Nocturnal Lagophthalmos and Sleep Quality in Patients with Dry Eye Disease
Abstract
1. Introduction
2. Methods
2.1. Patient Recruitment
2.2. Ethics Statements
2.3. Questionnaire
2.4. Data and Statistical Analyses
3. Results
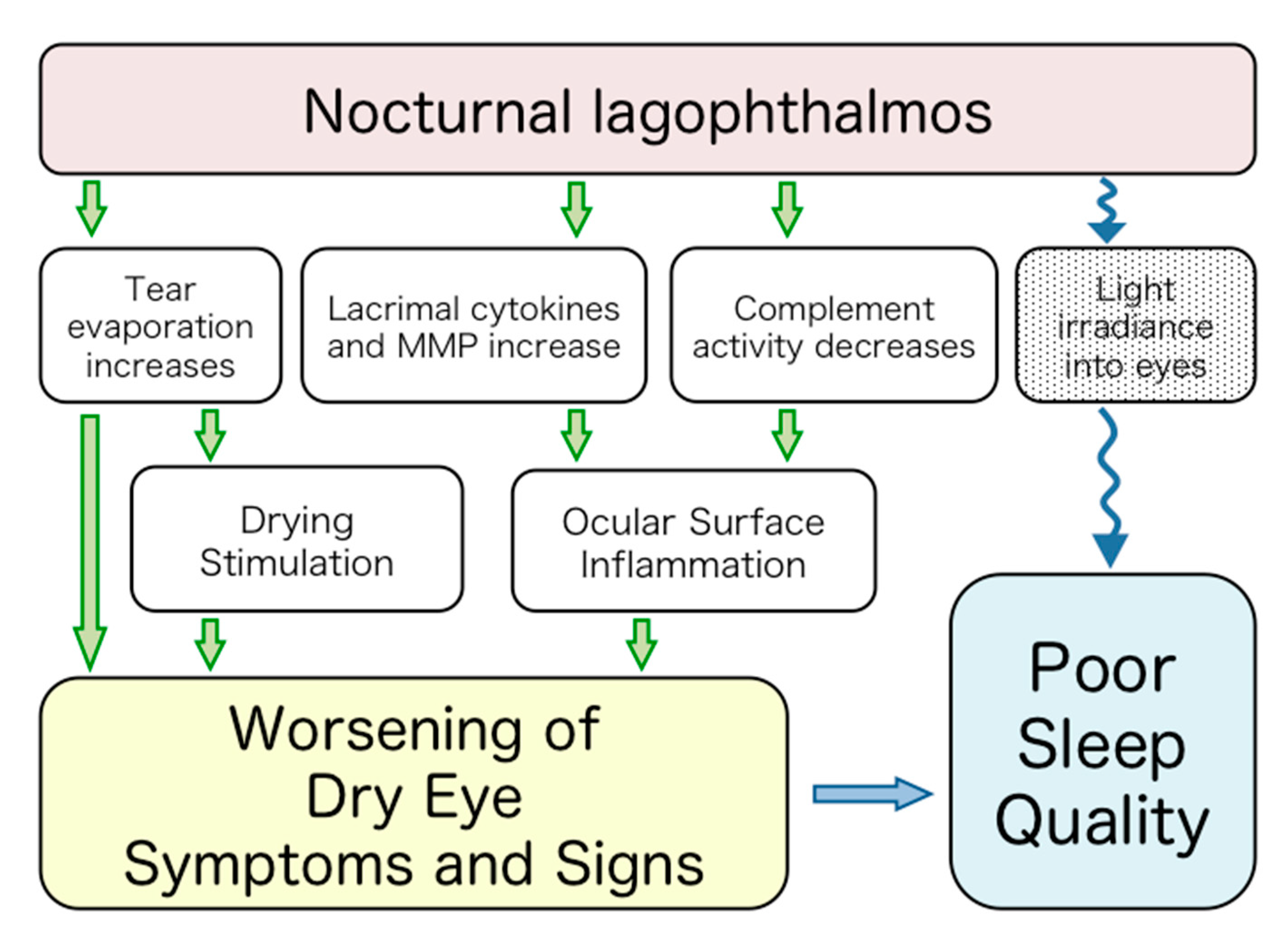
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, S.H.; Yeh, S.I.; Chen, L.J.; Wu, C.H.; Liao, S.L. Nocturnal Lagophthalmos. Int. J. Gerontol. 2009, 3, 89–95. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Matossian, C.; McDonald, M.; Donaldson, K.E.; Nichols, K.K.; MacIver, S.; Gupta, P.K. Dry Eye Disease: Consideration for Women’s Health. J. Women’s Health 2019, 28, 502–514. [Google Scholar] [CrossRef]
- Clayton, J.A.; Albeitz, J.; Begley, C.; Caffery, B.; Nichols, K.; Schaumberg, D.A.; Schein, O. The epidemiology of DED disease: Report of the Epidemiology Subcommittee of the International DED WorkShop. Ocul. Surf. 2007, 5, 93–107. [Google Scholar]
- Gulati, A.; Sullivan, R.; Buring, J.E.; Sullivan, D.A.; Dana, R.; Schaumberg, D.A. Validation and Repeatability of a Short Questionnaire for Dry Eye Syndrome. Am. J. Ophthalmol. 2006, 142, 125–131. [Google Scholar] [CrossRef]
- Lyons, C.J.; McNab, A.A. Symptomatic nocturnal lagophthalmos. Aust. N. Z. J. Ophthalmol. 1990, 18, 393–396. [Google Scholar] [CrossRef]
- Duke-Elder, S. System of Ophthalmology; Kimpton, H.: London, UK, 1964; p. 887. [Google Scholar]
- Sturrock, G.D. Nocturnal lagophthalmos and recurrent erosion. Br. J. Ophthalmol. 1976, 60, 97–103. [Google Scholar] [CrossRef]
- Fuchs, A.; Wu, F.C. Sleep with half-open eyes, physiologic lagophthalmus. Am. J. Ophthalmol. 1948, 31, 717–720. [Google Scholar] [CrossRef]
- Howitt, D.A.; Goldstein, J.H. Physiologic lagophthalmos. Am. J. Ophthalmol. 1969, 68, 355. [Google Scholar] [CrossRef]
- Mueller, F.O. Lagophthalmos during sleep. Br. J. Ophthalmol. 1967, 51, 246–248. [Google Scholar] [CrossRef] [PubMed]
