Takayasu’s Arteritis Diagnosed in an Adolescent Patient with Crohn’s Disease: Management of Biologicals
Abstract
:1. Introduction
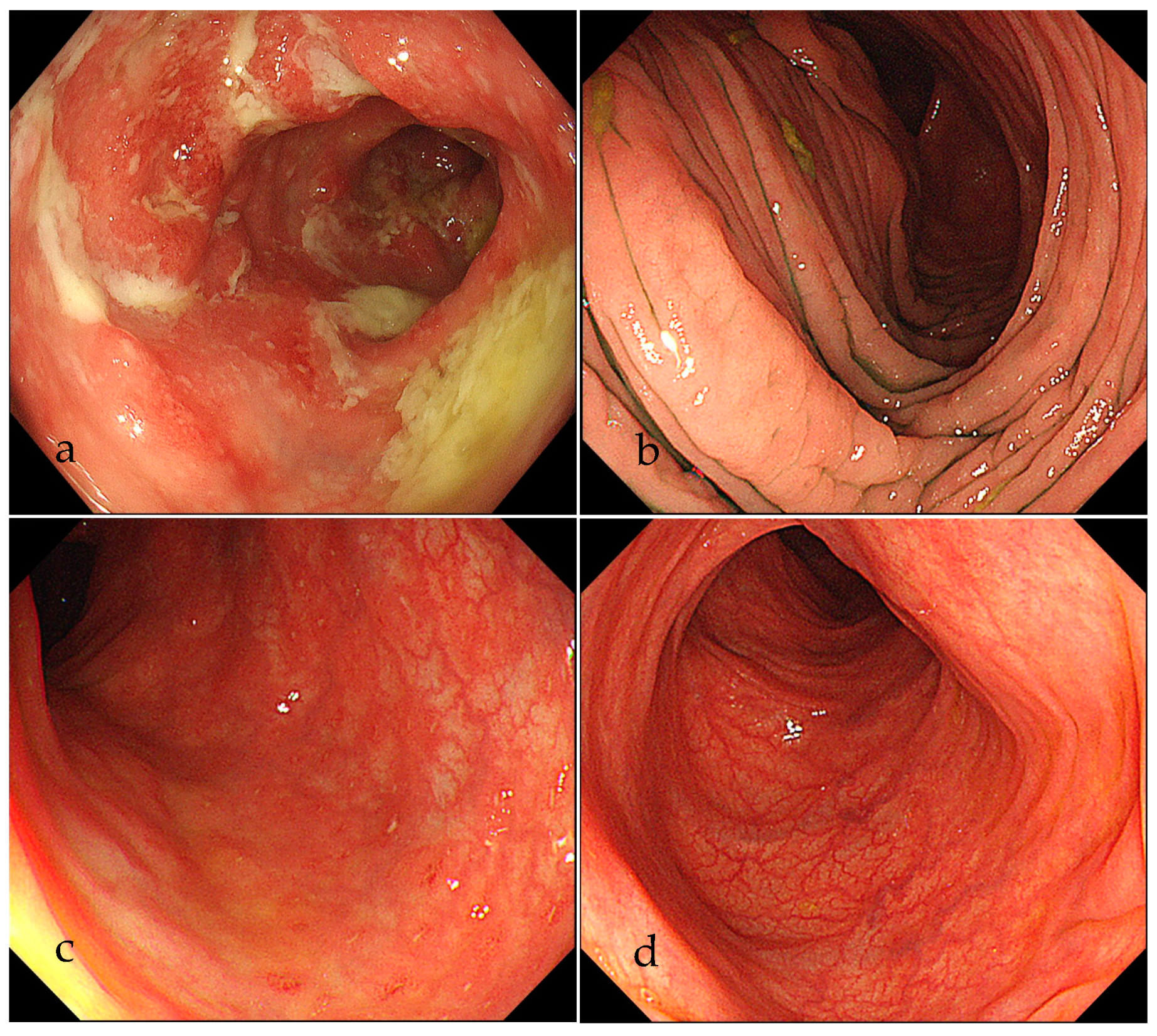
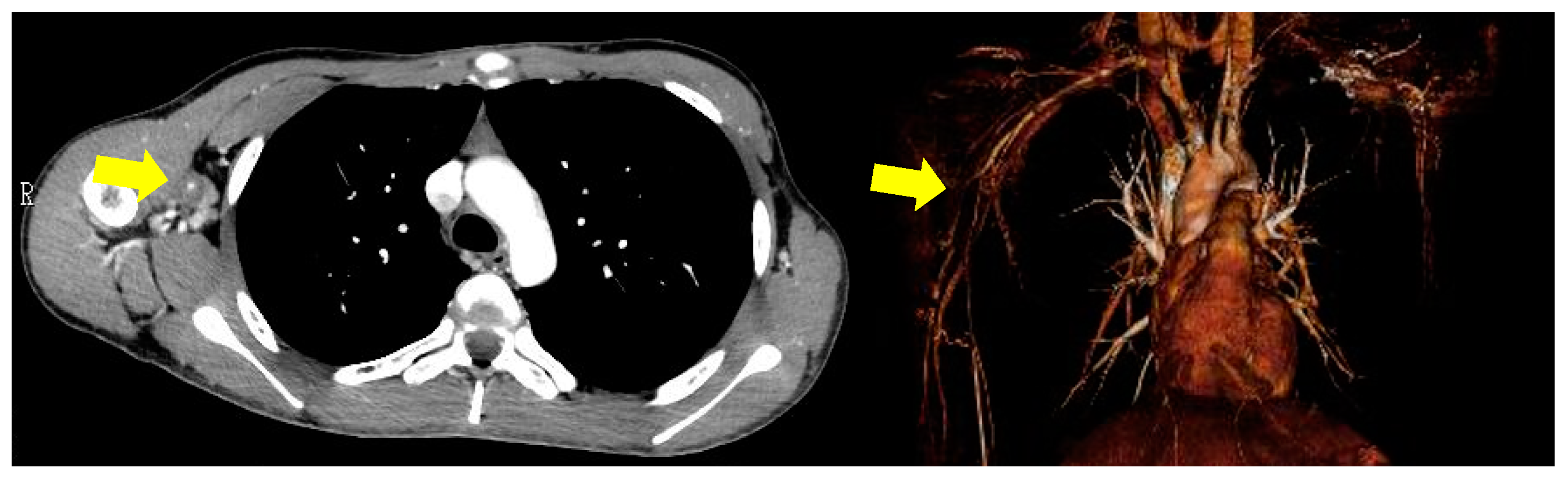
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.R.; Bruneval, P.; Angelini, A.; Bartoloni, G.; Basso, C.; Batoroeva, L.; Buja, L.M.; Butany, J.; Amati, G.; Fallon, J.T.; et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc. Pathol. 2015, 24, 267–278. [Google Scholar] [CrossRef]
- Yassinger, S.; Adelman, R.; Cantor, D.; Halsted, C.H.; Bolt, R.J. Association of inflammatory bowel disease and large vascular lesions. Gastroenterology 1976, 71, 844–846. [Google Scholar] [CrossRef]
- Kusunoki, R.; Ishihara, S.; Sato, M.; Sumita, Y.; Mishima, Y.; Okada, M.; Tada, Y.; Oka, A.; Fukuba, N.; Oshima, N.; et al. Rare Case of Takayasu’s Arteritis Associated with Crohn’s Disease. Intern. Med. 2011, 50, 1581–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, N.K.; Gupta, P.C.; Nityanand, S. High TNF-alpha and low IL-2 producing T cells characterize active disease in Takayasu’s arteritis. Clin. Immunol. 2006, 118, 154–158. [Google Scholar] [CrossRef]
- Pallone, F.; Monteleone, G. Mechanisms of tissue damage in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2001, 17, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Direskeneli, G.S.; Bicakcigil, M.; Yimaz, V.; Kamali, S.; Aksu, K.; Fresko, I.; Akkoc, N.; Kiraz, S.; Ozer, H.T.E.; Tunc, E.; et al. Interleukin (IL)-12, IL-2, and IL-6 gene polymorphisms in Takayasu’s arteritis from Turkey. Hum. Immunol. 2006, 67, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.S.; Merkel, P.A.; Brasington, R.D.; Lenschow, D.J.; Liang, P. Anti-Tumor Necrosis Factor Therapy in Patients With Difficult to Treat Takayasu Arteritis. Arthritis Rheum. 2004, 50, 2296–2304. [Google Scholar] [CrossRef]
