active copper ion, on the lumen side of the thylakoid membrane |
| Pheo | Pheophytin (chlorophyll without the Mg2+ ion) near P680 |
| Pheo−˙ | Pheophytin that has taken up an electron; a radical anion |
| PQ | Free plastoquinone, a benzoquinone derivative similar to ubiquinone (coenzyme Q) |
| PQH2 | Plastoquinol, the hydrogenated (fully reduced) form of PQ |
| PSI | Photosystem I, a protein complex in the thylakoid membrane |
| PSII | Photosystem II, a protein complex in the thylakoid membrane |
| QA or PQ-A | Protein-bound plastoquinone near pheophytin in PSII |
| QA−˙ | QA that has taken up an electron; a radical anion |
| QB or PQ-B | Loosely bound plastoquinone in PSII |
| QBH2 | The hydrogenated (fully reduced) form of QB |
| Rieske Fe3+ in [Fe2S2] | Oxidized form [Fe3+2S2] of the [Fe2S2] cluster in a Rieske protein |
| S0–S4 | States of the Mn4CaO5 water-splitting complex (oxygen-evolving complex, OEC) and associated H2O molecules, of increasing oxidation number and energy, in the S-cycle or Kok cycle |
| TyrZ | A tyrosine residue near P680 in PSII |
| TyrZ˙ | TyrZ after removal of the hydrogen (H+ + e−) from the OH group; a radical |
References
- Alberty, R.A. Thermodynamics of Biochemical Reactions; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; Worth Publishers: New York, NY, USA, 2013. [Google Scholar]
- Schmidt-Rohr, K. Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics. ACS Omega 2020, 5, 2221–2233. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 2nd ed.; Wiley Blackwell: Chichester, UK, 2014. [Google Scholar]
- Hill, R.; Bendall, F. Function of the two cytochrome components in chloroplasts: A working hypothesis. Nature 1960, 186, 136–137. [Google Scholar] [CrossRef]
- Joliot, P.; Kok, B. Oxygen Evolution in Photosynthesis. In Energetics of Photosynthesis; Govindjee, Ed.; Academic Press: New York, NY, USA, 1975; pp. 387–412. [Google Scholar]
- Du Ysens, L.N.M. The discovery of the two photosynthetic systems: A personal account. Photosynth. Res. 1989, 21, 61–79. [Google Scholar] [CrossRef]
- Junge, W. Oxygenic photosynthesis: History, status and perspective. Q. Rev. Biophys. 2019, 52, e1. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science/Taylor & Francis: New York, NY, USA, 2008. [Google Scholar]
- Hardin, J.; Betoni, G.P. Becker’s World of the Cell, 9th ed.; Pearson: Boston, MA, USA, 2015. [Google Scholar]
- Reece, B.J.; Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Campbell Biology, 7th ed.; Pearson/Benjamin Cummings: Boston, MA, USA, 2011. [Google Scholar]
- Morris, J.; Hartl, D.; Knoll, A.; Lue, R.; Michael, M.; Berry, A.; Biewener, A.; Farrell, B.; Holbrook, N.M.; Heitz, J.; et al. Biology: How Life Works, 3rd ed.; W. H. Freeman: New York, NY, USA, 2019. [Google Scholar]
- Karp, G. Cell and Molecular Biology, 7th ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics, 2nd ed.; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Schmidt-Rohr, K. Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2. J. Chem. Educ. 2015, 92, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Govindjee; Shevela, D.; Björn, L.O. Evolution of the Z-scheme of photosynthesis: A perspective. Photosynth. Res. 2017, 133, 5–15. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 7th ed.; W. H. Freeman: New York, NY, USA, 2012. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Johnson, M.P. Photosynthesis. Essays Biochem. 2016, 60, 255–273. [Google Scholar] [CrossRef]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy Conversion in Natural and Artificial Photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [Green Version]
- Cardona, T.; Sedoud, A.; Cox, N.; Rutherford, A.W. Charge separation in Photosystem II: A comparative and evolutionary overview. Biochim. Biophys. Acta (BBA)—Bioenerg. 2012, 1817, 26–43. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the Oxygen-Evolving Center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Caffarri, S.; Tibiletti, T.; Jennings, R.C.; Santabarbara, S. A Comparison Between Plant Photosystem I and Photosystem II Architecture and Functioning. Curr. Protein Pept. Sci. 2014, 15, 296–331. [Google Scholar] [CrossRef]