- McNab, A.A. The eye and sleep. Clin. Exp. Ophthalmol. 2005, 33, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bierman, A.; Figueiro, M.G.; Rea, M.S. Measuring and predicting eyelid spectral transmittance. J. Biomed. Opt. 2011, 16, 067011. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.; Morris, C.A.; Thakur, A.; Sack, R.A.; Wickson, J.; Boey, W. Complement and complement regulatory proteins in human tears. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1–8. [Google Scholar]
- Sack, R.A.; Beaton, A.; Sathe, S.; Morris, C.; Willcox, M.; Bogart, B. Towards a closed eye model of the pre-ocular tear layer. Prog. Retinal Eye Res. 2000, 19, 649–668. [Google Scholar] [CrossRef]
- Sack, R.A.; Conradi, L.; Krumholz, D.; Beaton, A.; Sathe, S.; Morris, C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: Angiogenin and other defense system constituents. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Kaufman, H.E. Corneal exposure during sleep (nocturnal lagophthalmos). Arch. Ophthalmol. (Chicago IL. 1960) 1977, 95, 449–453. [Google Scholar] [CrossRef]
- Small, R.G.; Fransen, S.R.; Adams, R.; Owen, W.L.; Taylor, R.B., 3rd. The effect of phenylephrine on Müller muscle. A blepharogram study of eyelid motion. Ophthalmolog 1995, 102, 599–606. [Google Scholar] [CrossRef]
- Takamoto, K.; Hori, E.; Urakawa, S.; Katayama, M.; Nagashima, Y.; Yada, Y.; Ono, T.; Nishijo, H. Thermotherapy to the facial region in and around the eyelids altered prefrontal hemodynamic responses and autonomic nervous activity during mental arithmetic. Psychophysiology 2013, 50, 35–47. [Google Scholar] [CrossRef]
- Tanida, M.; Niijima, A.; Shen, J.; Nakamura, T.; Nagai, K. Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neurosci. Lett. 2006, 398, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ely, B.R.; Francisco, M.A.; Halliwill, J.R.; Bryan, S.D.; Comrada, L.N.; Larson, E.A.; Brunt, V.E.; Minson, C.T. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in obese women with polycystic ovary syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, 630–640. [Google Scholar] [CrossRef]
