Further Characterization of the Pseudo-Symmetrical Ribosomal Region
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. On the Stability of the Structural Features of SymR During Translation
3.2. The Conservation of SymR over the Three Domains of Life
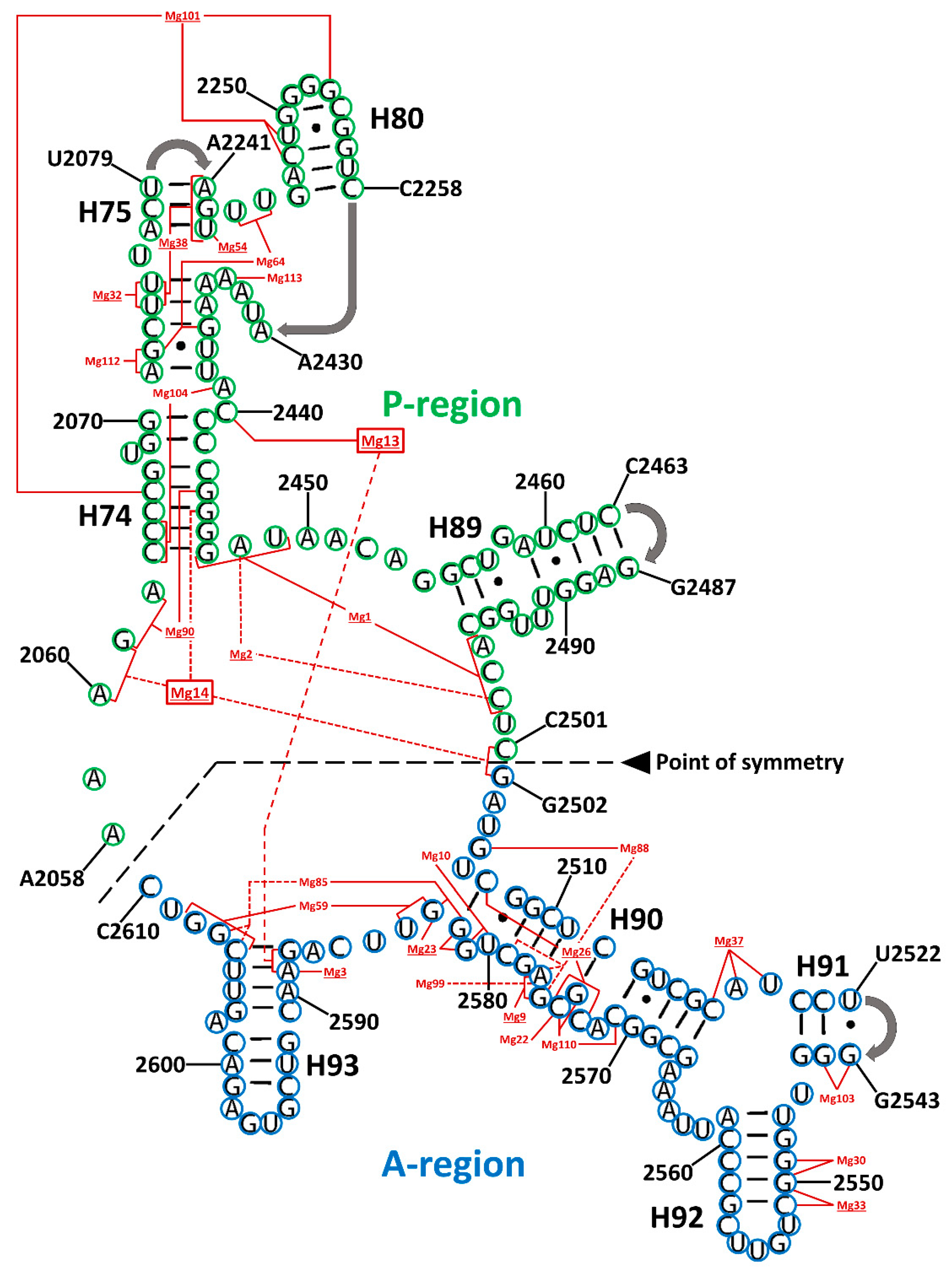
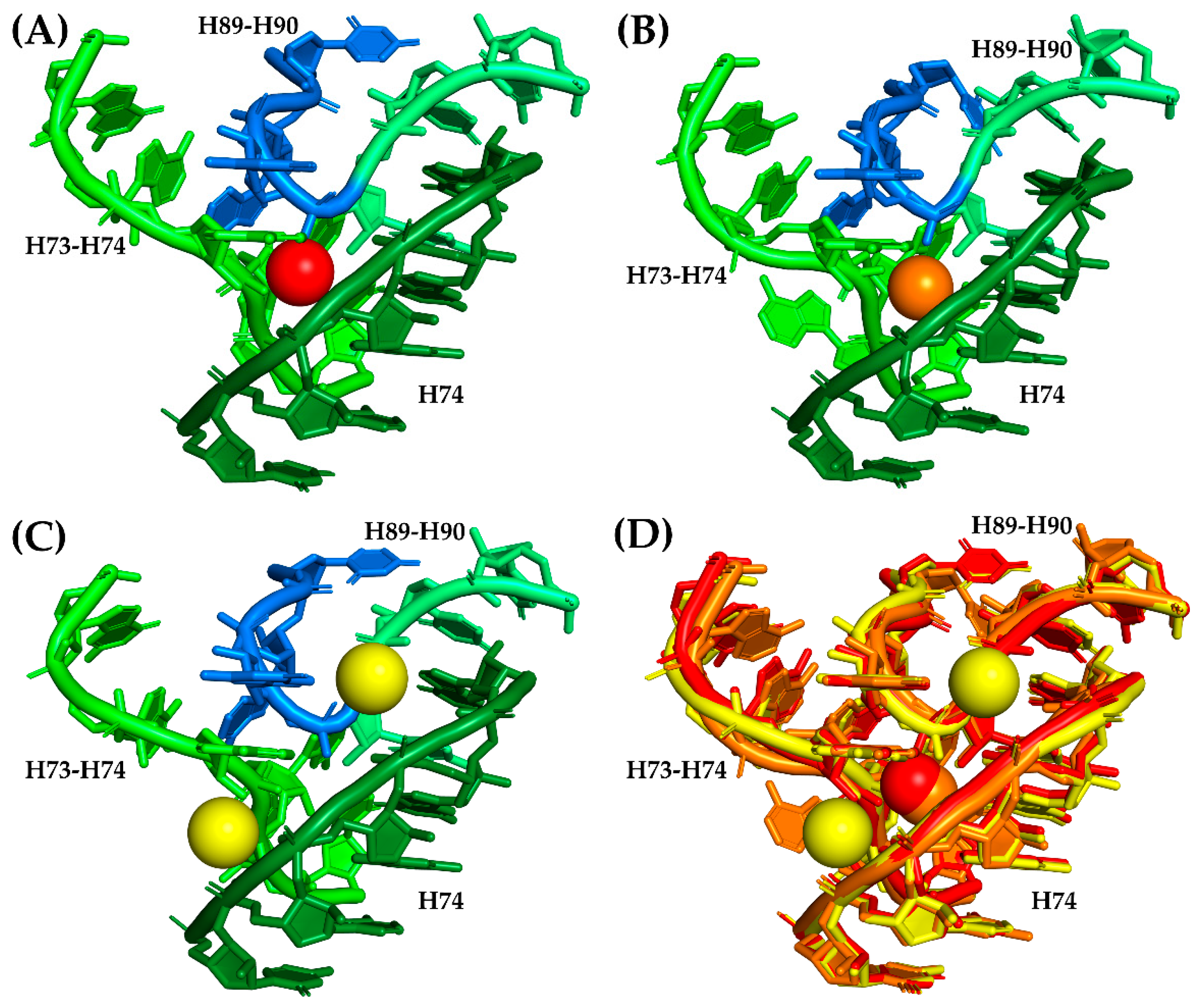
3.3. Magnesium Role in Folding and Evolution of the SymR
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Bashan, A.; Zarivach, R.; Schluenzen, F.; Agmon, I.; Harms, J.; Auerbach, T.; Baram, D.; Berisio, R.; Bartels, H.; Hansen, H.A.S.; et al. Ribosomal crystallography: Peptide bond formation and its inhibition. Biopolymers 2003, 70, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Polacek, N.; Mankin, A.S. The Ribosomal Peptidyl Transferase Center: Structure, Function, Evolution, Inhibition. Crit. Rev. Biochem. Mol. Boil. 2005, 40, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.; Auerbach, T.; Baram, D.; Bartels, H.; Bashan, A.; Berisio, R.; Fucini, P.; Hansen, H.A.S.; Harms, J.; Kessler, M.; et al. On Peptide Bond Formation, Translocation, Nascent Protein Progression and the Regulatory Properties of Ribosomes: Delivered on 20 October 2002 at the 28th FEBS Meeting in Istanbul. Eur. J. Biochem. 2003, 270, 2543–2556. [Google Scholar] [CrossRef]
- Fox, G.E.; Tran, Q.; Yonath, A. An Exit Cavity Was Crucial to the Polymerase Activity of the Early Ribosome. Astrobiology 2012, 12, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Tran, Q.; E Fox, G. Nanometer scale pores similar in size to the entrance of the ribosomal exit cavity are a common feature of large RNAs. RNA 2013, 19, 1349–1354. [Google Scholar] [CrossRef][Green Version]
- Milligan, R.A.; Unwin, P.N.T. Location of exit channel for nascent protein in 80S ribosome. Nature 1986, 319, 693–695. [Google Scholar] [CrossRef]
- Yonath, A.; Leonard, K.R.; Wittmann, H.G. A tunnel in the large ribosomal subunit revealed by three-dimensional image reconstruction. Science 1987, 236, 813–816. [Google Scholar] [CrossRef]
- Voss, N.R.; Gerstein, M.; Steitz, T.; Moore, P.B. The Geometry of the Ribosomal Polypeptide Exit Tunnel. J. Mol. Boil. 2006, 360, 893–906. [Google Scholar] [CrossRef]
- Fox, G.E.; Naik, A.K. The Evolutionary History of the Translations Machinery. In The Genetic Code and the Origin of Life; Springer: Boston, MA, USA, 2004; pp. 92–105. [Google Scholar]
