Frailty and Sleep Disorder in Chronic Liver Diseases
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. PSQI-J Score
2.3. Our Study
2.4. Statistics
3. Results
3.1. Patient Baseline Data
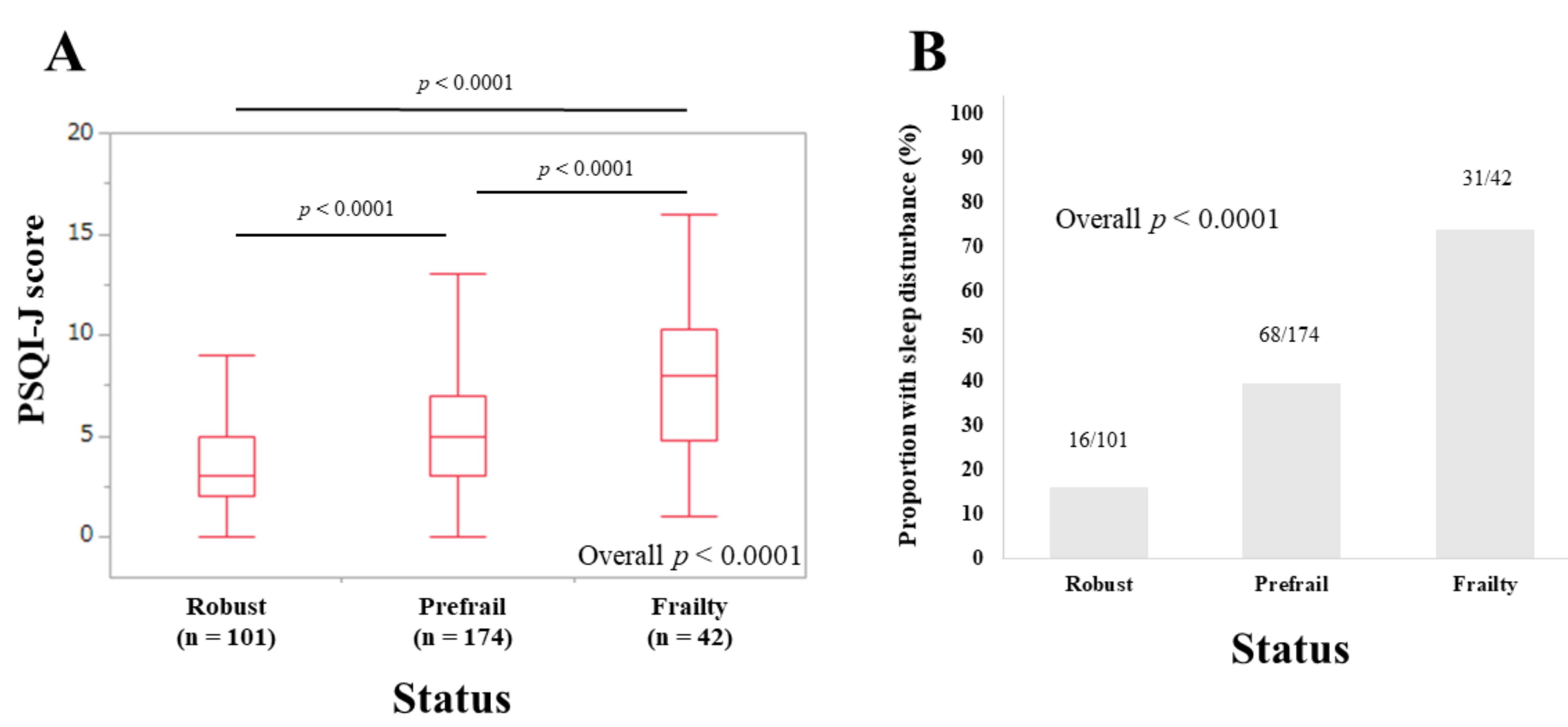
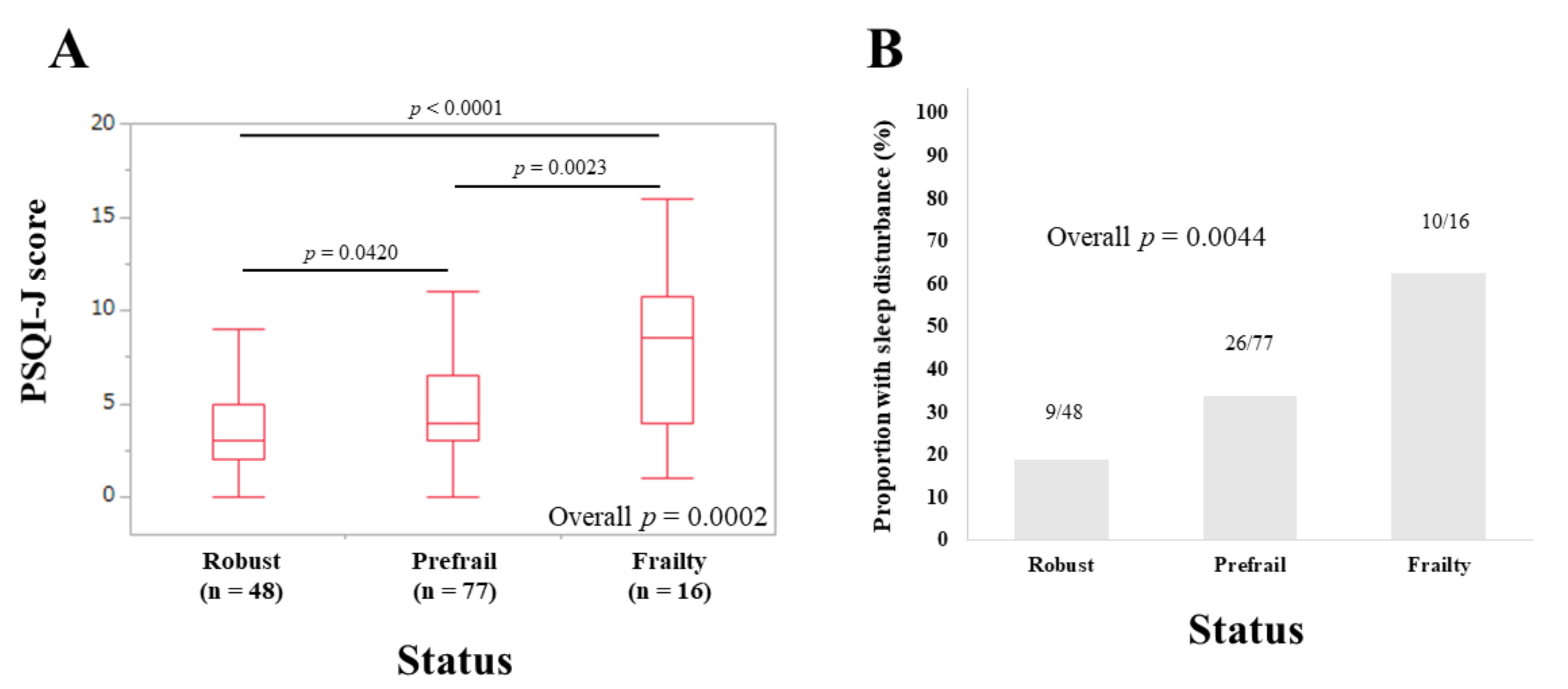
3.2. PSQI-J Scores for the Entire Cohort Stratified by Frailty Status
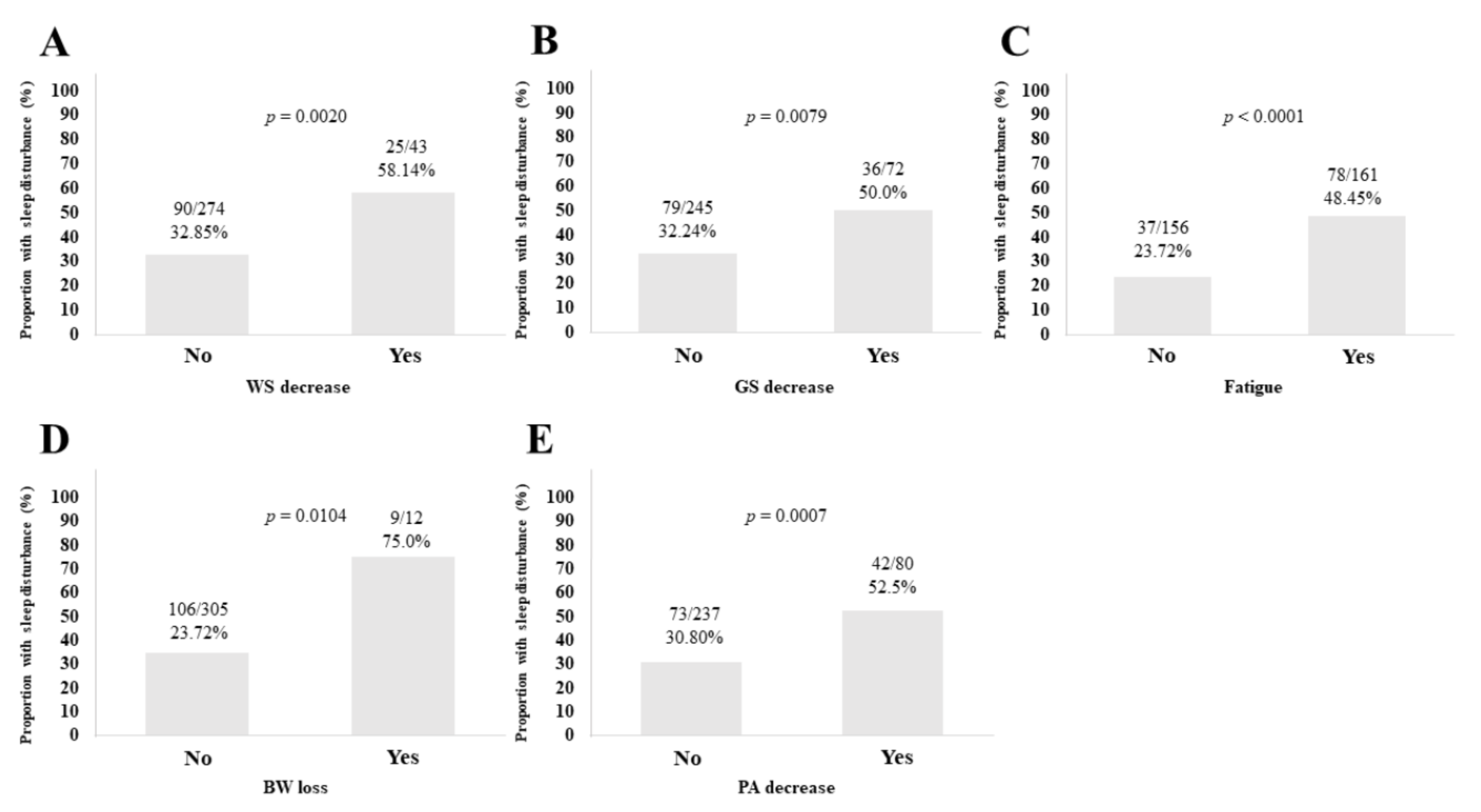
3.3. Sleep Disorder according to the Five Phenotypes of Frailty
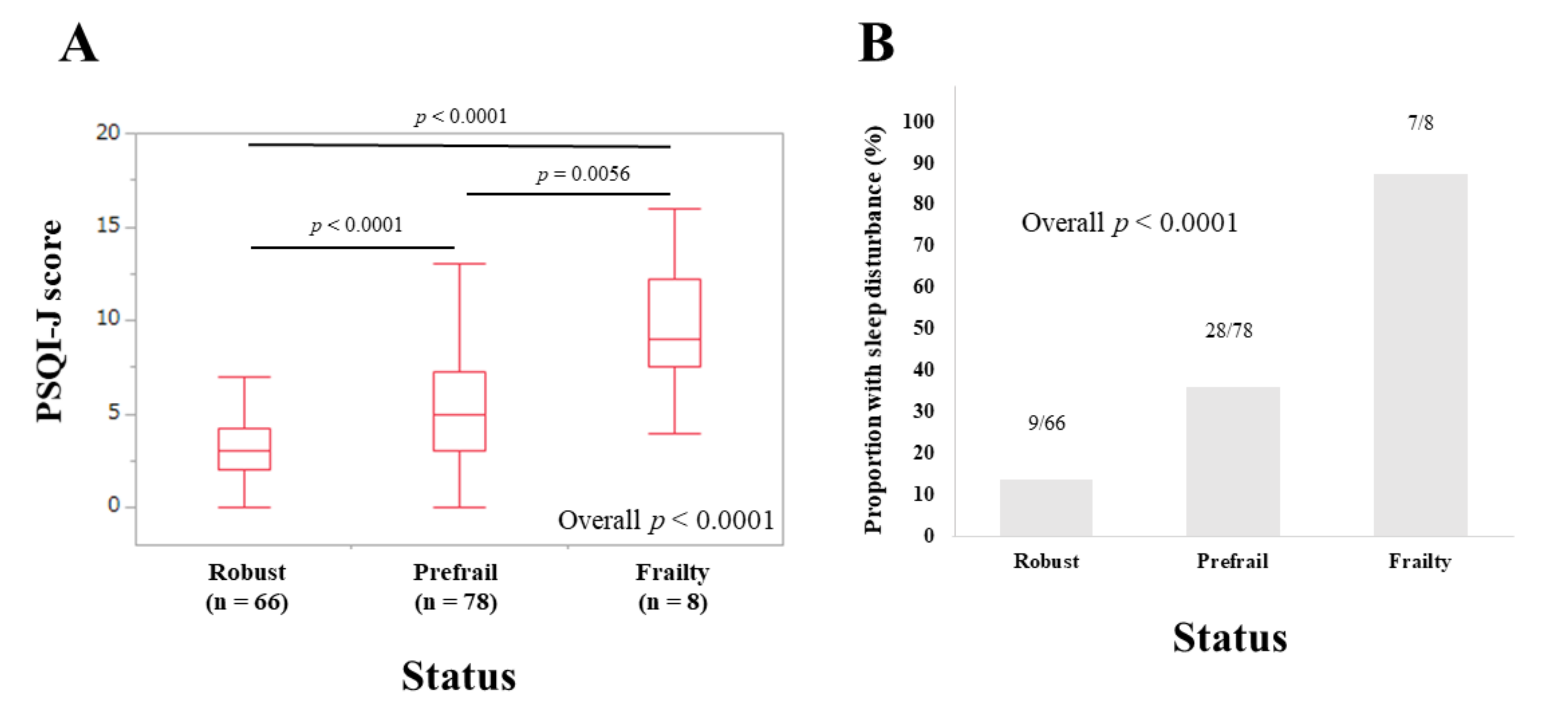
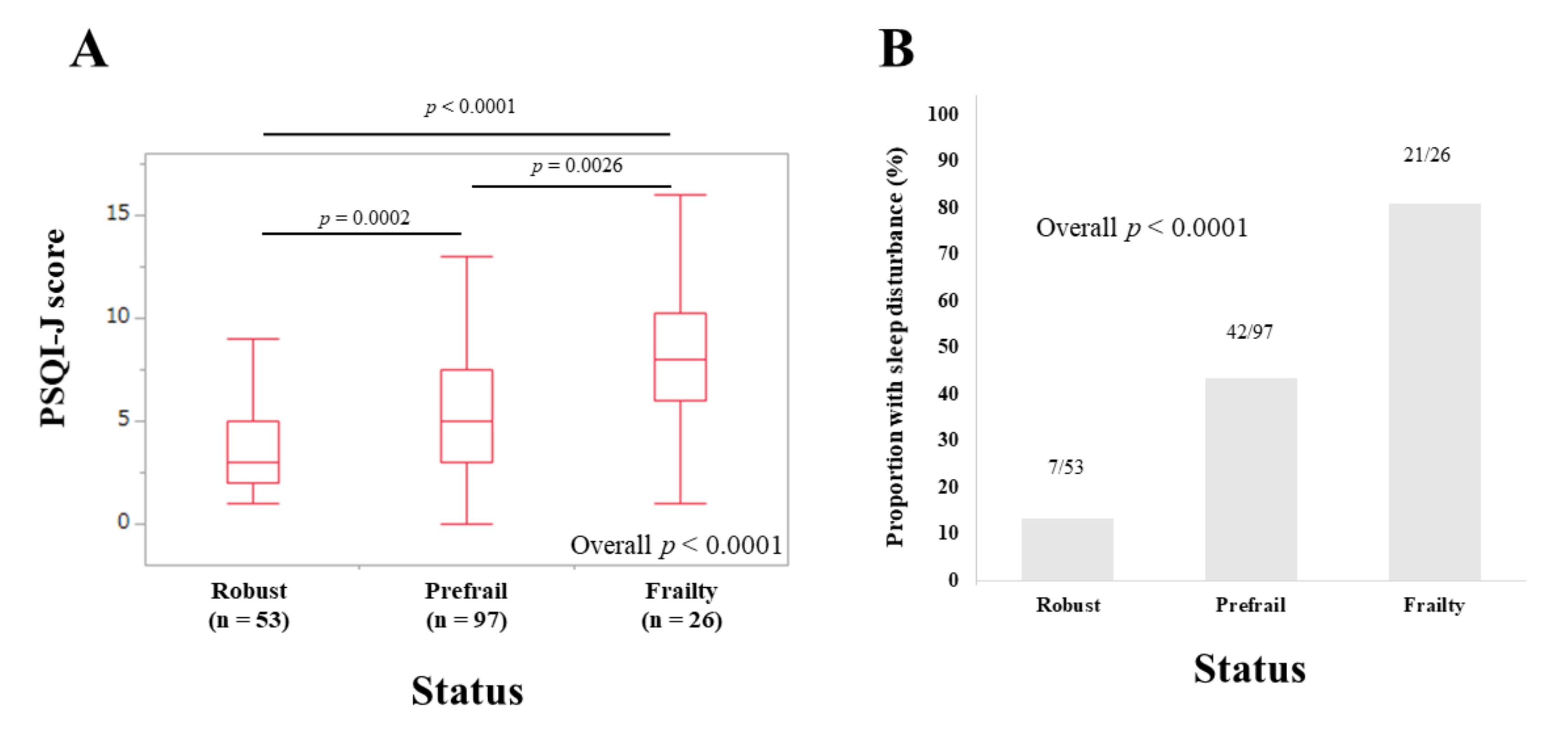
3.4. Subset Analysis 1: PSQI-J Scores for LC Patients Stratified by Frailty Status
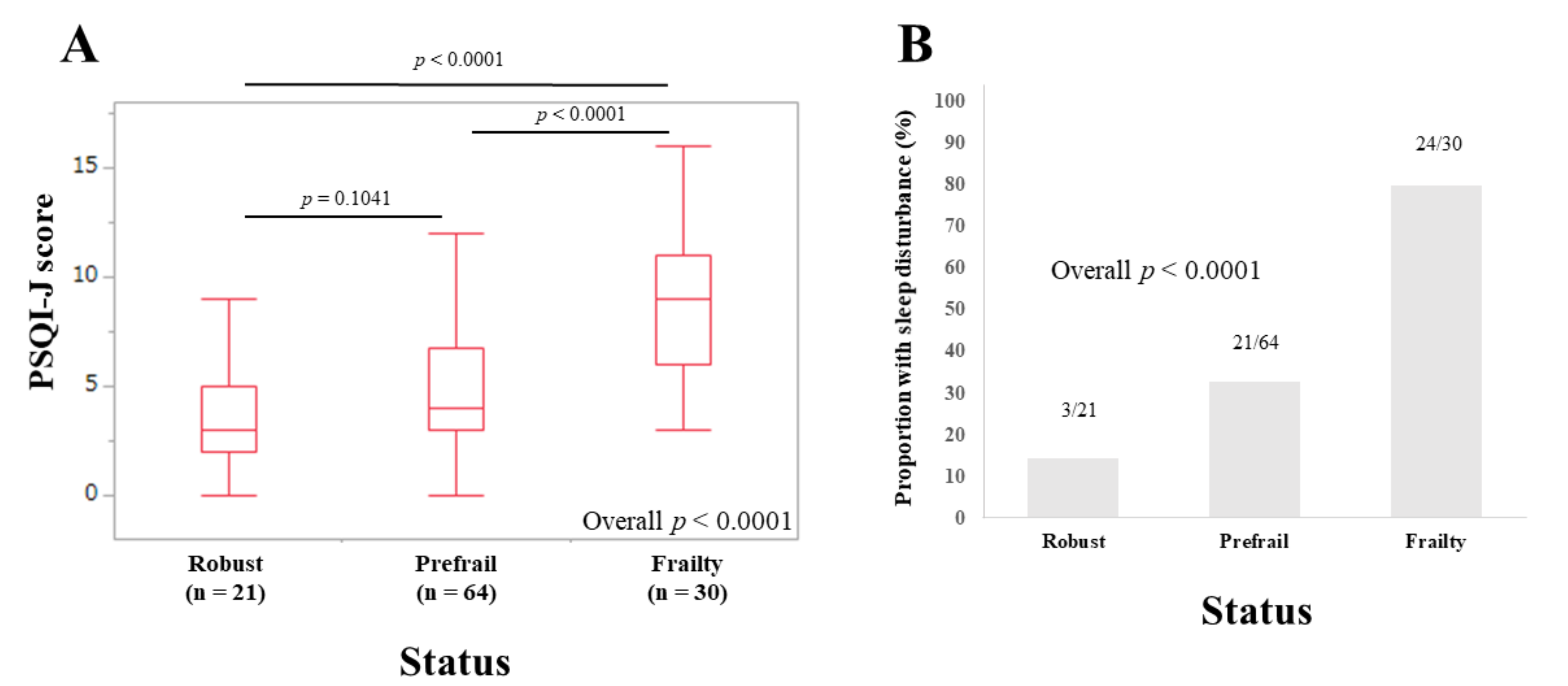
3.5. Subset Analysis 2: PSQI-J Scores for Non-LC Patients Stratified by Frailty Status
3.6. Subset Analysis 3: PSQI-J Scores for Elderly Patients (65 Years or Older) Stratified by Frailty Status
3.7. Subset Analysis 4: PSQI-J Scores for Non-Elderly Patients (<65 Years) Stratified by Frailty Status
3.8. Subset Analysis 5: PSQI-J Scores for Male Patients Stratified by Frailty Status
3.9. Subset Analysis 6: PSQI-J Scores for Female Patients Stratified by Frailty Status
3.10. Univariate and Multivariate Analyses of Factors Linked to Sleep Disorder for All Cases
3.11. Univariate and Multivariate Analyses of Factors Linked to Sleep Disorder according to the LC Status
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro, A.R.; Howlett, S.E.; Fernandes, A. Frailty-A promising concept to evaluate disease vulnerability. Mech. Ageing Dev. 2020, 187, 111217. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. Implications of frailty screening in clinical practice. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 4–10. [Google Scholar] [CrossRef]