- Farrand, K.F.; Fridman, M.; Stillman, I.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Kawashima, M.; Negishi, K.; Kishimoto, T.; Mimura, M.; Tsubota, K. Sleep and mood disorders in dry eye disease and allied irritating ocular diseases. Sci. Rep. 2016, 6, 22480. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Tsubota, K.; Kawashima, M.; Kishimoto, T.; Mimura, M.; Negishi, K. Sleep Disorders are a Prevalent and Serious Comorbidity in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 143–150. [Google Scholar] [CrossRef]
- Ayaki, M.; Kawashima, M.; Negishi, K.; Tsubota, K. High prevalence of sleep and mood disorders in dry eye patients: Survey of 1,000 eye clinic visitors. Neuropsychiatr. Dis. Treat. 2015, 11, 889–894. [Google Scholar] [CrossRef]
- Kawashima, M.; Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Tsubota, K. The association of sleep quality with dry eye disease: The Osaka study. Clinic. Ophthalmol. (Auckl. N. Z.) 2016, 10, 1015–1021. [Google Scholar] [CrossRef]
- Yu, X.; Guo, H.; Liu, X.; Wang, G.; Min, Y.; Chen, S.S.; Han, S.S.; Chang, R.T.; Zhao, X.; Hsing, A.; et al. Dry eye and sleep quality: A large community-based study in Hangzhou. Sleep 2019, 42, 160. [Google Scholar] [CrossRef]
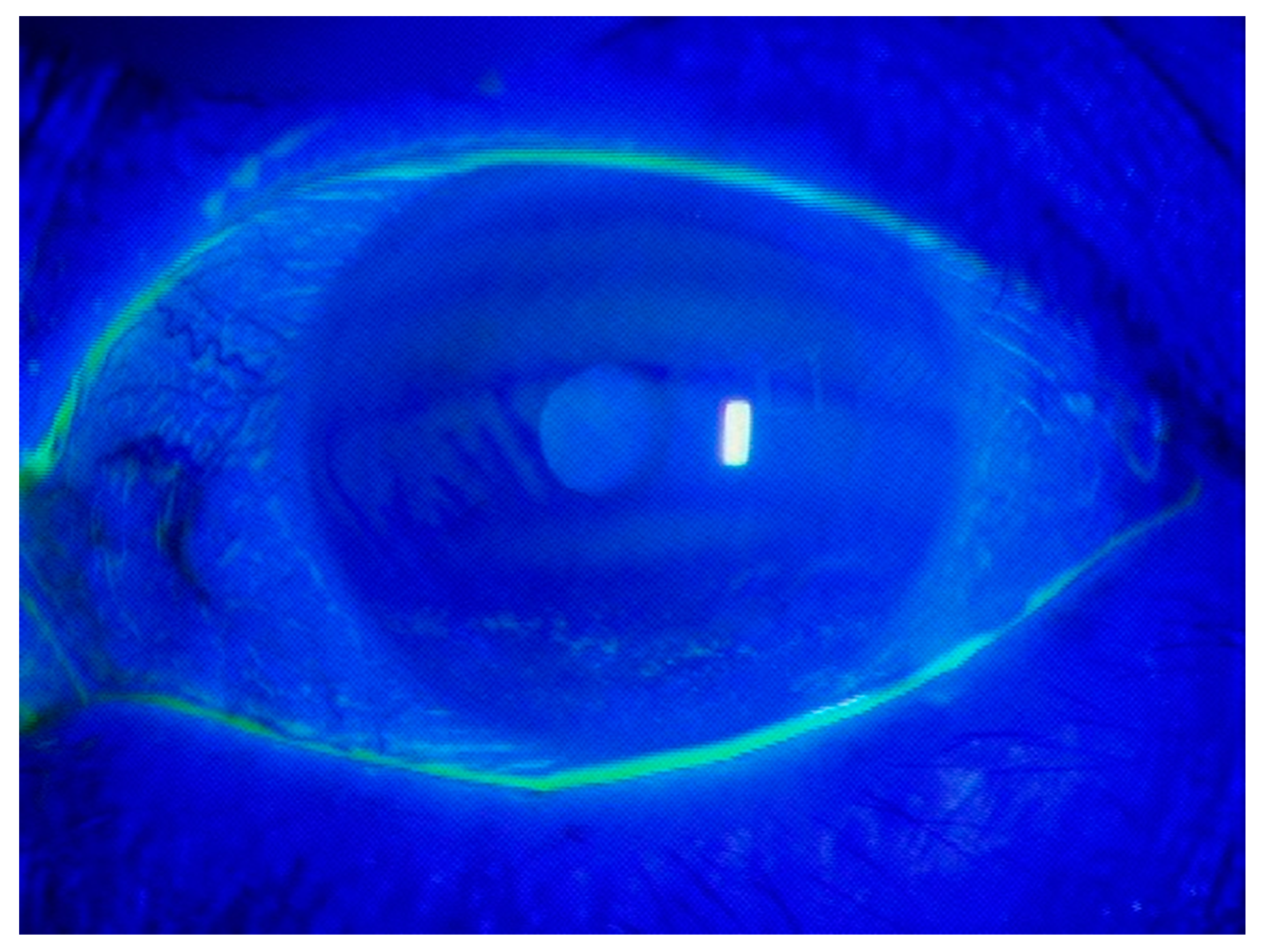
| Question | Possible Answers |
|---|---|
| 1. Height | m |
| 2. Weight | kg |
| Dry Eye | |
| D3. “How often do you feel dryness in your eye?” | 1: constantly, 2: sometimes, 3: rarely, 4: never |
| D4. “How often do you have a sensation of a foreign body in your eye?” | 1: constantly, 2: sometimes, 3: rarely, 4: never |
| D5. “Have you been diagnosed as having DED?” | 1: yes, 2: no |
| D6. “Do you have a dry mouth?” | 1: constantly, 2: sometimes; 3: rarely, 4: never |