- Molloy, E.S.; Langford, C.A.; Clark, T.M.; Gota, C.E.; Hoffman, G.S. Anti-tumor necrosis factor therapy in patients with refractory Takayasu arteritis: Long term follow-up. Ann. Rheum. Dis. 2008, 67, 1567–1569. [Google Scholar] [CrossRef]
- Targan, S.R.; Hanauer, S.B.; van Deventer, S.J.; Mayer, L.; Present, D.H.; Braakman, T.; DeWoody, K.L.; Schaible, T.H.; Rutgeerts, P.J. A short-Term Study of Chimeric Monoclonal Antibody cA2 to Tumor Necrosis Factor α for Crohn’s Disease. N. Engl. J. Med. 1997, 337, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomized trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Salvarani, C.; Magnani, L.; Catanoso, M.; Pipitone, N.; Versari, A.; Dardani, L.; Pulsatelli, L.; Meliconi, R.; Boiardi, L. Tocilizumab: A novel therapy for patients with large-vessel vasculitis. Rheumatology 2012, 51, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvarani, C.; Hatemi, G. Management of large-vessel vasculitis. Curr. Opin. Rheumatol. 2019, 31, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Takazoe, M.; Fukuda, Y.; Hibi, T.; Kusugami, K.; Andoh, A.; Matsumoto, T.; Yamamura, T.; Azuma, J.; Nishimoto, N.; et al. A Pilot Randomized Trial of a Human Anti-Interleukin-6 Receptor Monoclonal Antibody in Active Crohn’s Disease. Gastroenterology 2004, 126, 989–996. [Google Scholar] [CrossRef]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990, 33, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Keser, G.; Direskeneli, H.; Aksu, K. Management of Takayasu arteritis: A systematic review. Rheumatology 2014, 53, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comarmond, C.; Biard, L.; Lambert, M.; Mekinian, A.; Ferfar, Y.; Kahn, J.E.; Benhamou, Y.; Chiche, L.; Koskas, F.; Cluzel, P.; et al. Long-Term Outcomes and Prognostic Factors of Complications in Takayasu Arteritis. Circulation 2017, 136, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.S.; Leavitt, R.Y.; Kerr, G.S.; Rottem, M.; Sneller, M.S.; Fauci, A.S. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994, 37, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Valsakumar, A.K.; Valappil, U.C.; Jorapur, V.; Garg, N.; Nityanand, S.; Sinha, N. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu’s arteritis. J. Rheumatol. 2003, 30, 1793–1798. [Google Scholar]