- Sewo-Sampaio, P.Y.; Sampaio, R.A.; Yamada, M.; Arai, H. Systematic review of the Kihon Checklist: Is it a reliable assessment of frailty? Geriatr. Gerontol. Int. 2016, 16, 893–902. [Google Scholar] [CrossRef]
- Dunn, M.A.; Rogal, S.S.; Duarte-Rojo, A.; Lai, J.C. Physical Function, Physical Activity, and Quality of Life after Liver Transplantation. Liver Transplant. 2020, 26, 702–708. [Google Scholar] [CrossRef]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Dunn, M.A.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; McCulloch, C.E.; Haugen, C.E. Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J. Hepatol. 2020. (In Press) [CrossRef]
- Lai, J.C.; Dodge, J.L.; McCulloch, C.E.; Covinsky, K.E.; Singer, J.P. Frailty and the Burden of Concurrent and Incident Disability in Patients with Cirrhosis: A Prospective Cohort Study. Hepatol. Commun. 2019, 4, 126–133. [Google Scholar] [CrossRef]
- Hirota, K.; Kawaguchi, T.; Koya, S.; Nagamatsu, A.; Tomita, M.; Hashida, R.; Nakano, D.; Niizeki, T.; Matsuse, H.; Shiba, N.; et al. Clinical utility of the Liver Frailty Index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol. Res. 2020, 50, 330–341. [Google Scholar] [CrossRef]
- Lai, J.C.; Rahimi, R.S.; Verna, E.C.; Kappus, M.R.; Dunn, M.A.; McAdams-DeMarco, M.; Haugen, C.E.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; et al. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019, 156, 1675–1682. [Google Scholar] [CrossRef]