| Sleep | |
| S7. “How would you rate the quality of your sleep?” | 1: great, 2: good, 3: average, 4: not good, 5: poorly |
| S8. “At what time do you get up during weekdays?” | Time |
| S9. “At what time do you go to bed during weekdays?” | Time |
| S10. “How long does it take you to fall asleep after you go to bed?” | Duration (minutes) |
| S11. “Are your eyes open during sleep?” | 1: yes, 2: no, 3: not sure |
| Morning eye symptom | |
| M12. “Do you wake up to eye pain during sleep?” | 1: never, 2: once a week, 3: twice to four times a week, 4: every night |
| M13. “How do your eyes feel when you wake up?” | 1: good, 2: not good, 3: have difficulty opening, 4: feel foreign body sensation, 5: feel pain, 6: eye discharge, 7: lacrimation, 8: dry, 9: sensitive to light, 10: others, multiple answers allowed |
| M14. “Do you take eye drops after waking or for sleeping?” | 1: at waking, 2: at sleeping, 3: neither |
| Lifestyle | |
| L15. “How often do you have a night shift?” | Time per month |
| L16. “How long do you spend outside during daytime?” | 1: less than one h, 2: 1 h, 3: 2 h, 4: 3 h, 5: 4 h, 6: 5 h, 7: 6 h, 8: 7 h, 9: 8 h, 10: 9 h, 11: more than 10 h |
| L17. “How would you rate your happiness?” | 7–1: happiest–unhappiest |
| NL (+) n = 90 (5%) | NL (−) n = 1174 (59%) | p-Value | |
|---|---|---|---|
| Age (years) | 40.5 ± 13.8 | 44.8 ± 13.9 | 0.004 * |
| Gender (male/female) | 42/48 | 574/600 | 0.68 |
| BMI | 22.5 ± 3.8 | 22.2 ± 3.7 | 0.50 |
| Sleep Parameters | |||
| Sleep latency (minutes) | 26.4 ± 27.5 | 22.3 ± 22.1 | 0.10 |
| Sleep efficacy (%) | 93.3 ± 6.5 | 94.3 ± 5.7 | 0.13 |
| Sleep duration (minutes) | 370 ± 64 | 377 ± 73 | 0.37 |
| Subjective sleep quality | 2.83 ± 1.07 | 2.63 ± 1.02 | 0.07 |
| Subjective happiness | 4.2 ± 1.4 | 4.4 ± 1.5 | 0.29 |
| Outdoor time (hours) | 3.5 ± 2.3 | 3.1 ± 2.3 | 0.16 |
| Night shift (times/month) | 1.76 ± 5.4 | 0.70 ± 0.5 | 0.002 * |
