Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
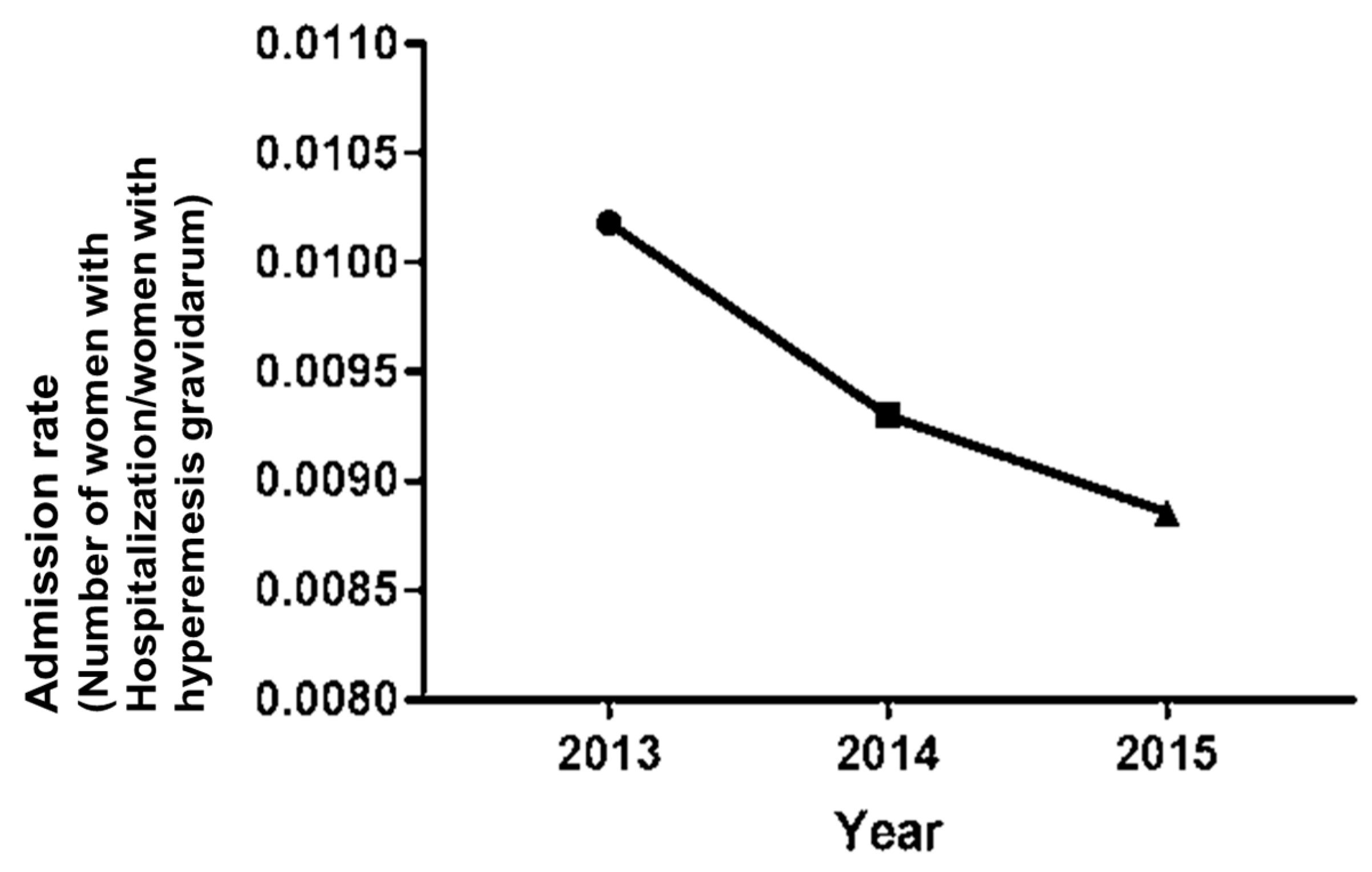
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiossi, G.; Neri, I.; Cavazzuti, M.; Basso, G.; Facchinetti, F. Hyperemesis gravidarum complicated by Wernicke encephalo-pathy: Background, case report, and review of the literature. Obstet. Gynecol. Surv. 2006, 61, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.B.; Yost, N.P.; Wendel, G.D. Acute Renal Failure in Association with Severe Hyperemesis Gravidarum. Obstet. Gynecol. 2002, 100, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Fejzo, M.S.; Magtira, A.; Schoenberg, F.P.; MacGibbon, K.; Mullin, P.; Romero, R.; Tabsh, K. Antihistamines and other prognos-tic factors for adverse outcome in hyperemesis gravidarum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fejzo, M.S.; Magtira, A.; Schoenberg, F.P.; MacGibbon, K.; Mullin, P.M. Neurodevelopmental delay in children exposed in utero to hyperemesis gravidarum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Fejzo, M.S.; Poursharif, B.; Korst, L.M.; Munch, S.; MacGibbon, K.; Romero, R.; Goodwin, T.M. Symptoms and Pregnancy Outcomes Associated with Extreme Weight Loss among Women with Hyperemesis Gravidarum. J. Women’s Health 2009, 18, 1981–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trogstad, L.I.; Stoltenberg, C.; Magnus, P.; Skjaerven, R.; Irgens, L.M.; Skjærven, R. Recurrence risk in hyperemesis gravidarum. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 1641–1645. [Google Scholar] [CrossRef]
- Rochelson, B.; Vohra, N.; Darvishzadeh, J.; Pagano, M. Low prepregnancy ideal weight:height ratio in women with hy-peremesis gravidarum. J. Reprod. Med. 2003, 48, 422–424. [Google Scholar]
- Hershman, J.M. Physiological and pathological aspects of the effect of human chorionic gonadotropin on the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 249–265. [Google Scholar] [CrossRef]
- Veenendaal, M.V.; van Abeelen, A.F.; Painter, R.C.; van der Post, J.A.; Roseboom, T.J. Consequences of hyperemesis gravi-darum for offspring: A systematic review and meta-analysis. BJOG 2011, 118, 1302–1313. [Google Scholar] [CrossRef]
- Minagawa, M.; Narita, J.; Tada, T.; Maruyama, S.; Shimizu, T.; Bannai, M.; Oya, H.; Hatakeyama, K.; Abo, T. Mechanisms un-derlying immunologic states during pregnancy: Possible association of the sympathetic nervous system. Cell Immunol. 1999, 196, 1–13. [Google Scholar] [CrossRef]