- Shelhamer, J.H.; Volkman, D.J.; Parrillo, J.E.; Lawley, T.J.; Johnston, M.R.; Fauci, A.S. Takayasu’s arteritis and its therapy. Ann. Intern. Med. 1985, 103, 121–126. [Google Scholar] [CrossRef]
- Mekinian, A.; Neel, A.; Sibilia, J.; Cohen, P.; Connault, J.; Lambert, M.; Federici, L.; Berthier, S.; Fiessinger, J.N.; Godeau, B.; et al. Efficacy and tolerance of infliximab in refractory Takayasu arteritis: French multicenter study. Rheumatology 2012, 51, 882–886. [Google Scholar] [CrossRef] [Green Version]
- Noris, M.; Daina, E.; Gamba, S.; Bonazzola, S.; Remuzzi, G. Interleukin-6 and RANTES in Takayasu arteritis: A guide for therapeutic decisions? Circulation 1999, 100, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaoka, Y.; Isobe, M.; Takei, S.; Tanaka, Y.; Ishii, T.; Yokota, S.; Nomura, A.; Yoshida, S.; Nishimoto, N. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: Results from a randomized, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann. Rheum. Dis. 2018, 77, 348–354. [Google Scholar] [CrossRef] [Green Version]
- D’Haens, G.; Baert, F.; van Assche, G.; Caenepeel, P.; Vergauwe, P.; Tuynman, H.; Vos, M.D.; van Deventer, S.; Stitt, L.; Donner, A.; et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomized trial. Lancet 2008, 371, 660–667. [Google Scholar] [CrossRef]
- Renna, S.; Cottone, M.; Orlando, A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 9675–9690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reny, J.L.; Paul, J.F.; Lefèbvre, C.; Champion, K.; Emmerich, J.; Blétry, O.; Piette, J.C.; Fiessinger, J.N. Association of Takayasu’s arteritis and Crohn’s disease. Results of a study on 44 Takayasu patients and review of the literature. Ann. Med. Interne 2003, 154, 85–90. [Google Scholar]