- Hury, J.; Nagaswamy, U.; Larios-Sanz, M.; Fox, G.E. Ribosome origins: The relative age of 23S rRNA domains. Orig. Life Evol. Biosphere 2006, 36, 421–429. [Google Scholar] [CrossRef]
- Fox, G.E. Origin and Evolution of the Ribosome. Cold Spring Harb. Perspect. Boil. 2010, 2, a003483. [Google Scholar] [CrossRef]
- Hsiao, C.; Mohan, S.; Kalahar, B.K.; Williams, L.D. Peeling the Onion: Ribosomes are Ancient Molecular Fossils. Mol. Boil. Evol. 2009, 26, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.S.; Bernier, C.R.; Hsiao, C.; Norris, A.M.; Kovacs, N.A.; Waterbury, C.C.; Stepanov, V.G.; Harvey, S.C.; Fox, G.E.; Wartell, R.M.; et al. Evolution of the ribosome at atomic resolution. Proc. Natl. Acad. Sci. USA 2014, 111, 10251–10256. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.S.; Gulen, B.; Norris, A.M.; Kovacs, N.A.; Bernier, C.R.; Lanier, K.A.; Fox, G.E.; Harvey, S.C.; Wartell, R.M.; Hud, N.V.; et al. History of the ribosome and the origin of translation. Proc. Natl. Acad. Sci. USA 2015, 112, 15396–15401. [Google Scholar] [CrossRef] [PubMed]
- Harish, A.; Caetano-Anollés, G. Ribosomal History Reveals Origins of Modern Protein Synthesis. PLoS ONE 2012, 7, e32776. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. Comparisons between small ribosomal RNA and theoretical minimal RNA ring secondary structures confirm phylogenetic and structural accretion histories. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Agmon, I.; Bashan, A.; Zarivach, R.; Yonath, A. Symmetry at the active site of the ribosome: Structural and functional implications. Boil. Chem. 2005, 386, 833–844. [Google Scholar] [CrossRef]
- Agmon, I. The Dimeric Proto-Ribosome: Structural Details and Possible Implications on the Origin of Life. Int. J. Mol. Sci. 2009, 10, 2921–2934. [Google Scholar] [CrossRef]
- Davidovich, C.; Belousoff, M.; Wekselman, I.; Shapira, T.; Krupkin, M.; Zimmerman, E.; Bashan, A.; Yonath, A. The Proto-Ribosome: An Ancient Nano-Machine for Peptide Bond Formation. Isr. J. Chem. 2010, 50, 29–35. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Root-Bernstein, M. The Ribosome as a Missing Link in Prebiotic Evolution III: Over-Representation of tRNA- and rRNA-Like Sequences and Plieofunctionality of Ribosome-Related Molecules Argues for the Evolution of Primitive Genomes from Ribosomal RNA Modules. Int. J. Mol. Sci. 2019, 20, 140. [Google Scholar] [CrossRef]