- Dunn, M.A.; Josbeno, D.A.; Tevar, A.D.; Rachakonda, V.; Ganesh, S.R.; Schmotzer, A.R.; Kallenborn, E.A.; Behari, J.; Landsittel, D.P.; DiMartini, A.F.; et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am. J. Gastroenterol. 2016, 111, 1768–1775. [Google Scholar] [CrossRef]
- Anderson, K.; Jones, D.E.; Wilton, K.; Newton, J.L. Restless leg syndrome is a treatable cause of sleep disturbance and fatigue in primary biliary cirrhosis. Liver Int. 2013, 33, 239–243. [Google Scholar] [CrossRef]
- Ozsahin, M.; Gonen, I.; Ermis, F.; Oktay, M.; Besir, F.H.; Kutlucan, A.; Sahin, A.; Ataoglu, S. The prevalence of fibromyalgia among patients with hepatitis B virus infection. Int. J. Clin. Exp. Med. 2013, 6, 804–808. [Google Scholar] [PubMed]
- Pennisi, M.; Bertino, G.; Gagliano, C.; Malaguarnera, M.; Bella, R.; Borzì, A.M.; Madeddu, R.; Drago, F.; Malaguarnera, G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients 2017, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; et al. Comparison of sleep disorders in chronic hepatitis C patients treated with interferon-based therapy and direct acting antivirals using actigraphy. Hepatol. Res. 2016, 46, 1358–1366. [Google Scholar] [CrossRef]
- Mir, H.M.; Stepanova, M.; Afendy, H.; Cable, R.; Younossi, Z.M. Association of Sleep Disorders with Nonalcoholic Fatty Liver Disease (NAFLD): A Population-based Study. J. Clin. Exp. Hepatol. 2013, 3, 181–185. [Google Scholar] [CrossRef]
- Erlangsen, A.; Runeson, B.; Bolton, J.M.; Wilcox, H.C.; Forman, J.L.; Krogh, J.; Shear, M.K.; Nordentoft, M.; Conwell, Y. Association Between Spousal Suicide and Mental, Physical, and Social Health Outcomes: A Longitudinal and Nationwide Register-Based Study. JAMA Psychiatry 2017, 74, 456–464. [Google Scholar] [CrossRef]