- Mungan, C.E. Radiation thermodynamics with applications to lasing and fluorescent cooling. Am. J. Phys. 2005, 73, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, R.G. Physical Chemistry, 3rd ed.; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Delgado-Bonal, A. Entropy of radiation: The unseen side of light. Sci. Rep. 2017, 7, 1642. [Google Scholar] [CrossRef] [Green Version]
- Yourgrau, W.; Merwe, A.V.D. Entropy Balance in Photosynthesis. Proc. Natl. Acad. Sci. USA 1968, 59, 734–737. [Google Scholar] [CrossRef] [Green Version]
- Knox, R.S. Thermodynamics and the Primary Processes of Photosynthesis. Biophys. J. 1969, 9, 1351–1362. [Google Scholar] [CrossRef] [Green Version]
- Spanner, D.C. Introduction to Thermodynamics; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Baierlein, R. Thermal Physics; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Albarrán-Zavala, E.; Angulo-Brown, F. A Simple Thermodynamic Analysis of Photosynthesis. Entropy 2007, 9, 152–168. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K. How Batteries Store and Release Energy: Explaining Basic Electrochemistry. J. Chem. Educ. 2018, 95, 1801–1810. [Google Scholar] [CrossRef]
- Ross, R.T.; Calvin, M. Thermodynamics of Light Emission and Free-Energy Storage in Photosynthesis. Biophys. J. 1967, 7, 595–614. [Google Scholar] [CrossRef] [Green Version]
- Dill, K.A.; Bromberg, S. Molecular Driving Forces, 2nd ed.; Garland Science: London, UK; New York, NY, USA, 2011. [Google Scholar]
- De Causmaecker, S.; Douglass, J.S.; Fantuzzi, A.; Nitschke, W.; Rutherford, A.W. Energetics of the exchangeable quinone, QB, in Photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 19458–19463. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Atkins, P.; Jones, L.; Laverman, L. Chemical Principles: The Quest for Insight, 7th ed.; Freeman: New York, NY, USA, 2016. [Google Scholar]
- Bockris, J.O.; Reddy, A.K.N.; Gamboa-Aldeco, M. Modern Electrochemistry 2A, Fundamentals of Electrodics, 2nd ed.; Kluwer Academic/Plenum: New York, NY, USA, 2001. [Google Scholar]
- Oxtoby, D.W.; Gillis, H.P.; Butler, L.J. Principles of Modern Chemistry, 8th ed.; CENGAGE Learning: Boston, MA, USA, 2015. [Google Scholar]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolyis via Homogenous to Biological Catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Britt, R.D.; Marchiori, D.A. Photosystem II, poised for O2 formation. Science 2019, 366, 305–306. [Google Scholar] [CrossRef]
- Ishikita, H.; Saenger, W.; Biesiadka, J.; Loll, B.; Knapp, E. How photosynthetic reaction centers control oxidation power in chlorophyll pairs P680, P700, and P870. Proc. Natl. Acad. Sci. USA 2006, 103, 9855–9860. [Google Scholar] [CrossRef] [Green Version]
- Goodsell, D.S. The Machinery of Life, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Szent-Györgyi, A. Introduction to a Submolecular Biology; Academic Press: New York, NY, USA, 1960. [Google Scholar]
- Mentzer, A.P. What Is NADPH in Photosynthesis? Available online: https://sciencing.com/nadph-photosynthesis-5799755.html (accessed on 31 July 2019).
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, Topic 8.4: Energy Demands for Photosynthesis in Land Plants, 6th ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt-Rohr, K. O2 and Other High-Energy Molecules in Photosynthesis: Why Plants Need Two Photosystems. Life 2021, 11, 1191. https://doi.org/10.3390/life11111191
Schmidt-Rohr K. O2 and Other High-Energy Molecules in Photosynthesis: Why Plants Need Two Photosystems. Life. 2021; 11(11):1191. https://doi.org/10.3390/life11111191
Chicago/Turabian StyleSchmidt-Rohr, Klaus. 2021. "O2 and Other High-Energy Molecules in Photosynthesis: Why Plants Need Two Photosystems" Life 11, no. 11: 1191. https://doi.org/10.3390/life11111191
APA StyleSchmidt-Rohr, K. (2021). O2 and Other High-Energy Molecules in Photosynthesis: Why Plants Need Two Photosystems. Life, 11(11), 1191. https://doi.org/10.3390/life11111191