- Seko, Y. Giant cell and Takayasu arteritis. Curr. Opin. Rheumatol. 2007, 19, 39–43. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zeron, P.; Cuadrado, M.J.; Khamashta, M.A. Vasculitis induced by tumor necrosis factor-targeted therapies. Curr. Rheumatol. Rep. 2008, 10, 442–448. [Google Scholar] [CrossRef]
- Narazaki, M.; Kishimoto, T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwabara, S.; Tanimura, S.; Matsumoto, S.; Nakamura, H.; Horita, T. Successful remission with tofacitinib in a patient with refractory Takayasu arteritis complicated by ulcerative colitis. Ann. Rheum. Dis. 2020, 79, 1125–1126. [Google Scholar] [CrossRef] [PubMed]
- Terao, C.; Yoshifuji, H.; Nakajima, T.; Yukawa, N.; Matsuda, F.; Mimori, T. Ustekinumab as a therapeutic option for Takayasu arteritis: From genetic findings to clinical application. Scand. J. Rheumatol. 2016, 45, 80–82. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishimoto, K.; Nozaki, Y.; Sakurai, T.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Takayasu’s Arteritis Diagnosed in an Adolescent Patient with Crohn’s Disease: Management of Biologicals. Life 2021, 11, 1019. https://doi.org/10.3390/life11101019
Kishimoto K, Nozaki Y, Sakurai T, Kinoshita K, Funauchi M, Matsumura I. Takayasu’s Arteritis Diagnosed in an Adolescent Patient with Crohn’s Disease: Management of Biologicals. Life. 2021; 11(10):1019. https://doi.org/10.3390/life11101019
Chicago/Turabian StyleKishimoto, Kazuya, Yuji Nozaki, Toshiharu Sakurai, Koji Kinoshita, Masanori Funauchi, and Itaru Matsumura. 2021. "Takayasu’s Arteritis Diagnosed in an Adolescent Patient with Crohn’s Disease: Management of Biologicals" Life 11, no. 10: 1019. https://doi.org/10.3390/life11101019
APA StyleKishimoto, K., Nozaki, Y., Sakurai, T., Kinoshita, K., Funauchi, M., & Matsumura, I. (2021). Takayasu’s Arteritis Diagnosed in an Adolescent Patient with Crohn’s Disease: Management of Biologicals. Life, 11(10), 1019. https://doi.org/10.3390/life11101019