- Owe, K.M.; Støer, N.; Wold, B.H.; Magnus, M.C.; Nystad, W.; Vikanes, Å.V. Leisure-time physical activity before pregnancy and risk of hyperemesis gravidarum: A population-based cohort study. Prev. Med. 2019, 125, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-C.; Lee, F.-K.; Wang, P.-H. Hyperemesis gravidarum, pregnancy and bone loss. J. Chin. Med. Assoc. 2018, 81, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-C.; Tsui, K.-H.; Wang, P.-H. Hyperemesis gravidarum. J. Chin. Med. Assoc. 2018, 81, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Verberg, M.; Gillott, D.; Al-Fardan, N.; Grudzinskas, J. Hyperemesis gravidarum, a literature review. Hum. Reprod. Updat. 2005, 11, 527–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fell, D.B.; Dodds, L.; Joseph, K.S.; Allen, V.M.; Butler, B. Risk Factors for Hyperemesis Gravidarum Requiring Hospital Admission During Pregnancy. Obstet. Gynecol. 2006, 107, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; Ravelli, A.C.; van der Post, J.A.; Painter, R.C. Maternal characteristics largely explain poor pregnancy out-come after hyperemesis gravidarum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 56–59. [Google Scholar] [CrossRef]
- Lee, J.; Einarson, A.; Gallo, M.; Okotore, B.; Koren, G. Longitudinal change in the treatment of nausea and vomiting of pregnancy in Ontario. Can. J. Clin. Pharmacol. J. Can. Pharmacol. Clin. 2000, 7, 205–208. [Google Scholar]
- Fell, D.B.; Joseph, K.S.; Dodds, L.; Allen, A.C.; Jangaard, K.; Van den Hof, M. Changes in maternal characteristics in Nova Sco-tia, Canada from 1988 to 2001. Can. J. Public Health 2005, 96, 234–238. [Google Scholar] [CrossRef]
- Castillo, M.J.; Phillippi, J.C. Hyperemesis gravidarum: A holistic overview and approach to clinical assessment and man-agement. J. Perinat. Neonatal. Nurs. 2015, 29, 12–22. [Google Scholar] [CrossRef]
- Cedergren, M.; Brynhildsen, J.; Josefsson, A.; Sydsjö, A.; Sydsjö, G. Hyperemesis gravidarum that requires hospitalization and the use of antiemetic drugs in relation to maternal body composition. Am. J. Obstet. Gynecol. 2008, 198, 412.e1–412.e5. [Google Scholar] [CrossRef]