- Chou, Y.T.; Cheng, H.J.; Wu, J.S.; Yang, Y.C.; Chou, C.Y.; Chang, C.J.; Lu, F.H. The association of sleep duration and sleep quality with non-alcoholic fatty liver disease in a Taiwanese population. Obes. Res. Clin. Pract. 2018, 12, 500–505. [Google Scholar] [CrossRef]
- Labenz, C.; Baron, J.S.; Toenges, G.; Schattenberg, J.M.; Nagel, M.; Sprinzl, M.F.; Nguyen-Tat, M.; Zimmermann, T.; Huber, Y.; Marquardt, J.U.; et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment. Pharmacol. Ther. 2018, 48, 313–321. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Weisskopf, D.M.; Pflueger, M.O.; Mosimann, J.; Campana, B.; Terracciano, L.; Beglinger, C.; Heim, M.H.; Cajochen, C. Sleep Disruption and Daytime Sleepiness Correlating with Disease Severity and Insulin Resistance in Non-Alcoholic Fatty Liver Disease: A Comparison with Healthy Controls. PLoS ONE 2015, 10, e0143293. [Google Scholar] [CrossRef]
- Iwasa, M.; Karino, Y.; Kawaguchi, T.; Nakanishi, H.; Miyaaki, H.; Shiraki, M.; Nakajima, T.; Sawada, Y.; Yoshiji, H.; Okita, K.; et al. Relationship of muscle cramps to quality of life and sleep disturbance in patients with chronic liver diseases: A nationwide study. Liver Int. 2018, 38, 2309–2316. [Google Scholar] [CrossRef]
- Ghabril, M.; Jackson, M.; Gotur, R.; Weber, R.; Orman, E.; Vuppalanchi, R.; Chalasani, N. Most Individuals With Advanced Cirrhosis Have Sleep Disturbances, Which Are Associated With Poor Quality of Life. Clin. Gastroenterol. Hepatol. 2017, 15, 1271–1278. [Google Scholar] [CrossRef]