- Prosdocimi, F.; Zamudio, G.S.; Palacios-Pérez, M.; De Farias, S.T.; José, M.V. The Ancient History of Peptidyl Transferase Center Formation as Told by Conservation and Information Analyses. Life 2020, 10, 134. [Google Scholar] [CrossRef]
- Huang, L.; Krupkin, M.; Bashan, A.; Yonath, A.; Massa, L. Protoribosome by quantum kernel energy method. Proc. Natl. Acad. Sci. USA 2013, 110, 14900–14905. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.J.; Moore, P.B.; Steitz, T.A. The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA 2004, 10, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.; Williams, L.D. A recurrent magnesium-binding motif provides a framework for the ribosomal peptidyl transferase center. Nucleic Acids Res. 2009, 37, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.S.; Bernier, C.R.; Hsiao, C.; Okafor, C.D.; Tannenbaum, E.; Stern, J.; Gaucher, E.; Schneider, D.; Hud, N.V.; Harvey, S.C.; et al. RNA–Magnesium–Protein Interactions in Large Ribosomal Subunit. J. Phys. Chem. B 2012, 116, 8113–8120. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-J.; Bussemaker, H.J.; Olson, W.K. DSSR: An integrated software tool for dissecting the spatial structure of RNA. Nucleic Acids Res. 2015, 43, e142. [Google Scholar] [CrossRef]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Leontis, N.B.; Westhof, E. Geometric nomenclature and classification of RNA base pairs. RNA 2001, 7, 499–512. [Google Scholar] [CrossRef]
- Bernier, C.R.; Petrov, A.S.; Waterbury, C.C.; Jett, J.; Li, F.; Freil, L.E.; Xiong, X.; Wang, L.; Migliozzi, B.; Hershkovits, E.; et al. RiboVision suite for visualization and analysis of ribosomes. Faraday Discuss. 2014, 169, 195–207. [Google Scholar] [CrossRef]
- Mohan, S.; Noller, H.F. Recurring RNA structural motifs underlie the mechanics of L1 stalk movement. Nat. Commun. 2017, 8, 14285. [Google Scholar] [CrossRef]
- Jaeger, L.; Michel, F.; Westhof, E. Involvement of a GNRA tetraloop in long-range RNA tertiary interactions. J. Mol. Boil. 1994, 236, 1271–1276. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Svetlov, V.; Nudler, E. Basic mechanism of transcription by RNA polymerase II. Biochim. Biophys. Acta 2013, 1829, 20–28. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Transition State | Classic PRE | Chimeric Hybrid | Hybrid | Classical POST | ||||