- Ioannidou, P.G.; Papanikolaou, D.; Mikos, T.; Mastorakos, G.; Goulis, D.G. Predictive factors of Hyperemesis Gravidarum: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.-H.; Yang, Y.-H.; Kuo, S.-H.; Chan, T.-F.; Yang, M.-S. Relationships among Smoking, Drinking, Betel Quid Chewing and Pregnancy-Related Nausea and Vomiting in Taiwanese Aboriginal Women. Kaohsiung J. Med. Sci. 2009, 25, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cai, W.W. Severe vomiting during pregnancy: Antenatal correlates and fetal outcomes. Epidemiology 1991, 2, 454–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikanes, Å.; Grjibovski, A.M.; Vangen, S.; Gunnes, N.; Samuelsen, S.O.; Magnus, P. Maternal Body Composition, Smoking, and Hyperemesis Gravidarum. Ann. Epidemiol. 2010, 20, 592–598. [Google Scholar] [CrossRef] [PubMed]
- DePue, R.H.; Bernstein, L.; Ross, R.K.; Judd, H.L.; Henderson, B.E. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and ther maternal factors: A seroepidemiologic study. Am. J. Obstet. Gynecol. 1987, 156, 1137–1141. [Google Scholar] [CrossRef]
- Uomori, T.; Horimoto, Y.; Mogushi, K.; Matsuoka, J.; Saito, M. Relationship between alcohol metabolism and chemothera-py-induced emetic events in beast cancer patients. Breast Cancer 2017, 24, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Stoicea, N.; Gan, T.J.; Joseph, N.; Uribe, A.A.; Pandya, J.; Dalal, R.; Bergese, S.D. Alternative Therapies for the Prevention of Postoperative Nausea andVomiting. Front. Med. 2015, 2, 87. [Google Scholar] [CrossRef] [Green Version]
- Beadle, K.L.; Helbling, A.R.; Love, S.L.; April, M.D.; Hunter, C.J. Isopropyl Alcohol Nasal Inhalation for Nausea in the Emer-gency Department: A Randoized Controlled Trial. Ann. Emerg. Med. 2016, 68, 1–9 e1. [Google Scholar] [CrossRef]
- Couwenbergs, C.J. Acute effects of drinking beer or wine on the steroid hormones of healthy men. J. Steroid Biochem. 1988, 31, 467–473. [Google Scholar] [CrossRef]
- Sarkola, T.; Fukunaga, T.; Mäkisalo, H.; Eriksson, C.J.P. Acute effect of alcohol on androgens in premenopausal women. Alcohol Alcohol. 2000, 35, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Poursharif, B.; Korst, L.M.; Fejzo, M.S.; MacGibbon, K.; Romero, R.; Goodwin, T.M. The psychosocial burden of hyperemesis gravidarum. J. Perinatol. 2007, 28, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| No Hospitalization (n = 214,163) | Hospitalization (n = 2210) | p-Value | |