- Cordoba, J.; Cabrera, J.; Lataif, L.; Penev, P.; Zee, P.; Blei, A.T. High prevalence of sleep disturbance in cirrhosis. Hepatology 1998, 27, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Effect of Sarcopenia on Sleep Disturbance in Patients with Chronic Liver Diseases. J. Clin. Med. 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Vaz Fragoso, C.A.; Gahbauer, E.A.; Van Ness, P.H.; Gill, T.M. Sleep-wake disturbances and frailty in community-living older persons. J. Am. Geriatr. Soc. 2009, 57, 2094–2100. [Google Scholar] [CrossRef]
- Cochen, V.; Arbus, C.; Soto, M.E.; Villars, H.; Tiberge, M.; Montemayor, T.; Hein, C.; Veccherini, M.F.; Onen, S.H.; Ghorayeb, I.; et al. Sleep disorders and their impacts on healthy, dependent, and frail older adults. J. Nutr. Health Aging. 2009, 13, 322–329. [Google Scholar] [CrossRef]
- Endeshaw, Y.W.; Unruh, M.L.; Kutner, M.; Newman, A.B.; Bliwise, D.L. Sleep-disordered breathing and frailty in the Cardiovascular Health Study Cohort. Am. J. Epidemiol. 2009, 170, 193–202. [Google Scholar] [CrossRef]
- Nakakubo, S.; Doi, T.; Makizako, H.; Tsutsumimoto, K.; Kurita, S.; Kim, M.; Ishii, H.; Suzuki, T.; Shimada, H. Association of sleep condition and social frailty in community-dwelling older people. Geriatr. Gerontol. Int. 2019, 19, 885–889. [Google Scholar] [CrossRef]
- Sampaio, R.A.; Sewo-Sampaio, P.Y.; Yamada, M.; Tsuboyama, T.; Arai, H. Self-reported quality of sleep is associated with bodily pain, vitality and cognitive impairment in Japanese older adults. Geriatr. Gerontol. Int. 2014, 14, 628–635. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. Chapter 1 Frailty: Definition, diagnosis, epidemiology. Geriatr. Gerontol. Int. 2020, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Auyeung, T.W.; Leung, J.; Chan, D.; Kwok, T.; Woo, J.; Wing, Y.K. Long sleep duration is associated with higher mortality in older people independent of frailty: A 5-year cohort study. J. Am. Med. Dir. Assoc. 