|---|---|---|---|---|---|---|---|---|
| PDB ID (Chain) | 4WPO (CA) | 5F8K (2A) | 4W29 (BA) | 4V9L (BA) | 4V9H (BA) | 4V90 (BA) | 4V67 (BA) | 4V51 (BA) |
| Base-Base interactions | 89 | 86 | 85 | 79 | 86 | 87 | 83 | 85 |
| Multiplets | 17 | 15 | 13 | 14 | 14 | 16 | 15 | 14 |
| Helices | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Stems | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Isolated WC/wobble pairs | 5 | 6 | 5 | 7 | 5 | 5 | 5 | 5 |
| Atom-base-capping interactions | 14 | 13 | 13 | 14 | 12 | 11 | 10 | 15 |
| Splayed-apart dinucleotides | 33 | 31 | 33 | 32 | 36 | 34 | 31 | 35 |
| Hairpin loops | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Bulges | 7 | 8 | 7 | 8 | 8 | 8 | 8 | 8 |
| Internal loops | 3 | 3 | 3 | 4 | 2 | 2 | 2 | 2 |
| Junctions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Non-loop single-stranded segments | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| A-minor (types I and II) motifs | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| eXtended A-minor (type X) motifs | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| Ribose zippers | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 |
| Symmetrical Region Contacts | Mg2+ Ion Number | Class |
|---|---|---|
| P-region | 1 | I |
| 90 | ||
| 101 | ||
| 38 | II | |
| 2 | III | |
| 32 | ||
| 54 | ||
| P-region and A-region | 14 | I |
| 13 | III | |
| A-region | 23 | I |
| 26 | ||
| 9 | II | |
| 37 | ||
| 3 | III | |
| 30 | ||
| 33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, M.; Fox, G.E. Further Characterization of the Pseudo-Symmetrical Ribosomal Region. Life 2020, 10, 201. https://doi.org/10.3390/life10090201
Rivas M, Fox GE. Further Characterization of the Pseudo-Symmetrical Ribosomal Region. Life. 2020; 10(9):201. https://doi.org/10.3390/life10090201
Chicago/Turabian StyleRivas, Mario, and George E. Fox. 2020. "Further Characterization of the Pseudo-Symmetrical Ribosomal Region" Life 10, no. 9: 201. https://doi.org/10.3390/life10090201
APA StyleRivas, M., & Fox, G. E. (2020). Further Characterization of the Pseudo-Symmetrical Ribosomal Region. Life, 10(9), 201. https://doi.org/10.3390/life10090201