| Number of symptoms of eyes upon waking | 1.09 ± 1.3 | 0.71 ± 0.92 | <0.001 * |
| Symptoms in Detail | |||
| None | 39 (43.3%) | 592 (50.5%) | 0.189 |
| Difficulty opening eyes | 13 (14.4%) | 63 | <0.001 ** |
| Foreign body sensation | 18 | 95 | <0.001 ** |
| Eye pain | 6 | 29 | 0.020 ** |
| Discharge | 22 | 276 | 0.85 |
| Tears | 8 | 57 | 0.10 |
| Dryness | 8 | 103 | 0.97 |
| Photophobia | 3 | 98 | 0.09 |
| DED (+) n = 890 (45%) | DED (−) n = 1110 (56%) | p-Value | |
|---|---|---|---|
| Age (years) | 44.0 ± 13.8 | 45.2 ± 13.9 | 0.05 |
| Gender (male/female) | 359/531 | 641/469 | <0.001 ** |
| BMI (kg/m2) | 22.2 ± 4.0 | 22.4 ± 3.7 | 0.26 |
| Sleep Parameters | |||
| Sleep latency (minutes) | 25.5 ± 24.6 | 21.6 ± 21.3 | <0.001 * |
| Sleep efficacy (%) | 93.4 ± 6.5 | 94.5 ± 5.7 | <0.001 * |
| Sleep duration (minutes) | 368 ± 74 | 377 ± 76 | 0.008 * |
| Subjective sleep quality | 2.91 ± 1.02 | 2.60 ± 1.04 | <0.001 *** |
| Subjective happiness | 4.2 ± 1.5 | 4.3 ± 1.5 | 0.04 * |
| Outdoor activity time (hours) | 3.3 ± 2.5 | 3.1 ± 2.4 | 0.05 |
| Night shift (times/month) | 0.96 ± 3.2 | 0.63 ± 2.9 | 0.02 * |
| Nocturnal lagophthalmos | 49 (5.4%) | 41(3.7%) | 0.007 * |
| Dryness of mouth | 398 (44.7%) | 415 (37.4%) | 0.001 ** |
| Number of symptoms of eyes upon waking | 1.24 ± 1.1 | 0.47 ± 0.43 | <0.001 * |
| Symptoms in Detail | |||
| None | 225 (24.7%) | 687 (75.3%) | <0.001 ** |
| Difficulty opening eyes | 90 | 36 | <0.001 ** |
| Foreign body sensation | 171 | 32 | <0.001 ** |
| Eye pain | 14 | 49 | <0.001 ** |
| Discharge | 301 | 204 | <0.001 ** |
| Tears | 69 | 45 | <0.001 ** |
| Dryness | 145 | 41 | <0.001 ** |
| Photophobia | 102 | 56 | <0.001 ** |
| Variables | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age # | 0.97 | 0.96–0.99 | 0.005 * |
| Gender (1: male, 0: female) # | 0.94 | 0.58–1.35 | 0.78 |
| BMI (kg/m2) | 1.03 | 0.97–1.09 | 0.30 |