2014, 15, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Blackwell, T.L.; Ancoli-Israel, S.; Redline, S.; Cawthon, P.M.; Paudel, M.L.; Dam, T.T.; Stone, K.L. Sleep disturbances and risk of frailty and mortality in older men. Sleep. Med. 2012, 13, 1217–1225. [Google Scholar] [CrossRef]
- Moreno-Tamayo, K.; Manrique-Espinoza, B.; Rosas-Carrasco, O.; Pérez-Moreno, A.; Salinas-Rodríguez, A. Sleep complaints are associated with frailty in Mexican older adults in a rural setting. Geriatr. Gerontol. Int. 2017, 17, 2573–2578. [Google Scholar] [CrossRef]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; O’Connor, P.J.; Dishman, R.K. The effects of acute exercise on sleep: A quantitative synthesis. Sleep 1997, 20, 203–214. [Google Scholar] [CrossRef]
- Singh, N.A.; Clements, K.M.; Fiatarone, M.A. A randomized controlled trial of the effect of exercise on sleep. Sleep 1997, 20, 95–101. [Google Scholar] [CrossRef]


| Variable | All Cases (n = 317) | |
|---|---|---|
| Age, years | Median (IQR) | 65 (54, 72) |
| Gender | Male Female | 141 (44.5%) 176 (55.5%) |
| Liver disease etiology | HCV HBV Other | 169 (53.3%) 56 (17.7%) 92 (29.0%) |
| Presence of frailty | Yes No | 42 (13.2%) 275 (86.8%) |
| Presence of LC | Yes No | 115 (36.3%) 202 (63.7%) |
| Presence of ascites | Yes | 13 (4.1%) |
| No | 304 (95.9%) | |
| Presence of overt hepatic encephalopathy | Yes | 0 |
| No | 317 (100%) | |
| Body mass index, kg/m2 | Median (IQR) | 22.9 (20.55, 25.8) |