|---|---|---|---|
| Age (years) | 30.51 ± 3.68 | 30.68 ± 3.77 | 0.038 |
| Nulliparity (%) | 69.96 | 72.31 | 0.016 |
| Multiple pregnancies (%) | 2.09 | 5.02 | <0.001 |
| Neonatal sex-female (%) | 48.41 | 55.38 | <0.001 |
| Smoking history (%) | 0.0035 | ||
| Never | 92.85 | 94.62 | |
| Past | 3.76 | 3.12 | |
| Current | 3.39 | 2.26 | |
| Alcohol (%) (frequency/week) | <0.001 | ||
| 0/week | 53.73 | 63.12 | |
| 1–2/week | 40.64 | 32.90 | |
| ≥3/week | 5.64 | 3.98 | |
| BMI (kg/m2) | ± | ± | <0.001 |
| Underweight | 14.62 | 17.47 | |
| Normal | 75.72 | 73.89 | |
| Obese | 9.66 | 8.64 | |
| WC (cm) | 70.49 ± 7.79 | 69.87 ± 7.51 | <0.001 |
| Hb (mg/dL) | 13.02 ± 1.00 | 12.95 ± 1.01 | 0.002 |
| Adjusted ORs | 95% CI | |
|---|---|---|
| Age (years) | 0.99 | 0.98, 1.00 |
| Nulliparity | 1.18 | 1.06, 1.30 |
| Multiple pregnancy | 2.43 | 2.00, 2.95 |
| Neonatal gender-female | 1.34 | 1.23, 1.46 |
| Smoking history (%) | ||
| Never | 1 | |
| Past | 0.93 | 0.73, 1.18 |
| Current | 0.77 | 0.58, 1.02 |
| Alcohol (frequency/week) | ||
| 0/week | 1 | |
| 1–2/week | 0.70 | 0.64, 0.76 |
| ≥3/week | 0.64 | 0.52, 0.80 |
| BMI (kg/m2) | ||
| Underweight | 1.16 | 1.03, 1.31 |
| Normal | 1 | |
| Obese | 1.04 | 0.87, 1.24 |
| WC (cm) | 1.01 | 1.00, 1.02 |
| Hb (mg/dL) | 1.06 | 1.00, 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.Y.; Cho, G.J.; Kim, S.Y.; Lee, K.-M.; Ahn, K.H.; Han, S.W.; Hong, S.-C.; Ryu, H.M.; Oh, M.-J.; Kim, H.-J.; et al. Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study. Life 2021, 11, 12. https://doi.org/10.3390/life11010012
Kim HY, Cho GJ, Kim SY, Lee K-M, Ahn KH, Han SW, Hong S-C, Ryu HM, Oh M-J, Kim H-J, et al. Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study. Life. 2021; 11(1):12. https://doi.org/10.3390/life11010012
Chicago/Turabian StyleKim, Ho Yeon, Geum Joon Cho, So Yeon Kim, Kyu-Min Lee, Ki Hoon Ahn, Sung Won Han, Soon-Cheol Hong, Hyun Mee Ryu, Min-Jeong Oh, Hai-Joong Kim, and et al. 2021. "Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study" Life 11, no. 1: 12. https://doi.org/10.3390/life11010012
APA StyleKim, H. Y., Cho, G. J., Kim, S. Y., Lee, K.-M., Ahn, K. H., Han, S. W., Hong, S.-C., Ryu, H. M., Oh, M.-J., Kim, H.-J., & Kim, S. C. (2021). Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study. Life, 11(1), 12. https://doi.org/10.3390/life11010012