| DED (1: (+), 0: (−)) | 1.75 | 1.12–2.71 | 0.01 * |
| Sleep parameters | |||
| Sleep latency (minutes) | 1.00 | 0.99–1.01 | 0.55 |
| Sleep efficacy (%) | 1.00 | 0.96–1.04 | 0.94 |
| Sleep duration (minutes) | 1.00 | 0.99–1.00 | 0.11 |
| Subjective sleep quality | 1.16 | 0.94–1.43 | 0.16 |
| Subjective happiness | 1.07 | 0.92–1.26 | 0.35 |
| Outdoor time | 1.06 | 0.98–1.15 | 0.16 |
| Night shift (times/month) | 0.98 | 0.91–1.06 | 0.62 |
| Dryness of mouth (1: (+), 0: (−)) | 1.00 | 0.65–1.56 | 0.99 |
| Number of symptoms of eyes upon awakening | 1.23 | 1.03–1.47 | 0.04 * |
| Symptoms in detail | |||
| Difficulty opening eyes (1: (+), 0: (−)) | 1.99 | 1.00–3.95 | 0.049 * |
| Foreign body sensation (1: (+), 0: (−)) | 1.00 | 0.47–2.14 | 1.00 |
| Eye pain (1: (+), 0: (−)) | 2.71 | 1.09–6.75 | 0.03 * |
| Discharge (1: (+), 0: (−)) | 1.37 | 0.85–2.19 | 0.20 |
| Tears (1: (+), 0: (−)) | 0.82 | 0.29–2.30 | 0.70 |
| Dryness (1: (+), 0: (−)) | 1.73 | 0.94–3.19 | 0.08 |
| Photophobia (1: (+), 0: (−)) | 1.11 | 0.52–2.37 | 0.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, A.; Negishi, K.; Ayaki, M.; Uchino, M.; Tsubota, K. Nocturnal Lagophthalmos and Sleep Quality in Patients with Dry Eye Disease. Life 2020, 10, 105. https://doi.org/10.3390/life10070105
Takahashi A, Negishi K, Ayaki M, Uchino M, Tsubota K. Nocturnal Lagophthalmos and Sleep Quality in Patients with Dry Eye Disease. Life. 2020; 10(7):105. https://doi.org/10.3390/life10070105
Chicago/Turabian StyleTakahashi, Aya, Kazuno Negishi, Masahiko Ayaki, Miki Uchino, and Kazuo Tsubota. 2020. "Nocturnal Lagophthalmos and Sleep Quality in Patients with Dry Eye Disease" Life 10, no. 7: 105. https://doi.org/10.3390/life10070105
APA StyleTakahashi, A., Negishi, K., Ayaki, M., Uchino, M., & Tsubota, K. (2020). Nocturnal Lagophthalmos and Sleep Quality in Patients with Dry Eye Disease. Life, 10(7), 105. https://doi.org/10.3390/life10070105