| Walking speed, m/s | Median (IQR) | 1.32 (1.14, 1.46) |
| Grip strength, kg, male | Median (IQR) | 33.8 (28.3, 39.9) |
| Grip strength, kg, female | Median (IQR) | 20.9 (17.6, 24.5) |
| Total bilirubin, mg/dL | Median (IQR) | 0.8 (0.7, 1.1) |
| Serum albumin, g/dL | Median (IQR) | 4.3 (4.0, 4.5) |
| ALBI score | Median (IQR) | −2.9 (−3.1, −2.6) |
| ALBI grade, n (%) | 1 2 3 | 241 (76.0%) 69 (21.8%) 7 (2.2%) |
| Prothrombin time, % | Median (IQR) | 91.1 (80.55, 98.6) |
| Platelet count, ×104/mm3 | Median (IQR) | 17.7 (12.6, 21.85) |
| AST, IU/L | Median (IQR) | 25 (19.5, 33.5) |
| ALT, IU/L | Median (IQR) | 19 (14, 33) |
| Total cholesterol, mg/dL | Median (IQR) | 182.5 (152, 214.75) |
| PSQI-J score | Median (IQR) | 4 (3, 7) |
| Variables | Univariate p-Value | Multivariate (OR, Per One Unit) | ||
|---|---|---|---|---|
| OR | 95%CI | p-Value | ||
| Age, Years | 0.0111 | 1.048 | 0.988–1.030 | 0.4049 |
| Gender, male/female | 0.1597 | |||
| Presence of LC, yes/no | 0.1452 | |||
| Etiology, HBV/HCV/others | 0.7782 | |||
| Body mass index, kg/m2 | 0.3497 | |||
| Total bilirubin, mg/dL | 0.5499 | |||
| Serum albumin, g/dL | 0.0708 | |||
| ALBI score | 0.0825 | 1.020 | 0.619–1.683 | 0.9372 |
| Prothrombin time, % | 0.8128 | |||
| Platelet count, ×104/mm3 | 0.8237 | |||
| AST, IU/L | 0.8981 | |||
| ALT, IU/L | 0.5496 | |||
| Total cholesterol, mg/dL | 0.8548 | |||
| Frailty score | <0.0001 | 1.939 | 1.505–2.498 | <0.0001 |
| LC Patients | Non-LC Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate p-Value | Multivariate (OR, Per One Unit) | Univariate p-Value | Multivariate (OR, Per One Unit) | |||||
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |||
| Age, years | 0.2562 | 0.0557 | 1.010 | 0.984-1.037 | 0.4549 | |||
| Gender, male/female | 0.1293 | 0.4502 | ||||||
| Etiology, HBV/HCV/others | 0.2306 | 0.6555 | ||||||
| Body mass index, kg/m2 | 0.4048 | 0.6501 | ||||||
| Total bilirubin, mg/dL | 0.9895 | 0.9537 | ||||||
| Serum albumin, g/dL | 0.0898 | 0.954 | 0.454–2.006 | 0.9012 | 0.3658 | |||
| ALBI score | 0.2494 | 0.3755 | ||||||
| Prothrombin time, % | 0.4484 | 0.8401 | ||||||
| Platelet count, ×104/mm3 | 0.1005 | 0.6948 | ||||||
| AST, IU/L | 0.7656 | 0.5099 | ||||||
| ALT, IU/L | 0.7553 | 0.3735 | ||||||
| Total cholesterol, mg/dL | 0.5783 | 0.9321 | ||||||
| Frailty score | <0.0001 | 2.476 | 1.626–3.772 | <0.0001 | 0.0002 | 1.711 | 1.212–2.416 | 0.0023 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Yoh, K.; Enomoto, H.; Iwata, Y.; Nishimura, T.; Nishiguchi, S.; Iijima, H. Frailty and Sleep Disorder in Chronic Liver Diseases. Life 2020, 10, 137. https://doi.org/10.3390/life10080137
Nishikawa H, Yoh K, Enomoto H, Iwata Y, Nishimura T, Nishiguchi S, Iijima H. Frailty and Sleep Disorder in Chronic Liver Diseases. Life. 2020; 10(8):137. https://doi.org/10.3390/life10080137
Chicago/Turabian StyleNishikawa, Hiroki, Kazunori Yoh, Hirayuki Enomoto, Yoshinori Iwata, Takashi Nishimura, Shuhei Nishiguchi, and Hiroko Iijima. 2020. "Frailty and Sleep Disorder in Chronic Liver Diseases" Life 10, no. 8: 137. https://doi.org/10.3390/life10080137
APA StyleNishikawa, H., Yoh, K., Enomoto, H., Iwata, Y., Nishimura, T., Nishiguchi, S., & Iijima, H. (2020). Frailty and Sleep Disorder in Chronic Liver Diseases. Life, 10(8), 137. https://doi.org/10.3390/life10080137




